Glycemic Status Assessment by the Latest Glucose Monitoring Technologies
Abstract
:1. Introduction
2. Data Management
2.1. Data Management: Diabetology Team “Perspective”
2.2. Patient “Perspective”: Data Management: Integrated Glycemic State
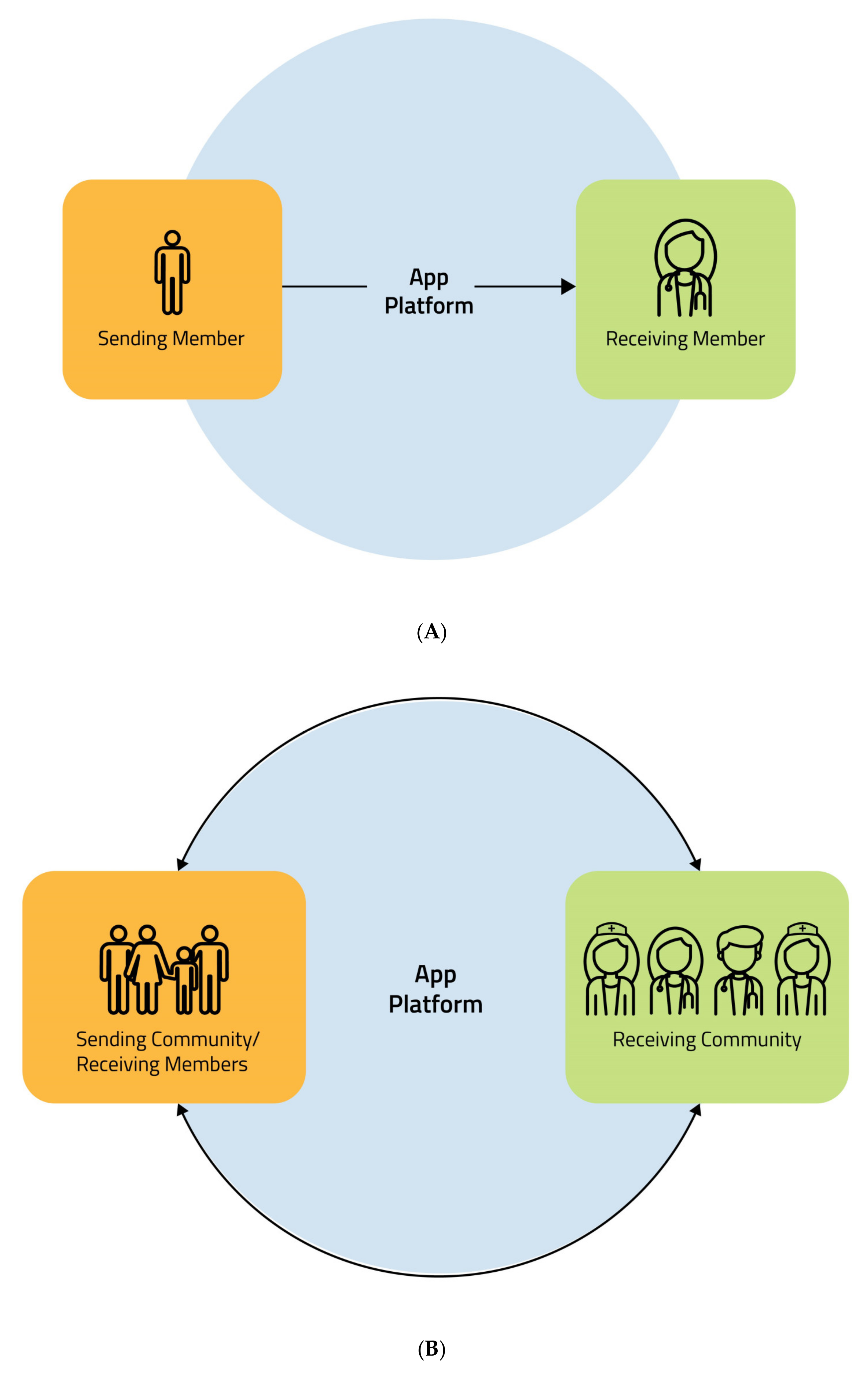
2.3. Data Management: “Circular Flow”
3. Current Glucose Monitoring Systems
4. Glucose Monitoring in Different Populations
4.1. T1D Children, Adolescents, and Youth Adults
4.2. T1D and T2D Insulin-Treated Adults
4.3. Pregnancy Insulin-Treated
5. Cost-Effectiveness
6. Emerging and Future Glucose Monitoring Systems
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajjan, R.A. How Can We Realize the Clinical Benefits of Continuous Glucose Monitoring? Diabetes Technol. Ther. 2017, 19, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F. 2019 ESC Guidelines on Diabetes, Pre-Diabetes and Cardiovascular Diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [PubMed] [Green Version]
- ADA. Diabetes Technology: Standards of Medical Care in Diabetes. Diabetes Care 2020, 43, S77–S88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergenstal, R.M.; Beck, R.W.; Close, K.L.; Grunberger, G.; Sacks, D.B.; Kowalski, A.; Brown, A.S.; Heinemann, L.; Aleppo, G.; Ryan, D.B.; et al. Glucose Management Indicator (GMI): A New Term for Estimating A1C from Continuous Glucose Monitoring. Diabetes Care 2018, 4, 2275–2280. [Google Scholar] [CrossRef] [Green Version]
- Frontoni, S.; Di Bartolo, P.; Avogaro, A.; Bosi, E.; Paolisso, G.; Ceriello, A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 102, 86–95. [Google Scholar] [CrossRef]
- Picconi, F.; Di Flaviani, A.; Malandrucco, I.; Giordani, I.; Frontoni, S. Impact of glycemic variability on cardiovascular outcomes beyond glycated hemoglobin. Evidence and clinical perspectives. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 691–696. [Google Scholar] [CrossRef]
- Ladyzynski, P.; Foltynski, P.; Bak, M.I.; Sabalinska, S.; Krzymien, J.; Kawiak, J. Validation of a hemoglobin A1c model in patients with type 1 and type 2 diabetes and its use to go beyond the averaged relationship of hemoglobin A1c and mean glucose level. J. Transl. Med. 2014, 12, 328. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Ma, X.; Zhang, L.; Mo, Y.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Zhou, J. Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J. Diabetes Investig. 2019, 10, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picconi, F.; Parravano, M.; Ylli, D.; Pasqualetti, P.; Coluzzi, S.; Giordani, I.; Malandrucco, I.; Lauro, D.; Scarinci, F.; Giorno, P.; et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: The role of glycemic variability. Acta Diabetol. 2017, 54, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.E.; Jin, S.M.; Baek, J.; Oh, S.; Hur, K.Y.; Lee, M.S.; Lee, M.K.; Kim, J.H. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 70. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zhao, L.H.; Su, J.B.; Chen, T.; Wang, X.Q.; Chen, J.F.; Wu, G.; Jin, Y.; Wang, X.H. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol. Metab. Syndr. 2014, 6, 139. [Google Scholar] [CrossRef] [Green Version]
- Di Flaviani, A.; Picconi, F.; Di Stefano, P.; Giordani, I.; Malandrucco, I.; Maggio, P.; Palazzo, P.; Sgreccia, F.; Peraldo, C.; Farina, F.; et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 2011, 34, 1605–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordani, I.; Di Flaviani, A.; Picconi, F.; Malandrucco, I.; Ylli, D.; Palazzo, P.; Altavilla, R.; Vernieri, F.; Passarelli, F.; Donno, S.; et al. Acute hyperglycemia reduces cerebrovascular reactivity: The role of glycemic variability. J. Clin. Endocrinol. Metab. 2014, 99, 2854–2860. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.W.; Bergenstal, R.M.; Cheng, P.; Kollman, C.; Carlson, A.L.; Johnson, M.L.; Rodbard, D. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J. Diabetes Sci. Technol. 2019, 13, 614–626. [Google Scholar] [CrossRef]
- Beck, R.W.; Bergenstal, R.M.; Riddlesworth, T.D.; Kollman, C.; Li, Z.; Brown, A.S.; Close, K.L. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care 2019, 42, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Ma, X.; Zhou, J.; Zhang, L.; Mo, Y.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Vigersky, R.A.; et al. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care 2018, 41, 2370–2376. [Google Scholar] [CrossRef] [Green Version]
- Bergenstal, R.M.; Ahmann, A.J.; Bailey, T.; Beck, R.W.; Bissen, J.; Buckingham, B.; Deeb, L.; Dolin, R.H.; Garg, S.K.; Goland, R.; et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: The Ambulatory Glucose Profile (AGP). Diabetes Technol. Ther. 2013, 15, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Vigersky, R.A.; Shin, J.; Jiang, B.; Siegmund, T.; McMahon, C.; Thomas, A. The Comprehensive Glucose Pentagon: A Glucose-Centric Composite Metric for Assessing Glycemic Control in Persons with Diabetes. J. Diabetes Sci. Technol. 2018, 12, 114–123. [Google Scholar] [CrossRef]
- Tauschmann, M.; Hovorka, R. Technology in the management of type 1 diabetes mellitus—Current status and future prospects. Nat. Rev. Endocrinol. 2018, 14, 464–475. [Google Scholar]
- Hommel, E.; Schmidt, S.; Vistisen, D.; Neergaard, K.; Gribhild, M.; Almdal, T.; Nørgaard, K. Effects of advanced carbohydrate counting guided by an automated bolus calculator in Type 1 diabetes mellitus (StenoABC): A 12-month, randomized clinical trial. Diabet. Med. 2017, 34, 708–715. [Google Scholar] [PubMed]
- Bailey, R.A.; Pfeifer, M.; Shillington, A.C.; Harshaw, Q.; Funnell, M.M.; VanWingen, J.; Col, N. Effect of a patient decision aid (PDA) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict. BMC Health Serv. Res. 2016, 16, 10. [Google Scholar]
- Ramchandani, N. Virtual Coaching to Enhance Diabetes Care. Diabetes Technol. Ther. 2019, 21, 248–251. [Google Scholar]
- O’Connor, P.J.; Sperl-Hillen, J.M. Current Status and Future Directions for Electronic Point-of-Care Clinical Decision Support to Improve Diabetes Management in Primary Care. Diabetes Technol. Ther. 2019, 21, 226–234. [Google Scholar]
- Clarke, S.F.; Foster, J.R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar]
- Hirsch, I.B.; Wright, E.E., Jr. Using Flash Continuous Glucose Monitoring in Primary Practice. Clin. Diabetes 2019, 37, 150–161. [Google Scholar]
- Hilliard, M.E.; Yi-Frazier, J.P.; Hessler, D.; Butler, A.M.; Anderson, B.J.; Jaser, S. Stress and A1c Among People with Diabetes Across the Lifespan. Curr. Diab. Rep. 2016, 16, 67. [Google Scholar]
- Powell, P.W.; Corathers, S.D.; Raymond, J.; Streisand, R. New approaches to providing individualized diabetes care in the 21st century. Curr. Diabetes Rev. 2015, 11, 222–230. [Google Scholar]
- Czupryniak, L.; Barkai, L.; Bolgarska, S.; Bronisz, A.; Broz, J.; Cypryk, K.; Honka, M.; Janez, A.; Krnic, M.; Lalic, N.; et al. Self-monitoring of blood glucose in diabetes: From evidence to clinical reality in Central and Eastern Europe--recommendations from the international Central-Eastern European expert group. Diabetes Technol. Ther. 2014, 16, 460–475. [Google Scholar] [PubMed]
- Funtanilla, V.D.; Candidate, P.; Caliendo, T.; Hilas, O. Continuous Glucose Monitoring: A Review of Available Systems. Pharm. Ther. 2019, 44, 550–553. [Google Scholar]
- Colin, I.M.; Paris, I. Glucose meters with built-in automated bolus calculator: Gadget or real value for insulin-treated diabetic patients? Diabetes Ther. 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Meldgaard, M.; Serifovski, N.; Storm, C.; Christensen, T.M.; Gade-Rasmussen, B.; Nørgaard, K. Use of an automated bolus calculator in MDI-treated type 1 diabetes: The BolusCal Study, a randomized controlled pilot study. Diabetes Care 2012, 35, 984–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbone, I.; Scaramuzza, A.E.; Ignaccolo, M.G.; Tinti, D.; Sicignano, S.; Redaelli, F.; De Angelis, L.; Bosetti, A.; Zuccotti, G.V.; Cerutti, F. Carbohydrate counting with an automated bolus calculator helps to improve glycaemic control in children with type 1 diabetes using multiple daily injection therapy: An 18-month observational study. Diabetes Res. Clin. Pract. 2014, 103, 388–394. [Google Scholar] [CrossRef]
- Doupis, J.; Festas, G.; Tsilivigos, C.; Efthymiou, V.; Kokkinos, A. Smartphone-Based Technology in Diabetes Management. Diabetes Ther. 2020, 11, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.W.; Bergenstal, R.M.; Laffel, L.M.; Pickup, J.C. Advances in technology for management of type 1 diabetes. Lancet 2019, 394, 1265–1273. [Google Scholar] [CrossRef]
- Bruttomesso, D.; Laviola, L.; Avogaro, A.; Bonora, E.; Del Prato, S.; Frontoni, S.; Orsi, E.; Rabbone, I.; Sesti, G.; Purrello, F. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: A consensus view of Italian diabetes experts using the Delphi method. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyagüez, I.; Merino-Torres, J.F.; Brito, M.; Bellido, V.; Cardona-Hernandez, R.; Gomez-Peralta, F.; Morales-Perez, F. Cost analysis of the flash monitoring system (FreeStyle Libre 2) in adults with type 1 diabetes mellitus. BMJ Open Diabetes. Res. 2020, 8, e001330. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef]
- Martin, C.T.; Criego, A.B.; Carlson, A.L.; Bergenstal, R.M. Advanced Technology in the Management of Diabetes: Which Comes First-Continuous Glucose Monitor or Insulin Pump? Curr. Diab. Rep. 2019, 19, 50. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, R.; Heidtmann, B.; Hilgard, D.; Hofer, S.; Rosenbauer, J.; Holl, R. DPV-Wiss-Initiative. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2011, 12, 11–17. [Google Scholar] [PubMed]
- Di Bartolo, P.; Nicolucci, A.; Cherubini, V.; Iafusco, D.; Scardapane, M.; Rossi, M.C. Young patients with type 1 diabetes poorly controlled and poorly compliant with self-monitoring of blood glucose: Can technology help? Results of the i-NewTrend randomized clinical trial. Acta Diabetol. 2017, 54, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, S.; Cohen, G.; Owen, K.R. Self-management of diabetes in children and young adults using technology and smartphone applications. Curr. Diabetes Rev. 2014, 10, 298–301. [Google Scholar]
- Battelino, T.; Phillip, M.; Bratina, N.; Nimri, R.; Oskarsson, P.; Bolinder, J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011, 34, 795–800. [Google Scholar]
- Battelino, T.; Conget, I.; Olsen, B.; Schütz-Fuhrmann, I.; Hommel, E.; Hoogma, R.; Schierloh, U.; Sulli, N.; Bolinder, J. SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: A randomised controlled trial. Diabetologia 2012, 55, 3155–3162. [Google Scholar]
- Mauras, N.; Beck, R.; Xing, D.; Ruedy, K.; Buckingham, B.; Tansey, M.; White, N.H.; Weinzimer, S.A.; Tamborlane, W.; Kollman, C. Diabetes Research in Children Network (DirecNet) Study Group. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care 2012, 35, 204–210. [Google Scholar]
- Bergenstal, R.M.; Tamborlane, W.V.; Ahmann, A.; Buse, J.B.; Dailey, G.; Davis, S.N.; Joyce, C.; Peoples, T.; Perkins, B.A.; Welsh, J.B.; et al. STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N. Engl. J. Med. 2010, 363, 311–320. [Google Scholar]
- Vettoretti, M.; Facchinetti, A. Combining continuous glucose monitoring and insulin pumps to automatically tune the basal insulin infusion in diabetes therapy: A review. Biomed. Eng. Online 2019, 18, 37. [Google Scholar]
- Battelino, T.; Nimri, R.; Dovc, K.; Phillip, M.; Bratina, N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: A randomized controlled trial. Diabetes Care 2017, 40, 764–770. [Google Scholar]
- Tauschmann, M.; Allen, J.M.; Wilinska, M.E.; Thabit, H.; Acerini, C.L.; Dunger, D.B.; Hovorka, R. Home Use of Day-and-Night Hybrid Closed-Loop Insulin Delivery in Suboptimally Controlled Adolescents with Type 1 Diabetes: A 3-Week, Free-Living, Randomized Crossover Trial. Diabetes Care 2016, 39, 2019–2025. [Google Scholar] [PubMed] [Green Version]
- Garg, S.K.; Weinzimer, S.A.; Tamborlane, W.V.; Buckingham, B.A.; Bode, B.W.; Bailey, T.S.; Brazg, R.L.; Ilany, J.; Slover, R.H.; Anderson, S.M.; et al. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1. Diabetes Technol. Ther. 2017, 19, 155–163. [Google Scholar]
- Dovc, K.; Macedoni, M.; Bratina, N.; Lepej, D.; Nimri, R.; Atlas, E.; Muller, I.; Kordonouri, O.; Biester, T.; Danne, T.; et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: A randomised controlled crossover trial. Diabetologia 2017, 60, 2157–2167. [Google Scholar] [CrossRef]
- Dougherty, J.P.; Lipman, T.H.; Hyams, S.; Montgomery, K.A. Telemedicine for adolescents with type 1 diabetes. West J. Nurs. Res. 2014, 36, 1199–1221. [Google Scholar]
- Xu, T.; Pujara, S.; Sutton, S.; Rhee, M. Telemedicine in the management of type 1 diabetes. Prev. Chronic. Dis. 2018, 15, E13. [Google Scholar]
- Wood, C.L.; Clements, S.A.; McFann, K.; Slover, R.; Thomas, J.F.; Wadwa, R.P. Use of telemedicine to improve adherence to American Diabetes Association standards in pediatric type 1 diabetes. Diabetes Technol. Ther. 2016, 18, 7–14. [Google Scholar] [PubMed]
- Carroll, A.E.; DiMeglio, L.A.; Stein, S.; Marrero, D.G. Using a cell phone-based glucose monitoring system for adolescent diabetes management. Diabetes Educator. 2011, 37, 59–66. [Google Scholar]
- Borus, J.S.; Blood, E.; Volkening, L.K.; Laffel, L.; Shrier, L.A. Momentary assessment of social context and glucose monitoring adherence in adolescents with type 1 diabetes. J. Adolesc. Health 2013, 52, 578–583. [Google Scholar] [PubMed] [Green Version]
- Landau, Z.; Mazor-Aronovitch, K.; Boaz, M.; Blaychfeld-Magnazi, M.; GraphBarel, C.; Levek-Motola, N.; Pinhas-Hamiel, O. The effectiveness of internet-based blood glucose monitoring system on improving diabetes control in adolescents with type 1 diabetes. Pediatric. Diabetes 2012, 13, 203–207. [Google Scholar]
- Evans, J.M.; Mackison, D.; Emslie-Smith, A.; Lawton, J. Self-monitoring of blood glucose in type 2 diabetes: Cross-sectional analyses in 1993, 1999 and 2009. Diabet. Med. 2012, 29, 792–795. [Google Scholar]
- Schütt, M.; Kern, W.; Krause, U.; Busch, P.; Dapp, A.; Grziwotz, R.; Mayer, I.; Rosenbauer, J.; Wagner, C.; Zimmermann, A.; et al. DPV Initiative. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp. Clin. Endocrinol. Diabetes 2006, 114, 384–388. [Google Scholar]
- Lalić, N.M.; Lalić, K.; Jotić, A.; Stanojević, D.; Živojinović, D.; Janićijević, A.; Parkin, C.; SPA-EDU Study Group. The Impact of Structured Self-Monitoring of Blood Glucose Combined with Intensive Education on HbA1c Levels, Hospitalizations, and Quality-of-Life Parameters in Insulin-Treated Patients with Diabetes at Primary Care in Serbia: The Multicenter SPA-EDU Study. J. Diabetes Sci. Technol. 2017, 11, 746–752. [Google Scholar] [CrossRef]
- Lee, A.A.; Piette, J.D.; Heisler, M.; Rosland, A.M. Diabetes Distress and Glycemic Control: The Buffering Effect of Autonomy Support from Important Family Members and Friends. Diabetes Care 2018, 41, 1157–1163. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults with Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef]
- Lind, M.; Polonsky, W.; Hirsch, I.B.; Heise, T.; Bolinder, J.; Dahlqvist, S.; Schwarz, E.; Ólafsdóttir, A.F.; Frid, A.; Wedel, H.; et al. Continuous Glucose Monitoring vs. Conventional Therapy for Glycemic Control in Adults with Type 1 Diabetes Treated with Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA 2017, 317, 379–387. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.D.; Ruedy, K.; Ahmann, A.; Haller, S.; Kruger, D.; McGill, J.B.; Polonsky, W.; Price, D.; Aronoff, S.; et al. Continuous Glucose Monitoring Versus Usual Care in Patients with Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann. Intern. Med. 2017, 167, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.; Foster, N.C.; Maahs, D.M.; Raghinaru, D.; Bergenstal, R.M.; Ahmann, A.J.; Peters, A.L.; Bode, B.W.; Aleppo, G.; Hirsch, I.B.; et al. T1D Exchange Clinic Network. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014, 37, 2702–2709. [Google Scholar]
- Little, S.A.; Leelarathna, L.; Walkinshaw, E.; Tan, H.K.; Chapple, O.; Lubina-Solomon, A.; Chadwick, T.J.; Barendse, S.; Stocken, D.D.; Brennand, C.; et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: A multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014, 37, 2114–2122. [Google Scholar] [CrossRef] [Green Version]
- Bolinder, J.; Antuna, R.; Geelhoed-Duijvestijn, P.; Kröger, J.; Weitgasser, R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Lancet 2016, 388, 2254–2263. [Google Scholar] [CrossRef]
- Haak, T.; Hanaire, H.; Ajjan, R.; Hermanns, N.; Riveline, J.P.; Rayman, G. Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: A Multicenter, Open-Label Randomized Controlled Trial. Diabetes Ther. 2017, 8, 55–73. [Google Scholar] [CrossRef] [Green Version]
- Yaron, M.; Roitman, E.; Aharon-Hananel, G.; Landau, Z.; Ganz, T.; Yanuv, I.; Rozenberg, A.; Karp, M.; Ish-Shalom, M.; Singer, J.; et al. Effect of Flash Glucose Monitoring Technology on Glycemic Control and Treatment Satisfaction in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 1178–1184. [Google Scholar] [CrossRef]
- Deshmukh, H.; Wilmot, E.G.; Gregory, R.; Barnes, D.; Narendran, P.; Saunders, S.; Furlong, N.; Kamaruddin, S.; Banatwalla, R.; Herring, R.; et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care 2020, 43, 2153–2160. [Google Scholar]
- Hermanides, J.; Nørgaard, K.; Bruttomesso, D.; Mathieu, C.; Frid, A.; Dayan, C.M.; Diem, P.; Fermon, C.; Wentholt, I.M.; Hoekstra, J.B.; et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet. Med. 2011, 28, 1158–1167. [Google Scholar] [PubMed]
- Ly, T.T.; Nicholas, J.A.; Retterath, A.; Lim, E.M.; Davis, E.A.; Jones, T.W. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: A randomized clinical trial. JAMA 2013, 310, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Forlenza, G.P.; Li, Z.; Buckingham, B.A.; Pinsker, J.E.; Cengiz, E.; Wadwa, R.P.; Ekhlaspour, L.; Church, M.M.; Weinzimer, S.A.; Jost, E.; et al. Predictive Low-Glucose Suspend Reduces Hypoglycemia in Adults, Adolescents, and Children with Type 1 Diabetes in an At-Home Randomized Crossover Study: Results of the PROLOG Trial. Diabetes Care 2018, 41, 2155–2161. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, A.; De Bock, M.I.; Jayawardene, D.; Loh, M.M.; Horsburgh, J.C.; Berthold, C.L.; Paramalingam, N.; Bach, L.A.; Colman, P.G.; Davis, E.A.; et al. Glycemia, Treatment Satisfaction, Cognition, and Sleep Quality in Adults and Adolescents with Type 1 Diabetes When Using a Closed-Loop System Overnight Versus Sensor-Augmented Pump with Low-Glucose Suspend Function: A Randomized Crossover Study. Diabetes Technol. Ther. 2016, 18, 772–783. [Google Scholar] [CrossRef]
- Benhamou, P.V.; Yves Reznik, S.F.; Thivolet, C.; Schaepelynck, P.; Renard, E.; Guerci, B.; Chaillous, L.; Lukas-Croisier, C.; Jeandidier, N.; Hanaire, H.; et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: A 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health 2019, 1, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kropff, J.; Del Favero, S.; Place, J.; Toffanin, C.; Visentin, R.; Monaro, M.; Messori, M.; Di Palma, F.; Lanzola, G.; Farret, A.; et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: A randomised crossover trial. Lancet Diabetes Endocrinol. 2015, 3, 939–947. [Google Scholar] [CrossRef]
- Brown, S.A.; Breton, M.D.; Anderson, S.M.; Kollar, L.; Keith-Hynes, P.; Levy, C.J.; Lam, D.W.; Levister, C.; Baysal, N.; Kudva, Y.C.; et al. Overnight Closed-Loop Control Improves Glycemic Control in a Multicenter Study of Adults with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 3674–3682. [Google Scholar] [CrossRef] [Green Version]
- Bergenstal, R.M.; Garg, S.; Weinzimer, S.A.; Buckingham, B.A.; Bode, B.W.; Tamborlane, W.V.; Kaufman, F.R. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients with Type 1 Diabetes. JAMA 2016, 316, 1407–1408. [Google Scholar] [CrossRef]
- Sherr, J.L.; Cengiz, E.; Palerm, C.C.; Clark, B.; Kurtz, N.; Roy, A.; Carria, L.; Cantwell, M.; Tamborlane, W.V.; Weinzimer, S.A. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care 2013, 36, 2909–2914. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.J.; El-Khatib, F.H.; Sinha, M.; Magyar, K.L.; McKeon, K.; Goergen, L.G.; Balliro, C.; Hillard, M.A.; Nathan, D.M.; Damiano, E.R. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N. Engl. J. Med. 2014, 371, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Lucidi, P.; Porcellati, F.; Bolli, G.B.; Fanelli, C.G. Prevention and Management of Severe Hypoglycemia and Hypoglycemia Unawareness: Incorporating Sensor Technology. Curr. Diab. Rep. 2018, 18, 83. [Google Scholar] [CrossRef]
- Chiu, C.J.; Chou, Y.H.; Chen, Y.J.; Du, Y.F. Impact of New Technologies for Middle-Aged and Older Patients: In-Depth Interviews with Type 2 Diabetes Patients Using Continuous Glucose Monitoring. JMIR Diabetes 2019, 4, e10992. [Google Scholar] [CrossRef]
- Dixon, R.F.; Zisser, H.; Layne, J.E.; Barleen, N.A.; Miller, D.P.; Moloney, D.P.; Majithia, A.R.; Gabbay, R.A.; Riff, J. A Virtual Type 2 Diabetes Clinic Using Continuous Glucose Monitoring and Endocrinology Visits. J. Diabetes Sci. Technol. 2019, 14, 908–911. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Sun, Z.; Yang, Y.; Yu, H.; Ding, H.; Wang, S. Effect of a CGMS and SMBG on Maternal and Neonatal Outcomes in Gestational Diabetes Mellitus: A Randomized Controlled Trial. Sci. Rep. 2016, 6, 19920. [Google Scholar] [CrossRef] [Green Version]
- Feig, D.S.; Donovan, L.E.; Corcoy, R.; Murphy, K.E.; Amiel, S.A.; Hunt, K.F.; Asztalos, E.; Barrett, J.; Sanchez, J.J.; de Leiva, A.; et al. CONCEPTT Collaborative Group. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet 2017, 390, 2347–2359. [Google Scholar] [CrossRef] [Green Version]
- Secher, A.L.; Ringholm, L.; Andersen, H.U.; Damm, P.; Mathiesen, E.R. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: A randomized controlled trial. Diabetes Care 2013, 36, 1877–1883. [Google Scholar] [CrossRef] [Green Version]
- Secher, A.L.; Madsen, A.B.; Ringholm, L.; Barfred, C.; Stage, E.; Andersen, H.U.; Damm, P.; Mathiesen, E.R. Patient satisfaction and barriers to initiating real-time continuous glucose monitoring in early pregnancy in women with diabetes. Diabet. Med. 2012, 29, 272–277. [Google Scholar] [CrossRef]
- Stewart, Z.A.; Wilinska, M.E.; Hartnell, S.; O’Neil, L.K.; Rayman, G.; Scott, E.M.; Barnard, K.; Farrington, C.; Hovorka, R.; Murphy, H.R. Day-and-Night Closed-Loop Insulin Delivery in a Broad Population of Pregnant Women with Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care 2018, 41, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Farrington, C.; Stewart, Z.A.; Barnard, K.; Hovorka, R.; Murphy, H.R. Experiences of closed-loop insulin delivery among pregnant women with Type 1 diabetes. Diabet. Med. 2017, 34, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Skandari, M.R.; Minc, A.; Nathan, A.G.; Winn, A.; Zarei, P.; O’Grady, M.; Huang, E.S. Cost-effectiveness of Continuous Glucose Monitoring for Adults with Type 1 Diabetes Compared with Self-Monitoring of Blood Glucose: The DIAMOND Randomized Trial. Diabetes Care 2018, 41, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Kamble, S.; Schulman, K.A.; Reed, S.D. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the United States. Value Health 2012, 15, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Roze, S.; Smith-Palmer, J.; de Portu, S.; Delbaere, A.; de Brouwer, B.; de Valk, H.W. Cost-effectiveness of sensor-augmented insulin pump therapy vs continuous subcutaneous insulin infusion in patients with type 1 diabetes in the Netherlands. Clinicoecon. Outcomes Res. 2019, 11, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemsma, R.; Corro Ramos, I.; Birnie, R.; Büyükkaramikli, N.; Armstrong, N.; Ryder, S.; Duffy, S.; Worthy, G.; Al, M.; Severens, J.; et al. Integrated sensor-augmented pump therapy systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: A systematic review and economic evaluation. Health Technol. Assess 2016, 20, 251. [Google Scholar] [CrossRef]
- Roze, S.; Smith-Palmer, J.; Valentine, W.J.; Cook, M.; Jethwa, M.; de Portu, S.; Pickup, J.C. Long-term health economic benefits of sensor-augmented pump therapy vs continuous subcutaneous insulin infusion alone in type 1 diabetes: A U.K. perspective. J. Med. Econ. 2016, 19, 236–242. [Google Scholar] [CrossRef]
- Ly, T.T.; Brnabic, A.J.; Eggleston, A.; Kolivos, A.; McBride, M.E.; Schrover, R.; Jones, T.W. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health 2014, 17, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Pease, A.J.; Zomer, E.; Liew, D.; Earnest, A.; Soldatos, G.; Ademi, Z.; Zoungas, S. Cost-effectiveness analysis of a hybrid closed-loop system versus multiple daily injections and capillary glucose testing for adults with type 1 diabetes. Diabetes Technol. Ther. 2020, 10, 1089. [Google Scholar] [CrossRef]
- Nørgaard, K.; Shin, J.; Welsh, J.B.; Gjessing, H. Performance and acceptability of a combined device for insulin infusion and glucose sensing in the home setting. J. Diabetes Sci. Technol. 2015, 9, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Tschaikner, M.; Powell, K.; Jungklaus, M.; Fritz, M.; Ellmerer, M.; Hovorka, R.; Lane, S.; Pieber, T.R.; Regittnig, W. Novel Single-Site Device for Conjoined Glucose Sensing and Insulin Infusion: Performance Evaluation in Diabetes Patients During Home-Use. Trans. Biomed. Eng. 2020, 67, 323–332. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.G.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.Q.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2017, 12, 5996–6022. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.J.; Gao, C.M.; Lu, S.Y.; Duan, H.G.; Jing, N.N.; Dong, D.; Shi, C.F.; Liu, M.Z. Anti-photobleaching flower-like microgels as optical nanobiosensors with high selectivity at physiological conditions for continuous glucose monitoring. J. Mater. Chem. B 2014, 2, 5452–5460. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Gao, W.; Brooks, G.A.; Klonoff, D.C. Wearable physiological systems and technologies for metabolic monitoring. J. Appl. Physiol. 2018, 124, 548–556. [Google Scholar] [CrossRef]
- Lipani, L.; Dupont, B.; Doungmene, F.; Marken, F.; Tyrrell, R.M.; Guy, R.H.; Ilie, A. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 2018, 13, 504–511. [Google Scholar] [CrossRef]

| References | Population | Technology | Cost-Effective |
|---|---|---|---|
| [92] | T1D with suboptimal glycemic control | CGM vs. SMBG | Yes |
| [93] | T1D | SAP vs. MDI | No |
| [94] | T1D with suboptimal glycemic control or with frequent severe hypoglycemic events | SAP vs. CSII+SMBG | Yes |
| [95] | T1D | SAP LGS vs. MDI, CSII, CGM, SAP | No |
| [96] | Poorly controlled T1D | SAP LGS vs. CSII | Yes |
| [97] | T1D with unaware hypoglycemia | SAP LGS vs. CSII+SMBG | Yes |
| [98] | T1D | Hybrid closed-loop vs. MDI+SMBG | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malandrucco, I.; Russo, B.; Picconi, F.; Menduni, M.; Frontoni, S. Glycemic Status Assessment by the Latest Glucose Monitoring Technologies. Int. J. Mol. Sci. 2020, 21, 8243. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218243
Malandrucco I, Russo B, Picconi F, Menduni M, Frontoni S. Glycemic Status Assessment by the Latest Glucose Monitoring Technologies. International Journal of Molecular Sciences. 2020; 21(21):8243. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218243
Chicago/Turabian StyleMalandrucco, Ilaria, Benedetta Russo, Fabiana Picconi, Marika Menduni, and Simona Frontoni. 2020. "Glycemic Status Assessment by the Latest Glucose Monitoring Technologies" International Journal of Molecular Sciences 21, no. 21: 8243. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218243





