Idiopathic Osteoporosis and Nephrolithiasis: Two Sides of the Same Coin?
Abstract
:1. Introduction
2. Definition and Epidemiological Data
3. Natural History and Statistical Evidence
4. Pathogenesis
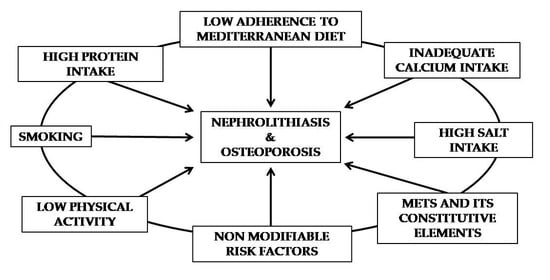
4.1. Modifiable Risk Factors
4.2. Non-Modifiable Risk Factors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Golden, S.H.; Robinson, K.A.; Saldanha, I.; Anton, B.; Ladenson, P.W. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: A comprehensive review. J. Clin. Endocrinol. Metab. 2009, 94, 1853–1878. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 8, 1137–1141. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation Facts and Statistics. Available online: https://www.iofbonehealth.org/facts-statistics (accessed on 2 May 2020).
- Ward, L.M.; Weber, D.R.; Munns, C.F.; Högler, W.; Zemel, B.S. A Contemporary View of the Definition and Diagnosis of Osteoporosis in Children and Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, dgz294. [Google Scholar] [CrossRef] [PubMed]
- Centres de Référence des Maladies Rares du Calcium et du Phosphate Protocole National de Diagnostic et de Soins (PNDS) Fragilités Osseuses Secondaires de L’enfant. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2019-10/pnds_fragilites_osseuses-27-09-2019.pdf (accessed on 31 July 2020).
- Bianchi, M.L.; Baim, S.; Bishop, N.J.; Gordon, C.M.; Hans, D.B.; Langman, C.B.; Leonard, M.B.; Kalkwarf, H.J. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr. Nephrol. 2010, 25, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; El-Hajj Fuleihan, G.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef]
- Laine, C.M.; Laine, T. Diagnosis of osteoporosis in children and adolescents. Eur. Endocrinol. 2013, 9, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Gambaro, G.; Croppi, E.; Coe, F.; Lingeman, J.; Moe, O.; Worcester, E.; Buchholz, N.; Bushinsky, D.; Curhan, G.C.; Ferraro, P.M.; et al. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: A consensus statement. J. Nephrol. 2016, 29, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, J.B.; Matlaga, B.R. Epidemiology and economics of nephrolithiasis. Investig. Clin. Urol. 2017, 58, 299–306. [Google Scholar] [CrossRef]
- European Association of Urology Guidelines 2018 Edition. 2019. Available online: https://uroweb.org/wp-content/uploads/Guidelines_WebVersion_Complete-1.pdf (accessed on 2 May 2020).
- Sas, D.J. Dietary risk factors for urinary stones in children. Curr. Opin. Pediatr. 2020, 32, 284–287. [Google Scholar] [CrossRef]
- Habbig, S.; Beck, B.B.; Hoppe, B. Nephrocalcinosis and urolithiasis in children. Kidney Int. 2011, 80, 1278–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copelovitch, L. Urolithiasis in children: Medical approach. Pediatr. Clin. N. Am. 2012, 59, 881–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhe, M.; Hangm, Z. Nephrolithiasis as a risk factor of chronic kidney disease: A meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis 2017, 45, 441–448. [Google Scholar] [CrossRef]
- Lucato, P.; Trevisan, C.; Stubbs, B.; Zanforlini, B.M.; Solmi, M.; Luchini, C.; Girotti, G.; Pizzato, S.; Manzato, E.; Sergi, G.; et al. Nephrolithiasis, bone mineral density, osteoporosis, and fractures: A systematic review and comparative meta-analysis. Osteoporos. Int. 2016, 27, 3155–3164. [Google Scholar] [CrossRef]
- Sakhaee, K.; Maalouf, N.M.; Poindexter, J.; Adams-Huet, B.; Moe, O.W. Relationship between urinary calcium and bone mineral density in patients with calcium nephrolithiasis. J. Urol. 2017, 197, 1472–1477. [Google Scholar] [CrossRef]
- Chou, P.S.; Kuo, C.N.; Hung, K.S.; Chang, W.C.; Liao, Y.C.; Chi, Y.C.; Chou, W.P.; Tsai, S.J.; Liu, M.E.; Lai, C.L.; et al. Osteoporosis and the risk of symptomatic nephrolithiasis: A population-based 5-year follow-up study in Taiwan. Calcif. Tissue Int. 2014, 95, 317–322. [Google Scholar] [CrossRef]
- Prochaska, M.; Taylor, E.; Vaidya, A.; Curhan, G. Low bone density and bisphosphonate use and the risk of kidney stones. Clin. J. Am. Soc. Nephrol. 2017, 12, 1284–1290. [Google Scholar] [CrossRef]
- Rendina, D.; D’Elia, L.; Evangelista, M.; De Filippo, G.; Giaquinto, A.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Osteoporosis is a predictive factor for nephrolithiasis in an adult free-living Caucasian population from southern Italy: A longitudinal retrospective study based on a general practice database Calcif. Tissue Int. 2020, in press. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and dietary patterns related to osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanozzi, A.; Avallone, S.; Barbato, A.; Iacone, R.; Russo, O.; De Filippo, G.; D’Angelo, G.; Pensabene, L.; Malamisura, B.; Cecere, G.; et al. High sodium and low potassium intake among Italian children: Relationship with age, body mass and blood pressure. PLoS ONE 2015, 10, e0121183. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 2 May 2020).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/salt/reduce_sodium_tips.htm (accessed on 2 May 2020).
- World Action on Salt and Health. Available online: http://www.worldactiononsalt.com/ (accessed on 2 May 2020).
- Società Italiana Nutrizione Umana. Available online: https://sinu.it/meno-sale-piu-salute/ (accessed on 2 May 2020).
- Cappuccio, F.P.; Kalaitzidis, R.; Duneclift, S.; Eastwood, J.B. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J. Nephrol. 2000, 13, 169–177. [Google Scholar]
- Van der Wijst, J.; Tutakhel, O.A.Z.; Bos, C.; Danser, A.H.J.; Hoorn, E.J.; Hoenderop, J.G.J.; Bindels, R.J.M. Effects of a high-sodium/low-potassium diet on renal calcium, magnesium, and phosphate handling. Am. J. Physiol. Ren. Physiol. 2018, 315, F110–F122. [Google Scholar] [CrossRef]
- Blackwood, A.M.; Sagnella, G.A.; Cook, D.G.; Cappuccio, F.P. Urinary calcium excretion, sodium intake and blood pressure in a multi-ethnic population: Results of the Wandsworth Heart and Stroke Study. J. Hum. Hypertens. 2001, 15, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Timio, F.; Kerry, S.M.; Anson, K.M.; Eastwood, J.B.; Cappuccio, F.P. Calcium urolithiasis, blood pressure and salt intake. Blood Press. 2003, 12, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Haberle, D.A.; von Baeyer, D.H. Characteristics of glomerulotubular balance. Am. J. Physiol. 1983, 244, F355–F366. [Google Scholar] [CrossRef]
- Friedman, P.A.; Gesek, F.A. Calcium transport in renal epithelial cells. Am. J. Physiol. 1993, 264, F181–F198. [Google Scholar] [CrossRef]
- Kleeman, C.R.; Bohannan, J.; Bernstein, D.; Ling, S.; Maxwell, M.H. Effects of variations in sodium intake on calcium excretion in normal humans. Proc. Soc. Exp. Biol. Med. 1964, 115, 29–32. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Maalouf, N.M.; Borghi, L.; Meschi, T. Salt and nephrolithiasis. Nephrol. Dial. Transplant. 2016, 31, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Nordin, B.E.C.; Need, A.G.; Steurer, T.; Morris, H.A.; Chatterton, B.E.; Horowitz, M. Nutrition, osteoporosis, and aging. Ann. N. Y. Acad. Sci. 1998, 854, 336–351. [Google Scholar] [CrossRef]
- Cirillo, M.; Ciacci, C.; Laurenzi, M.; Mellone, M.; Mazzacca, G.; De Santo, N.G. Salt intake, urinary sodium, and hypercalciuria. Miner. Electrolyte Metab. 1997, 23, 265–268. [Google Scholar] [PubMed]
- Sakhaee, K.; Harvey, J.A.; Padalino, P.K.; Whitson, P.; Pak, C.Y. The potential role of salt abuse on the risk for kidney stone formation. J. Urol. 1993, 150, 310–312. [Google Scholar] [CrossRef]
- Moe, O.W.; Preisig, P.A. Hypothesizing on the evolutionary origins of salt-induced hypercalciuria. Curr. Opin. Nephrol. Hypertens. 2005, 14, 368–372. [Google Scholar] [CrossRef]
- Caudarella, R.; Vescini, F.; Buffa, A.; Stefoni, S. Citrate and mineral metabolism: Kidney stones and bone disease. Front. Biosci. 2003, 8, s1084–s1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frings-Meuthen, P.; Baecker, N.; Heer, M. Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption. J. Bone Miner. Res. 2008, 23, 517–524. [Google Scholar] [CrossRef]
- Kirejczyk, J.K.; Korzeniecka-Kozerska, A.; Baran, M.; Porowska, H.; Porowski, T.; Wasilewska, A. Dyslipidaemia in overweight children and adolescents is associated with an increased risk of kidney stones. Acta Paediatr. 2015, 104, e407–e413. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Kiremit, M.C.; Sag, A.A.; Tarim, K.; Acar, O.; Esen, T.; Solak, Y.; Covic, A.; Kanbay, M. The role of sodium intake in nephrolithiasis: Epidemiology, pathogenesis, and future directions. Eur. J. Intern. Med. 2016, 35, 16–19. [Google Scholar] [CrossRef]
- Rendina, D.; De Filippo, G.; D’Elia, L.; Strazzullo, P. Metabolic syndrome and nephrolithiasis: A systematic review and meta-analysis of the scientific evidence. J. Nephrol. 2014, 27, 371–376. [Google Scholar] [CrossRef]
- Rendina, D.; D’Elia, L.; Evangelista, M.; De Filippo, G.; Giaquinto, A.; Abate, V.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Metabolic syndrome is associated to an increased risk of low bone mineral density in free-living women with suspected osteoporosis. J. Endocrinol. Investig. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; De Filippo, G.; Zampa, G.; Muscariello, R.; Mossetti, G.; Strazzullo, P. Characteristic clinical and biochemical profile of recurrent calcium-oxalate nephrolithiasis in patients with metabolic syndrome. Nephrol. Dial. Transplant. 2011, 26, 2256–2263. [Google Scholar] [CrossRef] [Green Version]
- Fatahi, S.; Namazi, N.; Larijani, B.; Azadbakht, L. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2018, 37, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Edwards, D.G.; Farquhar, W.B. The influence of dietary salt beyond blood pressure. Curr. Hypertens. Rep. 2019, 21, 42. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization. Calcium Intake Levels in the United States: Issues and Considerations 2019. Available online: http://www.fao.org/3/W7336T/w7336t06.htm (accessed on 2 May 2020).
- Gaddi, A.; Cicero, A.F.; Wani, F.O.; Dormi, A.; Pasquarelli, V.; D’Addato, S. The realization of a project aimed at reducing the plasmatic lipid level in a large Italian population improves the mean calcium daily intake: The Brisighella Study. Eur. J. Clin. Nutr. 2001, 55, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N. Engl. J. Med. 1993, 328, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Marengo, S.R.; Romani, A.M. Oxalate in renal stone disease: The terminal metabolite that just won’t go away. Nat. Clin. Pract. Nephrol. 2008, 4, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Schianchi, T.; Meschi, T.; Guerra, A.; Allegri, F.; Maggiore, U.; Novarini, A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N. Engl. J. Med. 2002, 346, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Tremaine, W.J.; De Simone, C.; O’Connor, H.M.; Li, X.; Bergstralh, E.J.; Goldfarb, D.S. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 2010, 78, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNicolantonio, J.J.; Mehta, V.; Zaman, S.B.; O’Keefe, J.H. Not salt but sugar as aetiological in osteoporosis: A review. Mo. Med. 2018, 115, 247–252. [Google Scholar]
- Ericsson, Y.; Angmar-Månsson, B.; Flores, M. Urinary mineral ion loss after sugar ingestion. Bone Miner. 1990, 9, 233–237. [Google Scholar] [CrossRef]
- Douard, V.; Sabbagh, Y.; Lee, J.; Patel, C.; Kemp, F.W.; Bogden, J.D.; Lin, S.; Ferraris, R.P. Excessive fructose intake causes 1,25-(OH)(2)D(3)-dependent inhibition of intestinal and renal calcium transport in growing rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1303–E1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, M.; Inaba, M.; Yano, Y.; Hasuma, T.; Nishizawa, Y.; Morii, H.; Otani, S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone 1998, 22, 17–23. [Google Scholar] [CrossRef]
- Merlotti, D.; Gennari, L.; Dotta, F.; Lauro, D.; Nuti, R. Mechanisms of impaired bone strength in type 1 and 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 683–690. [Google Scholar] [CrossRef]
- Wyshak, G.; Frisch, R.E. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J. Adolesc. Health 1994, 15, 210–215. [Google Scholar] [CrossRef]
- Wyshak, G. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch. Pediatr. Adolesc. Med. 2000, 154, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Jones, G. Soft drink and milk consumption, physical activity, bone mass, and upper limb fractures in children: A population-based case-control study. Calcif. Tissue Int. 2004, 75, 286–291. [Google Scholar] [CrossRef]
- Petridou, E.; Karpathios, T.; Dessypris, N.; Simou, E.; Trichopoulos, D. The role of dairy products and non alcoholic beverages in bone fractures among schoolage children. Scand. J. Soc. Med. 1997, 25, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Thom, J.A.; Morris, J.E.; Bishop, A.; Blacklock, N.J. The influence of refined carbohydrate on urinary calcium excretion. Br. J. Urol. 1978, 50, 459–464. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Soda and other beverages and the risk of kidney stones. Clin. J. Am. Soc. Nephrol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denova-Gutiérrez, E.; Méndez-Sánchez, L.; Muñoz-Aguirre, P.; Tucker, K.L.; Clark, P. Dietary patterns, bone mineral density, and risk of fractures: A systematic review and meta-analysis. Nutrients 2018, 10, 1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odermatt, A. The Western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Curhan, G.C.; Gambaro, G.; Taylor, E.N.; Ferraro, P.M. Mediterranean diet adherence and risk of incident kidney stones. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Fernández-Montero, A.; de la Fuente-Arrillaga, C.; Martínez-González, M.Á.; Bertoli, S.; Battezzati, A.; Bes-Rastrollo, M. Adherence to the mediterranean dietary pattern and incidence of nephrolithiasis in the Seguimiento Universidad de Navarra Follow-up (SUN) Cohort. Am. J. Kidney Dis. 2017, 70, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Bertoli, S.; Terranegra, A.; Brasacchio, C.; Mingione, A.; Dogliotti, E.; Raspini, B.; Leone, A.; Frau, F.; Vignati, L.; et al. Relevance of Mediterranean diet and glucose metabolism for nephrolithiasis in obese subjects. J. Transl. Med. 2014, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomeras-Vilches, A.; Viñals-Mayolas, E.; Bou-Mias, C.; Jordà-Castro, M.; Agüero-Martínez, M.; Busquets-Barceló, M.; Pujol-Busquets, G.; Carrion, C.; Bosque-Prous, M.; Serra-Majem, L.; et al. Adherence to the mediterranean diet and bone fracture risk in middle-aged women: A case control study. Nutrients 2019, 11, 2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmir, H.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Adherence to mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2018, 57, 2147–2160. [Google Scholar] [CrossRef]
- UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 2 May 2020).
- Keys, A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995, 61, 1321S–1323S. [Google Scholar] [CrossRef] [Green Version]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of Fruits and Vegetables with Major Cardiometabolic Risk Factors, Markers of Oxidation, and Inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef]
- Khan, S.R. Stress oxidative: Nephrolithiasis and chronic kidney diseases. Minerva Med. 2013, 104, 23–30. [Google Scholar] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 2, 115–137. [Google Scholar] [CrossRef] [Green Version]
- Manolagas, S.C.; Kousteni, S.; Jilka, R.L. Sex steroids and bone. Recent Prog. Horm. Res. 2002, 57, 385–409. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Liu, A.L.; Zhang, Z.M.; Li, X.M.; Zou, Z.P.; Zeng, W.S.; Cheng, B.L.; Luo, S.Q. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J. Biol. Chem. 2005, 280, 17497–17506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, P.; Kontek, B.; Skalski, B.; Król, A.; Różański, W.; Olas, B. Oxidative Stress and Hemostatic Parameters in Patients with Nephrolithiasis before and After Ureteroscopic Lithotripsy. Front. Physiol. 2019, 21, 799. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, D.; Ji, M.F.; Liu, T.; Mei, C.L.; Tang, X.J. Activation of liver X receptor suppresses osteopontin expression and ameliorates nephrolithiasis. J. Cell. Physiol. 2019, 234, 14109–14122. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Li, C.; Lu, Y.; Hu, H.; Qin, B.; Xun, Y.; Zhu, Y.; Wu, Y.; Zhang, J.; et al. Inhibiting inflammation and modulating oxidative stress in oxalate-induced nephrolithiasis with the Nrf2 activator dimethyl fumarate. Free Radic. Biol. Med. 2019, 134, 9–22. [Google Scholar] [CrossRef]
- Woźniak, P.; Kontek, B.; Różański, W.; Olas, B. The lipid peroxidation in patients with nephrolithiasis before and after extracorporeal shock wave lithotripsy. Future Med. Chem. 2018, 23, 2685–2693. [Google Scholar] [CrossRef]
- Yasui, T.; Okada, A.; Hamamoto, S.; Ando, R.; Taguchi, K.; Tozawa, K.; Kohri, K. Pathophysiology-based treatment of urolithiasis. Int. J. Urol. 2017, 24, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, M.D.; Chi, T.; Shara, N.M.; Wang, H.; Hsi, R.S.; Orchard, T.; Kahn, A.J.; Jackson, R.D.; Miller, J.; Reiner, A.P.; et al. Activity, energy intake, obesity, and the risk of incident kidney stones in postmenopausal women: A report from the Women’s Health Initiative. J. Am. Soc. Nephrol. 2014, 25, 362–369. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, G.; Trinchieri, A. Recent advances in managing and understanding nephrolithiasis/nephrocalcinosis. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronado-Zarco, R.; Olascoaga-Gómez de León, A.; García-Lara, A.; Quinzaños-Fresnedo, J.; Nava-Bringas, T.I.; Macías-Hernández, S.I. Nonpharmacological interventions for osteoporosis treatment: Systematic review of clinical practice guidelines. Osteoporos. Sarcopenia 2019, 5, 69–77. [Google Scholar] [CrossRef]
- Pathak, J.L.; Bravenboer, N.; Klein-Nulend, J. The Osteocyte as the New Discovery of Therapeutic Options in Rare Bone Diseases. Front. Endocrinol. 2020, 11, 405. [Google Scholar] [CrossRef]
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte Mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Carina, V.; Della Bella, E.; Costa, V.; Bellavia, D.; Veronesi, F.; Cepollaro, S.; Fini, M.; Giavaresi, G. Bone’s Response to Mechanical Loading in Aging and Osteoporosis: Molecular Mechanisms. Calcif. Tissue Int. 2020, 107, 301–318. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.; Shackelford, L.C.; Sibonga, J.D.; Spatz, J.; Pietrzyk, R.A.; Hudson, E.K.; Zwart, S.R. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015, 81, 712–720. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Heer, M.; Hudson, E.K.; Shackelford, L.; Morgan, J.L. Men and women in space: Bone loss and kidney stone risk after long-duration spaceflight. J. Bone Miner. Res. 2014, 29, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The effects of smoking on bone metabolism. Osteoporos. Int. 2012, 23, 2081–2092. [Google Scholar] [CrossRef]
- Cusano, N.E. Skeletal Effects of Smoking. Curr. Osteoporos. Rep. 2015, 13, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Huang, S.P.; Wu, W.J.; Chou, Y.H.; Juo, S.H.; Tsai, L.Y.; Huang, C.H.; Wu, M.T. The impact of cigarette smoking, alcohol drinking and betel quid chewing on the risk of calcium urolithiasis. Ann. Epidemiol. 2009, 19, 539–545. [Google Scholar] [CrossRef]
- Jones, P.; Karim Sulaiman, S.; Gamage, K.N.; Tokas, T.; Jamnadass, E.; Somani, B.K. Do lifestyle factors including smoking, alcohol, and exercise impact your risk of developing kidney stone disease? Outcomes of a systematic review. J. Endourol. 2020, in press. [Google Scholar] [CrossRef]
- Prezioso, D.; Strazzullo, P.; Lotti, T.; Bianchi, G.; Borghi, L.; Caione, P.; Carini, M.; Caudarella, R.; Ferraro, M.; Gambaro, G.; et al. Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group. Arch. Ital. Urol. Androl. 2015, 87, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, L.; Bonaccio, M.; Cairella, G.; Catani, M.V.; Costanzo, S.; D’Elia, L.; Giacco, R.; Rendina, D.; Sabino, P.; Savini, I.; et al. Working Group for Nutrition and Stroke Diet and primary prevention of stroke: Systematic review and dietary recommendations by the ad hoc Working Group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 309–334. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.A.; McKay, H.A.; Mirwald, R.L.; Crocker, P.R.; Faulkner, R.A. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The university of Saskatchewan bone mineral accrual study. J. Bone Miner. Res. 1999, 14, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; TmavaBerisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, in press. [Google Scholar] [CrossRef]
- Hagenau, T.; Vest, R.; Gissel, T.N.; Poulsen, C.S.; Erlandsen, M.; Mosekilde, L.; Vestergaard, P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos. Int. 2009, 20, 133–140. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D in childhood and adolescence. Postgrad. Med. J. 2007, 83, 230–235. [Google Scholar] [CrossRef]
- Girón-Prieto, M.S.; Del Carmen Cano-García, M.; Arrabal-Polo, M.Á.; Poyatos-Andujar, A.; Quesada-Charneco, M.; de Haro-Muñoz, T.; Arias-Santiago, S.; Arrabal-Martín, M. Analysis of vitamin D deficiency in calcium stone-forming patients. Int. Urol. Nephrol. 2016, 48, 1243–1246. [Google Scholar] [CrossRef]
- Singh, G.V.; Hampson, G.; Thomas, K.; Bultitude, M.; Willis, S. Vitamin D and kidney stones is there an association? BJU Int. 2019, 123, 751–752. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014, 10, CD007470. [Google Scholar] [CrossRef]
- Rendina, D.; De Filippo, G.; Merlotti, D.; Di Stefano, M.; Mingiano, C.; Giaquinto, A.; Evangelista, M.; Bo, M.; Arpino, S.; Faraonio, R.; et al. Increased prevalence of nephrolithiasis and hyperoxaluria in Paget’s disease of bone. J. Clin. Endocrinol. Metab. 2020, in press. [Google Scholar] [CrossRef]
- Rendina, D.; De Filippo, G.; Merlotti, D.; Di Stefano, M.; Succoio, M.; Muggianu, S.M.; Bianciardi, S.; D’Elia, L.; Coppo, E.; Faraonio, R.; et al. Vitamin D status in Paget disease of bone and efficacy-safety profile of cholecalciferol treatment in pagetic patients with hypovitaminosis D. Calcif. Tissue Int. 2019, 105, 412–422. [Google Scholar] [CrossRef]
- Ralston, S.H.; Uitterlinden, A.G. Genetics of osteoporosis. Endocr. Rev. 2010, 31, 629–662. [Google Scholar] [CrossRef] [Green Version]
- Howles, S.A.; Thakker, R.V. Genetics of kidney stone disease. Nat. Rev. Urol. 2020, 17, 407–421. [Google Scholar] [CrossRef]
- Di Nisio, A.; Rocca, M.S.; Ghezzi, M.; Ponce, M.R.; Taglianetti, S.; Plebani, M.; Ferlin, A.; Foresta, C. Calcium-sensing receptor polymorphisms increase the risk of osteoporosis in ageing males. Endocrine 2018, 61, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.B.; Guo, J.J.; Liu, Y.J.; Deng, F.Y.; Jiang, D.K.; Deng, H.W. The human calcium-sensing receptor and interleukin-6 genes are associated with bone mineral density in Chinese. Yi Chuan Xue Bao 2006, 33, 870–880. [Google Scholar] [CrossRef]
- Vezzoli, G.; Terranegra, A.; Aloia, A.; Arcidiacono, T.; Milanesi, L.; Mosca, E.; Mingione, A.; Spotti, D.; Cusi, D.; Hou, J.; et al. Decreased transcriptional activity of calcium-sensing receptor gene promoter 1 is associated with calcium nephrolithiasis. J. Clin. Endocrinol. Metab. 2013, 98, 3839–3847. [Google Scholar] [CrossRef] [Green Version]
- González-Castro, T.B.; Blachman-Braun, R.; Hernández-Díaz, Y.; Tovilla-Zárate, C.A.; Pérez-Hernández, N.; Moscardi, P.R.M.; Alam, A.; Borgonio-Cuadra, V.M.; Reyes-López, P.A.; Juárez-Rojop, I.E.; et al. Association of vitamin D receptor polymorphisms and nephrolithiasis: A meta-analysis. Gene 2019, 711, 143936. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; De Filippo, G.; Gianfrancesco, F.; Muscariello, R.; Schiano di Cola, M.; Strazzullo, P.; Esposito, T. Evidence for epistatic interaction between VDR and SLC13A2 genes in the pathogenesis of hypocitraturia in recurrent calcium oxalate stone formers. J. Nephrol. 2017, 30, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; Mossetti, G.; Viceconti, R.; Sorrentino, M.; Castaldo, R.; Manno, G.; Guadagno, V.; Strazzullo, P.; Nunziata, V. Association between vitamin D receptor gene polymorphisms and fasting idiopathic hypercalciuria in recurrent stone-forming patients. Urology 2004, 64, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mossetti, G.; Rendina, D.; Viceconti, R.; Manno, G.; Guadagno, V.; Strazzullo, P.; Nunziata, V. The relationship of 3′ vitamin D receptor haplotypes to urinary supersaturation of calcium oxalate salts and to age at onset and familial prevalence of nephrolithiasis. Nephrol. Dial. Transplant. 2004, 19, 2259–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mossetti, G.; Vuotto, P.; Rendina, D.; Numis, F.G.; Viceconti, R.; Giordano, F.; Cioffi, M.; Scopacasa, F.; Nunziata, V. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J. Intern. Med. 2003, 253, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Palsson, R.; Indridason, O.S.; Edvardsson, V.O.; Oddsson, A. Genetics of common complex kidney stone disease: Insights from genome-wide association studies. Urolithiasis 2019, 47, 11–21. [Google Scholar] [CrossRef]
- Peris, P.; González-Roca, E.; Rodríguez-García, S.C.; Del Mar López-Cobo, M.; Monegal, A.; Guañabens, N. Incidence of mutations in the ALPL, GGPS1, and CYP1A1 genes in patients with atypical femoral fractures. JBMR Plus 2018, 3, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Larraz-Prieto, B.; Berg, K.; Lambert, Z.; Redmond, P.; Harris, S.E.; Deary, I.J.; Pugh, C.; Prendergast, J.; Ralston, S.H. Loss-of-function mutations in the ALPL gene presenting with adult onset osteoporosis and low serum concentrations of total alkaline phosphatase. J. Bone Miner. Res. 2020, 35, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. Association between polymorphisms in osteopontin gene (SPP1) and first episode calcium oxalate urolithiasis. Urolithiasis 2013, 41, 303–313. [Google Scholar] [CrossRef]
- Gao, B.; Yasui, T.; Itoh, Y.; Li, Z.; Okada, A.; Tozawa, K.; Hayashi, Y.; Kohri, K. Association of osteopontin gene haplotypes with nephrolithiasis. Kidney Int. 2007, 72, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Thorleifsson, G.; Holm, H.; Edvardsson, V.; Walters, G.B.; Styrkarsdottir, U.; Gudbjartsson, D.F.; Sulem, P.; Halldorsson, B.V.; de Vegt, F.; d’Ancona, F.C.; et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat. Genet. 2009, 41, 926–930. [Google Scholar] [CrossRef]
- Tang, R.; Wei, Y.; Li, Z.; Chen, H.; Miao, Q.; Bian, Z.; Zhang, H.; Wang, Q.; Wang, Z.; Lian, M.; et al. A Common variant in CLDN14 is associated with primary biliary cirrhosis and bone mineral density. Sci. Rep. 2016, 6, 19877. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Choi, H.J.; Estrada, K.; Leo, P.J.; Li, J.; Pei, Y.F.; Zhang, Y.; Lin, Y.; Shen, H.; Liu, Y.Z.; et al. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum. Mol. Genet. 2014, 23, 1923–1933. [Google Scholar] [CrossRef] [Green Version]
- Prié, D.; Huart, V.; Bakouh, N.; Planelles, G.; Dellis, O.; Gérard, B.; Hulin, P.; Benqué-Blanchet, F.; Silve, C.; Grandchamp, B.; et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N. Engl. J. Med. 2002, 347, 983–989. [Google Scholar] [CrossRef]
- Rendina, D.; Esposito, T.; Mossetti, G.; De Filippo, G.; Gianfrancesco, F.; Perfetti, A.; Magliocca, S.; Formisano, P.; Prié, D.; Strazzullo, P. A functional allelic variant of the FGF23 gene is associated with renal phosphate leak in calcium nephrolithiasis. J. Clin. Endocrinol. Metab. 2012, 97, E840–E844. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, P.M.; Minucci, A.; Primiano, A.; De Paolis, E.; Gervasoni, J.; Persichilli, S.; Naticchia, A.; Capoluongo, E.; Gambaro, G. A novel CYP24A1 genotype associated to a clinical picture of hypercalcemia, nephrolithiasis and low bone mass. Urolithiasis 2017, 45, 291–294. [Google Scholar] [CrossRef]
- Wray, N.R.; Wijmenga, C.; Sullivan, P.F.; Yang, J.; Visscher, P.M. Common disease is more complex than implied by the core gene omnigenic model. Cell 2018, 173, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, M. Renal Ca2+ and Water Handling in Response to Calcium Sensing Receptor Signaling: Physiopathological Aspects and Role of CaSR-Regulated microRNAs. Int. J. Mol. Sci. 2019, 20, 5341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Arita, K.; Nanda, A.; Wessagowit, V.; Akiyama, M.; Alsaleh, Q.A.; McGrath, J.A. A novel mutation in the VDR gene in hereditary vitamin D-resistant rickets. Br. J. Dermatol. 2008, 158, 168–171. [Google Scholar] [CrossRef]
- Weiss, M.J.; Henthorn, P.S.; Lafferty, M.A.; Slaughter, C.; Raducha, M.; Harris, H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. USA 1986, 83, 7182–7186. [Google Scholar] [CrossRef] [Green Version]
- Zurutuza, L.; Muller, F.; Gibrat, J.F.; Taillandier, A.; Simon-Bouy, B.; Serre, J.L.; Mornet, E. Correlations of genotype and phenotype in hypophosphatasia. Hum. Mol. Genet. 1999, 8, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Ogbureke, K.U.; Fisher, L.W. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int. 2005, 68, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleberzon, J.S.; Liao, Y.; Mittler, S.; Goldberg, H.A.; Grohe, B. Incorporation of osteopontin peptide into kidney stone-related calcium oxalate monohydrate crystals: A quantitative study. Urolithiasis 2019, 47, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegård, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Hou, J. Claudins in barrier and transport function-the kidney. Pflugers Arch. 2017, 469, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Yamashita, T. FGF23 is a hormone-regulating phosphate metabolism—Unique biological characteristics of FGF23. Bone 2007, 40, 1190–1195. [Google Scholar] [CrossRef]
- Mossetti, G.; Rendina, D.; De Filippo, G.; Zampa, G.; Strazzullo, P. Phosphatonins: Novel insights and clinical perspectives. G. Ital. Nefrol. 2009, 26, 171–180. [Google Scholar]
- Rendina, D.; Mossetti, G.; De Filippo, G.; Cioffi, M.; Strazzullo, P. Fibroblast growth factor 23 is increased in calcium nephrolithiasis with hypophosphatemia and renal phosphate leak. J. Clin. Endocrinol. Metab. 2006, 91, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Prié, D.; Beck, L.; Silve, C.; Friedlander, G. Hypophosphatemia and calcium nephrolithiasis. Nephron Exp. Nephrol. 2004, 98, e50–e54. [Google Scholar] [CrossRef]
- Murer, H.; Hernando, N.; Forster, I.; Biber, J. Proximal tubular phosphate reabsorption: Molecular mechanisms. Physiol. Rev. 2000, 80, 1373–1409. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Irwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Kuwertz-Bröking, E.; Fehrenbach, H.; et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011, 365, 410–421. [Google Scholar] [CrossRef]
- Labuda, M.; Lemieux, N.; Tihy, F.; Prinster, C.; Glorieux, F.H. Human 25-hydroxyvitamin D 24-hydroxylase cytochrome P450 subunit maps to a different chromosomal location than that of pseudovitamin D-deficient rickets. J. Bone Miner. Res. 1993, 8, 1397–1406. [Google Scholar] [CrossRef] [PubMed]

| Gene | HGNC Symbol | Location | Ref. |
|---|---|---|---|
| Calcium-sensing receptor | CASR | 3q13.3-q21.1 | [124,125,126] |
| Vitamin D receptor | VDR | 12q13.11 | [127,128,129,130,131,132] |
| Alkaline phosphatase | ALPL | 1p36.12 | [133,134] |
| Osteopontin | SPP1 | 4q22.1 | [135,136] |
| Claudin 14 | CLDN14 | 21q22.13 | [137,138,139] |
| Type 2a sodium–phosphate cotransporter | SLC34A1 | 5q35.3 | [140] |
| Fibroblast growth factor 23 | FGF23 | 12p13.32 | [141] |
| 25(OH)D-24-hydroxylase | CYP24A1 | 20q13.2 | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendina, D.; De Filippo, G.; Iannuzzo, G.; Abate, V.; Strazzullo, P.; Falchetti, A. Idiopathic Osteoporosis and Nephrolithiasis: Two Sides of the Same Coin? Int. J. Mol. Sci. 2020, 21, 8183. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218183
Rendina D, De Filippo G, Iannuzzo G, Abate V, Strazzullo P, Falchetti A. Idiopathic Osteoporosis and Nephrolithiasis: Two Sides of the Same Coin? International Journal of Molecular Sciences. 2020; 21(21):8183. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218183
Chicago/Turabian StyleRendina, Domenico, Gianpaolo De Filippo, Gabriella Iannuzzo, Veronica Abate, Pasquale Strazzullo, and Alberto Falchetti. 2020. "Idiopathic Osteoporosis and Nephrolithiasis: Two Sides of the Same Coin?" International Journal of Molecular Sciences 21, no. 21: 8183. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218183