Metal Homeostasis and Gas Exchange Dynamics in Pisum sativum L. Exposed to Cerium Oxide Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
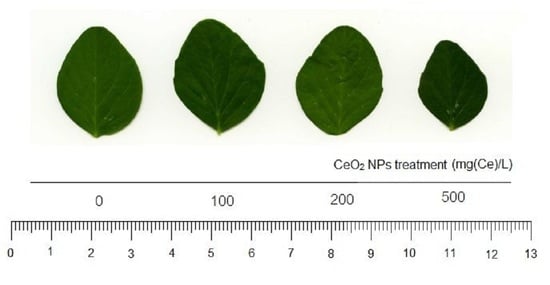
2.1. Plant Biomass
2.2. Cerium Migration and Accumulation
2.3. Gas Exchange
2.4. Leaf Photosynthetic Pigments
2.5. Plant Uptake and Accumulation of Mineral Nutrients
3. Materials and Methods
3.1. Cerium Oxide Nanoparticles
3.2. Plant Growth and Exposure
3.3. Biomass Determination
3.4. Gas Exchange Parameters
3.5. Photosynthetic Pigments
3.6. Metal Content Determination in Plant Tissues
3.7. Transfer Coefficient, Bioaccumulation Factor, Translocation Factor, and Tolerance Index
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ENPs | Engineered nanoparticles |
| NPs | Nanoparticles |
| FW | Fresh weights |
| DW | Dry weights |
| TI | Tolerance index |
| TC | Transfer coefficient |
| BAF | Bioaccumulation factor |
| TF | Translocation factor |
| A | Leaf net photosynthesis |
| Ci | Sub-stomatal CO2 concentration |
| E | Transpiration |
| Gs | Stomatal conductance |
| WUE | Water use efficiency |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| Car | Carotenoids |
| ROS | Reactive oxygen species |
| PARi | Photosynthetically active radiation |
References
- Uematsu, T.; Baba, M.; Oshima, Y.; Tsuda, T.; Torimoto, T.; Kuwabata, S. Atomic resolution imaging of gold nanoparticle generation and growth in ionic liquids. J. Am. Chem. Soc. 2014, 136, 13789–13797. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foss Hansen, S.; Heggelund, L.R.; Revilla Besora, P.; Mackevica, A.; Boldrin, A.; Baun, A. Nanoproducts—What is actually available to European consumers? Environ. Sci. Nano 2016, 3, 169–180. [Google Scholar] [CrossRef] [Green Version]
- The Nanodatabase. Available online: www.nanodb.dk (accessed on 20 July 2020).
- Zhang, J.; Guo, W.; Li, Q.; Wang, Z.; Liu, S. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ. Sci. Nano 2018, 5, 2482–2499. [Google Scholar] [CrossRef]
- Tong, T.; Wilke, C.M.; Wu, J.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.F.; Gray, K.A. Combined Toxicity of Nano-ZnO and Nano-TiO2: From Single- to Multinanomaterial Systems. Environ. Sci. Technol. 2015, 49, 8113–8123. [Google Scholar] [CrossRef]
- Rawat, S.; Pullagurala, V.L.R.; Adisa, I.O.; Wang, Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Factors affecting fate and transport of engineered nanomaterials in terrestrial environments. Curr. Opin. Environ. Sci. Health 2018, 6, 47–53. [Google Scholar] [CrossRef]
- Azzazy, H.M.E.; Mansour, M.M.H.; Samir, T.M.; Franco, R. Gold nanoparticles in the clinical laboratory: Principles of preparation and applications. Clin. Chem. Lab. Med. 2012, 50, 193–209. [Google Scholar] [CrossRef]
- Manna, I.; Bandyopadhyay, M. A review on the biotechnological aspects of utilizing engineered nanoparticles as delivery systems in plants. Plant Gene 2019, 17, 100167. [Google Scholar] [CrossRef]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafique, M.; Luo, X. Nanotechnology in transportation vehicles: An overview of its applications, environmental, health and safety concerns. Materials 2019, 12, 2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, P.K.; Kumar, V.; Lee, S.S.; Raza, N.; Kim, K.H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Int. 2018, 119, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, M.M.; Terekhova, V.A. Engineered nanomaterials in soil: Sources of entry and migration pathways. Moscow Univ. Soil Sci. Bull. 2013, 68, 129–134. [Google Scholar] [CrossRef]
- Fang, J.; Wang, M.H.; Lin, D.H.; Shen, B. Enhanced transport of CeO2 nanoparticles in porous media by macropores. Sci. Total Environ. 2016, 543, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; Von Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Combined cadmium-zinc interactions alter manganese, lead, copper uptake by Melissa officinalis. Sci. Rep. 2020, 10, 1675. [Google Scholar] [CrossRef] [Green Version]
- Skiba, E.; Wolf, W.M. Cerium Oxide Nanoparticles Affect Heavy Metals Uptake by Pea in a Divergent Way than Their Ionic and Bulk Counterparts. Water Air. Soil Pollut. 2019, 230, 248. [Google Scholar] [CrossRef] [Green Version]
- Montes, A.; Bisson, M.A.; Gardella, J.A.; Aga, D.S. Uptake and transformations of engineered nanomaterials: Critical responses observed in terrestrial plants and the model plant Arabidopsis thaliana. Sci. Total Environ. 2017, 607–608, 1497–1516. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Srivastava, A.K.; Karmakar, S. Nanomaterial toxicity for plants. Environ. Chem. Lett. 2018, 16, 85–100. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Oviedo, K.; Aga, D.S. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A. Soils and soil processes. In Trace Elements in Soils and Plants, 4th ed.; CRC Press Taylor & Francis Group: Amsterdam, The Netherlands, 2010; pp. 37–60. [Google Scholar] [CrossRef]
- Milenković, I.; Mitrović, A.; Algarra, M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Maksimović, V.; Spasić, S.Z.; Beškoski, V.P.; Radotić, K. Interaction of carbohydrate coated cerium-oxide nanoparticles with wheat and pea: Stress induction potential and effect on development. Plants 2019, 8, 478. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Singh, A.; Singh, N.B.; Singh, P. Plant-nanoceria interaction: Toxicity, accumulation, translocation and biotransformation. S. Afr. J. Bot. 2019, 121, 239–247. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Zhang, W.; Pei, H.; Chen, Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 2012, 4, 1105–1112. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, M.; Srivastav, A.; Gandhi, S.; Rao, S.; Roychoudhury, A.; Kumar, A.; Singhal, R.K.; Jha, S.K.; Singh, S.D. Monitoring of engineered nanoparticles in soil-plant system: A review. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100218. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Carbone, S.; Bosi, S.; Gatti, A.; Dinelli, G. Engineered nanoparticles effects in soil-plant system: Basil (Ocimum basilicum L.) study case. Appl. Soil Ecol. 2018, 123, 551–560. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Pan, H.; Wang, P.; Zhao, F.J. Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana. J. Hazard. Mater. 2017, 322, 292–300. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [Green Version]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants - Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef]
- Tamez, C.; Morelius, E.W.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J. Biochemical and physiological effects of copper compounds/ nanoparticles on sugarcane (Saccharum officinarum). Sci. Total Environ. 2019, 649, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.L.; De La Rosa, G.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 2010, 58, 3689–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, H. The Impact of Silver Nanoparticles on Plant Biomass and Chlorophyll Content. Res. Inven. Int. J. Eng. Sci. 2014, 4, 12–20. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Carmora, E.; Recio, G.; Nunes-Nesi, A.; Reyes-Diaz, M.; Alberdi, M.; Rengel, Z.; Inostroza-Blancheteau, C. Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant Physiol. Biochem. 2018, 130, 408–417. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Sarkar, A. Nanoparticles on Photosynthesis of Plants: Effects and Role. In Green Nanoparticles. Nanotechnology in the Life Sciences, 1st ed.; Patra, J., Fraceto, L., Das, G., Campos, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 273–288. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Genc, Y.; Hayes, J. The use of hydroponics in abiotic stress tolerance research. In Hydroponics—A Standard Methodology for Plant Biological Researches, 1st ed.; Asao, T., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 39–66. [Google Scholar] [CrossRef]
- Deng, Y.Q.; White, J.C.; Xing, B.S. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.; McInturf, S.A.; Mendoza-Cózatl, D.G. Hydroponics: A versatile system to study nutrient allocation and plant responses to nutrient availability and exposure to toxic elements. J. Vis. Exp. 2016, 113, 54317. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zheng, G.; Hamdani, S.; Essemine, J.; Song, Q.; Wang, H.; Chu, C.; Sirault, X.; Zhu, X.G. Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol. 2017, 175, 248–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, F.; Schulin, R.; Limbach, L.K.; Stark, W.; Bürge, D.; Nowack, B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere 2013, 91, 512–520. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Vilchis-Nestor, A.R.; Majumdar, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Citric acid modifies surface properties of commercial CeO2 nanoparticles reducing their toxicity and cerium uptake in radish (Raphanus sativus) seedlings. J. Hazard. Mater. 2013, 263, 677–684. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Mujtaba Munir, M.A.; Ahmed, R.; Rehman, A. Biochar-assisted transformation of engineered-cerium oxide nanoparticles: Effect on wheat growth, photosynthetic traits and cerium accumulation. Ecotoxicol. Environ. Saf. 2020, 187, 109845. [Google Scholar] [CrossRef]
- Gui, X.; Zhang, Z.; Liu, S.; Ma, Y.; Zhang, P.; He, X.; Li, Y.; Zhang, J.; Li, H.; Rui, Y.; et al. Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PLoS ONE 2015, 10, e0134261. [Google Scholar] [CrossRef]
- Morales, M.I.; Rico, C.M.; Hernandez-Viezcas, J.A.; Nunez, J.E.; Barrios, A.C.; Tafoya, A.; Flores-Marges, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J. Agric. Food Chem. 2013, 61, 6224–6230. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Hong, J.; Majumdar, S.; Niu, G.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environ. Sci. Technol. 2015, 49, 2921–2928. [Google Scholar] [CrossRef]
- Rico, C.M.; Barrios, A.C.; Tan, W.; Rubenecia, R.; Lee, S.C.; Varela-Ramirez, A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Physiological and biochemical response of soil-grown barley (Hordeum vulgare L.) to cerium oxide nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 10551–10558. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Yasmeen, F.; Komatsu, S. Nanoparticles: Synthesis, morphophysiological effects, and proteomic responses of crop plants. Int. J. Mol. Sci. 2020, 21, 3056. [Google Scholar] [CrossRef] [PubMed]
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maathuis, F. Solute Transport: Basic Concepts. In Plant Physiology and Function; Clemens, S., Ed.; Springer: New York, NY, USA, 2017; Volume 6, pp. 1–31. [Google Scholar] [CrossRef]
- Li, J.; Mu, Q.; Du, Y.; Luo, J.; Liu, Y.; Li, T. Growth and Photosynthetic Inhibition of Cerium Oxide Nanoparticles on Soybean (Glycine max). Bull. Environ. Contam. Toxicol. 2020, 105, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Komatsu, S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta 2016, 1864, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Chakraverty, N. Mechanism of Plant Tolerance in Response to Heavy Metals. In Molecular Stress Physiology of Plants; Rout, G., Das, A., Eds.; Springer: New Delhi, India, 2013; pp. 289–308. [Google Scholar] [CrossRef]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interactions between CeO2 Nanoparticles and the Desert Plant Mesquite: A Spectroscopy Approach. ACS Sustain. Chem. Eng. 2016, 4, 1187–1192. [Google Scholar] [CrossRef]
- Majumdar, S.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Sahi, S.; Gardea-Torresdey, J.L. Exposure of cerium oxide nanoparticles to kidney bean shows disturbance in the plant defense mechanisms. J. Hazard. Mater. 2014, 278, 279–287. [Google Scholar] [CrossRef]
- White, P.J. Ion Uptake Mechanisms of Individual Cells and Roots: Short-distance Transport. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 7–47. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Li, J.; Tappero, R.V.; Acerbo, A.S.; Yan, H.; Chu, Y.; Lowry, G.V.; Unrine, J.M. Effect of CeO2 nanomaterial surface functional groups on tissue and subcellular distribution of Ce in tomato (Solanum lycopersicum). Environ. Sci. Nano 2019, 6, 273–285. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Jamal, A. Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci. Total Environ. 2011, 409, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Clavel, M.; Meehan, K.; Samir, E.; Gaballah, S.; Salah, M. Enhanced erbium-doped ceria nanostructure coating to improve solar cell performance. Materials 2015, 8, 7663–7672. [Google Scholar] [CrossRef] [PubMed]
- Ver Sagun, J.; Badger, M.R.; Chow, W.S.; Ghannoum, O. Cyclic electron flow and light partitioning between the two photosystems in leaves of plants with different functional types. Photosynth. Res. 2019, 142, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreier, T.B.; Hibberd, J.M. Variations in the Calvin-Benson cycle: Selection pressures and optimization? J. Exp. Bot. 2019, 70, 1697–1701. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Tiwari, E.; Mondal, M.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Effect of the irrigation water type and other environmental parameters on CeO2 nanopesticide-clay colloid interactions. Environ. Sci. Process. Impacts 2020, 22, 84–94. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Shabala, S.; Giraldo, J.P. Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 2018, 5, 1567–1583. [Google Scholar] [CrossRef]
- Priester, J.H.; Moritz, S.C.; Espinosa, K.; Ge, Y.; Wang, Y.; Nisbet, R.M.; Schimel, J.P.; Susana Goggi, A.; Gardea-Torresdey, J.L.; Holden, P.A. Damage assessment for soybean cultivated in soil with either CeO2 or ZnO manufactured nanomaterials. Sci. Total Environ. 2017, 579, 1756–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Zhu, J.; Peralta-Videa, J.R.; Guo, H. Physiological and Biochemical Changes Imposed by CeO2 Nanoparticles on Wheat: A Life Cycle Field Study. Environ. Sci. Technol. 2015, 49, 11884–11893. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Hong, J.; Morales, M.I.; Zhao, L.; Barrios, A.C.; Zhang, J.Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on rice: A study involving the antioxidant defense system and in vivo fluorescence imaging. Environ. Sci. Technol. 2013, 47, 5635–5642. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Beaulieu, A.L.; Beaulieu, N.L.; Mazer, S.J.; Keller, A.A. Environmental Stresses Increase Photosynthetic Disruption by Metal Oxide Nanomaterials in a Soil-Grown Plant. ACS Nano 2015, 9, 11737–11749. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; García-Morales, S.; Pérez-Sato, J.A.; Gómez-Merino, F.C. Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Erratum: Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium Oxide Nanoparticles Decrease Drought-Induced Oxidative Damage in Sorghum Leading to Higher Photosynthesis and Grain Yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging Investigator Series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Rossi, L.; Zhang, W.; Ma, X. Cerium oxide nanoparticles alter the salt stress tolerance of Brassica napus L. by modifying the formation of root apoplastic barriers. Environ. Pollut. 2017, 229, 132–138. [Google Scholar] [CrossRef]
- McElroy, J.S.C.O.T.; Kopsell, D.A. Physiological role of carotenoids and other antioxidants in plants and application to turfgrass stress management. N. Z. J. Crop Hortic. Sci. 2009, 37, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2013, 64, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Kudla, J. Calcium decoding mechanisms in plants. Biochimie 2011, 93, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, H.; Guo, H.; Musante, C.; Coskun, S.H.; Nelson, B.C.; White, J.C.; Xing, B.; Dhankher, O.P. Defense mechanisms and nutrient displacement in: Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ. Sci. Nano 2016, 3, 1369–1379. [Google Scholar] [CrossRef]
- Pošćić, F.; Schat, H.; Marchiol, L. Cerium negatively impacts the nutritional status in rapeseed. Sci. Total Environ. 2017, 593–594, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Skiba, E.; Adamczyk-Szabela, D.; Wolf, W.M. Metal based nanoparticles interactions with plants. In Plant Responses to Nanomaterials. Recent Interventions and Physiological and Biochemical Responses; Singh, V.P., Singh, S., Prasad, S.M., Chauhan, D.K., Tripathi, D.K., Eds.; Springer: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.N.; Bruce, N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef] [Green Version]
- Borah, K.D.; Bhuyan, J. Magnesium porphyrins with relevance to chlorophylls. Dalton Trans. 2017, 46, 6497–6509. [Google Scholar] [CrossRef]
- Weston, J. Biochemistry of Magnesium. In The Chemistry of Organo-magnesium Compounds; Rappoport, Z., Marek, I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 315–367. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Stec, B. Structural mechanism of RuBisCO activation by carbamylation of the active site lysine. Proc. Natl. Acad. Sci. USA 2012, 109, 18785–18790. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yokosho, K.; Liu, S.; Cao, H.R.; Yamaji, N.; Zhu, X.G.; Liao, H.; Ma, J.F.; Chen, Z.C. Diel magnesium fluctuations in chloroplasts contribute to photosynthesis in rice. Nat. Plants 2020, 6, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Aery, N.C. Effect of cerium on growth, dry matter production, biochemical constituents and enzymatic activities of cowpea plants [Vigna unguiculata (L.) Walp.]. J. Soil Sci. Plant Nutr. 2012, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yuguan, Z.; Min, Z.; Luyang, L.; Zhe, J.; Chao, L.; Sitao, Y.; Yanmei, D.; Na, L.; Fashui, H. Effects of cerium on key enzymes of carbon assimilation of spinach under magnesium deficiency. Biol. Trace Elem. Res. 2009, 131, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Gong, X.; Wang, Y.; Liu, C.; Hong, M.; Wang, L.; Hong, F. Improvement of cerium of photosynthesis functions of maize under magnesium deficiency. Biol. Trace Elem. Res. 2011, 142, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.D.S.; de Souza, A.E.; Oliva, M.A.; Pereira, E.G. Oxidative damage and photosynthetic impairment in tropical rice cultivars upon exposure to excess iron. Sci. Agric. 2016, 73, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’S Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press, Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 191–248. [Google Scholar] [CrossRef]
- Van Oijen, T.; Van Leeuwe, M.A.; Gieskes, W.W.C.; De Baar, H.J.W. Effects of iron limitation on photosynthesis and carbohydrate metabolism in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). Eur. J. Phycol. 2004, 39, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, E. Introduction, Definition and Classification of Nutrients. In Marschner’S Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press, Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 3–5. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxidants Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.; Diaz, B.C.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef]
- Ma, C.; Rui, Y.; Liu, S.; Li, X.; Xing, B.; Liu, L. Phytotoxic mechanism of nanoparticles: Destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci. Rep. 2015, 5, 11618. [Google Scholar] [CrossRef] [Green Version]
- Rico, C.M.; Johnson, M.G.; Marcus, M.A.; Andersen, C.P. Intergenerational responses of wheat (Triticum aestivum L.) to cerium oxide nanoparticles exposure. Environ. Sci. Nano 2017, 4, 700–711. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.R.; Zia-ur-Rehman, M.; Sohail, M.I.; Anwar ul Haq, M.; Khalid, H.; Ayub, M.A.; Ishaq, G. Effects of Rare Earth Oxide Nanoparticles on Plants. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, K.D., Parvaiz, A., Sharma, S., Chauhan, D., Dubey, N.K., Eds.; Academic Press, Elsevier Inc.: London, UK, 2018; Volume 1, pp. 239–275. [Google Scholar] [CrossRef]
- Barrios, A.C.; Rico, C.M.; Trujillo-Reyes, J.; Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of uncoated and citric acid coated cerium oxide nanoparticles, bulk cerium oxide, cerium acetate, and citric acid on tomato plants. Sci. Total Environ. 2016, 563–564, 956–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios, A.C.; Medina-Velo, I.A.; Zuverza-Mena, N.; Dominguez, O.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Nutritional quality assessment of tomato fruits after exposure to uncoated and citric acid coated cerium oxide nanoparticles, bulk cerium oxide, cerium acetate and citric acid. Plant Physiol. Biochem. 2017, 110, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corral-Diaz, B.; Peralta-Videa, J.R.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Morales, M.I.; Osuna-Avila, P.; Niu, G.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L). Plant Physiol. Biochem. 2014, 84, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri Tirani, M.; Madadkar Haghjou, M.; Ismaili, A. Hydroponic grown tobacco plants respond to zinc oxide nanoparticles and bulk exposures by morphological, physiological and anatomical adjustments. Funct. Plant Biol. 2019, 46, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll-nutrient relationships identify nutritionally caused decline in Picea abies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Skiba, E.; Kobyłecka, J.; Wolf, W.M. Influence of 2,4-D and MCPA herbicides on uptake and translocation of heavy metals in wheat (Triticum aestivum L.). Environ. Pollut. 2017, 220, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yuan, X.; Li, T.; Hu, S.; Ji, J.; Wang, C. Characteristics of heavy metal transfer and their influencing factors in different soil-crop systems of the industrialization region, China. Ecotoxicol. Environ. Saf. 2016, 126, 193–201. [Google Scholar] [CrossRef]
- Zoufan, P.; Baroonian, M.; Zargar, B. ZnO nanoparticles-induced oxidative stress in Chenopodium murale L, Zn uptake, and accumulation under hydroponic culture. Environ. Sci. Pollut. Res. 2020, 27, 11066–11078. [Google Scholar] [CrossRef]
- Yashim, Z.I.; Kehinde Israel, O.; Hannatu, M. A Study of the Uptake of Heavy Metals by Plants near Metal-Scrap Dumpsite in Zaria, Nigeria. J. Appl. Chem. 2014, 2014, 394650. [Google Scholar] [CrossRef] [Green Version]
- Stefanowicz, A.M.; Stanek, M.; Woch, M.W.; Kapusta, P. The accumulation of elements in plants growing spontaneously on small heaps left by the historical Zn-Pb ore mining. Environ. Sci. Pollut. Res. 2016, 23, 6524–6534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buendía-González, L.; Orozco-Villafuerte, J.; Cruz-Sosa, F.; Barrera-Díaz, C.E.; Vernon-Carter, E.J. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010, 101, 5862–5867. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, M.S.; Amin, F. Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): Profitable phytoremediation with biofuel crops. Geol. Ecol. Landsc. 2018, 2, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Herber, R.F.M.; Sallé, H.J.A. Statistics and data evaluation. Tech. Instrum. Anal. Chem. 1994, 15, 257–271. [Google Scholar]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Fellet, G.; Zavalloni, C.; Carlino, E.; Musetti, R. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 322. [Google Scholar] [CrossRef] [Green Version]
- Viciedo, D.O.; de Mello Prado, R.; Lizcano Toledo, R.; dos Santos, L.C.N.; Calero Hurtado, A.; Nedd, L.L.T.; Castellanos Gonzalez, L. Silicon Supplementation Alleviates Ammonium Toxicity in Sugar Beet (Beta vulgaris L.). J. Soil Sci. Plant Nutr. 2019, 19, 413–419. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: www.R-project (accessed on 16 October 2020).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: github.com/taiyun/corrplot (accessed on 16 October 2020).


| CeO2 NPs Treatment (mg (Ce)/L) | Metal Contents (mg/kg DW) | ||||||
|---|---|---|---|---|---|---|---|
| Roots | |||||||
| Ce | Cu | Zn | Mn | Fe | Ca | Mg | |
| 0 | nd 1 | 12.54 ± 0.60 a | 97.8 ± 4.5 a | 102.1 ± 5.8 a | 320 ± 19 a | 4746 ± 383 c | 2379 ± 74 a |
| 100 | 15,216 ± 220 a | 9.40 ± 0.66 bc | 68.8 ± 2.7 b | 101.0 ± 9.6 a | 176 ± 10 b | 5268 ± 188 bc | 2044 ± 100 b |
| 200 | 18,478 ± 1143 a | 8.41 ± 0.33 c | 56.6 ± 4.2 c | 71.6 ± 6.4 b | 161 ± 14 b | 5723 ± 179 ab | 1965 ± 55 b |
| 500 | 26,040 ± 2901 b | 10.00 ± 0.62 b | 49.1 ± 2.1 d | 52.8 ± 2.5 c | 169 ± 7 b | 5953 ± 187 a | 2144 ± 100 a |
| ANOVA | *** | *** | *** | *** | *** | ** | ** |
| Shoot | |||||||
| Ce | Cu | Zn | Mn | Fe | Ca | Mg | |
| 0 | nd | 9.86 ± 0.32 a | 60.5 ± 2.2 a | 36.7 ± 3.8 a | 105 ± 11 a | 19,493 ± 512 a | 3891 ± 91 a |
| 100 | 101 ± 3 a | 8.69 ± 0.70 b | 51.7 ± 2.6 b | 30.8 ± 1.9 b | 93 ± 3 ab | 17,564 ± 327 b | 3546 ± 64 b |
| 200 | 198 ± 21 b | 8.38 ± 0.61 b | 52.2 ± 2.0 b | 26.8 ± 1.2 b | 81 ± 4 b | 17,165 ± 360 b | 3766 ± 65 ab |
| 500 | 243 ± 38 b | 8.97 ± 0.62 ab | 55.4 ± 0.9 b | 27.3 ± 2.7 b | 65 ± 8 c | 14,593 ± 835 c | 3873 ± 202 a |
| ANOVA | *** | * | *** | *** | *** | *** | * |
| CeO2 NPs Treatment (mg (Ce)/L) | A | Ci | E | gs | WUE |
|---|---|---|---|---|---|
| (µmol CO2/m2s) | (µmol/mol) | (mmol H2O/m2s) | (mmol H2O/m2s) | (mmol CO2/mol H2O) | |
| 0 | 12.9 ± 1.6 b | 293 ± 9 a | 8.49 ± 0.43 b | 407 ± 7 a | 1.53 ± 0.24 b |
| 100 | 18.1 ± 1.3 a | 277 ± 14 a | 10.22 ± 0.42 a | 554 ± 58 a | 1.98 ± 0.17 a |
| 200 | 14.8 ± 1.4 b | 292 ± 10 a | 10.63 ± 0.07 a | 548 ± 19 a | 1.50 ± 0.14 b |
| 500 | 14.4 ± 1.7 b | 299 ± 8 a | 7.73 ± 1.04 b | 488 ± 156 a | 1.54 ± 0.10 b |
| ANOVA | ** | ns 1 | *** | ns | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, E.; Pietrzak, M.; Gapińska, M.; Wolf, W.M. Metal Homeostasis and Gas Exchange Dynamics in Pisum sativum L. Exposed to Cerium Oxide Nanoparticles. Int. J. Mol. Sci. 2020, 21, 8497. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228497
Skiba E, Pietrzak M, Gapińska M, Wolf WM. Metal Homeostasis and Gas Exchange Dynamics in Pisum sativum L. Exposed to Cerium Oxide Nanoparticles. International Journal of Molecular Sciences. 2020; 21(22):8497. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228497
Chicago/Turabian StyleSkiba, Elżbieta, Monika Pietrzak, Magdalena Gapińska, and Wojciech M. Wolf. 2020. "Metal Homeostasis and Gas Exchange Dynamics in Pisum sativum L. Exposed to Cerium Oxide Nanoparticles" International Journal of Molecular Sciences 21, no. 22: 8497. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228497