Phytochemical Molecules from the Decarboxylation of Gomphrenins in Violet Gomphrena globosa L.—Floral Infusions from Functional Food
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of Gomphrenins during Tea Brewing of G. globosa in Aqueous Solutions
2.2. Generation and Identification of 17-Decarboxylated Derivatives of Gomphrenins in Aqueous Solutions
2.3. Concentration Profiles of 17-Decarboxylated-Gomphrenins in Aqueous Solutions
2.4. Generation and Identification of 2-Decarboxylated Derivatives of Gomphrenins in Aqueous Solutions
2.5. Concentration Profiles of 2-Decarboxylated-Gomphrenins in Aqueous Solutions
2.6. Generation and Identification of 15-Decarboxylated Derivatives of Gomphrenins in Aqueous Solutions
2.7. Concentration Profiles of 15-Decarboxylated-Gomphrenins in Aqueous Solutions
2.8. Generation of Bi-Decarboxylated Derivatives of Gomphrenins
2.9. Extraction of Gomphrenins during Tea Brewing of G. globosa in Aqueous Citric Acid Solutions
2.10. Generation of Decarboxylated Derivatives of Gomphrenins in Aqueous Citric Acid Solutions and Their Time-Dependent Concentration Profiles
2.11. Studies on Model Acylated Gomphrenins Isolated from G. globosa Extract
2.12. Identification of Thermally Mono-Decarboxylated Derivatives of Isolated Gomphrenins
2.13. Identification of Thermally Bi-Decarboxylated Derivatives of Isolated Acylated Gomphrenins
3. Materials and Methods
3.1. Plant Material
3.2. Reagents
3.3. Tea Brewing of Purple G. Globosa Flowers
3.4. Preparation of Plant Material for Semi-Preparative Chromatography
3.5. Semi-Preparative Chromatography
3.6. LC-DAD-ESI-MS/MS Analyses
3.7. Chromatographic Analyses with Detection by Ion-Trap Time-of-Flight System (LCMS-IT-TOF)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vieira, C.C.J.; Mercier, H.; Chu, E.P.; Figueiredo-Ribeiro, R.C.L. Gomphrena Species (Globe Amaranth). In Vitro Culture and Production of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 1994; pp. 257–270. [Google Scholar]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. HPLC-profiles of tocopherols, sugars, and organic acids in three medicinal plants consumed as infusions. Int. J. Food Sci. 2014, 2014, 241481. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Sutor, K.; Nemzer, B.; Pietrzkowski, Z.; Wybraniec, S. Thermal decarboxylation of betacyanins in red beet betalain-rich extract. Polish J. Food Nutr. Sci. 2020, 70, 7–14. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubia cordifolia: Structure-radical scavenginq activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosa L.) inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović, V.M.; Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Velićanski, A.S.; Cvetković, D.D.; Tumbas, V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Ben Haj Koubaier, H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta vulgaris L.conditiva) Roots and Stems Extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef] [Green Version]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Pietrzkowski, Z. Chlorination of Betacyanins in Several Hypochlorous Acid Systems. J. Agric. Food Chem. 2016, 64, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O.; Francis, F.J. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Coloring attributes of betalains: A key emphasis on stability and future applications. Food Funct. 2017, 8, 1357–1372. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globosa L. and Bougainvillea sp. by HPLC–DAD–ESI–MS n. Anal. Bioanal. Chem. 2007, 387, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Jerz, G.; Gebers, N.; Szot, D.; Szaleniec, M.; Winterhalter, P.; Wybraniec, S. Separation of amaranthine-type betacyanins by ion-pair high-speed countercurrent chromatography. J. Chromatogr. A 2014, 1344, 42–50. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent Advances in Betalain Research. ChemInform 2003, 34. [Google Scholar] [CrossRef]
- Wybraniec, S.; Platzner, I.; Geresh, S.; Gottlieb, H.E.; Haimberg, M.; Mogilnitzki, M.; Mizrahi, Y. Betacyanins from vine cactus Hylocereus polyrhizus. Phytochemistry 2001, 58, 1209–1212. [Google Scholar] [CrossRef]
- Wybraniec, S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 3483–3487. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Jagodzińska, J.; Wybraniec, S. Separation of betacyanins from purple flowers of Gomphrena globosa L. by ion-pair high-speed counter-current chromatography. J. Chromatogr. A 2017, 1489, 51–57. [Google Scholar] [CrossRef]
- Tuwalska, D.; Starzak, K.; Szot, D.; Wybraniec, S.; Winterhalter, P.; Jerz, G. Semi-synthesis of red beet betacyanin ethyl-esters by esterification. Challenges Mod. Technol. 2014, 5, 27–31. [Google Scholar]
- Kumorkiewicz, A.; Wybraniec, S. Thermal Degradation of Major Gomphrenin Pigments in the Fruit Juice of Basella alba L. (Malabar Spinach). J. Agric. Food Chem. 2017, 65, 7500–7508. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Identification and distribution of simple and acylated betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Heuer, S.; Richter, S.; Metzger, J.W.; Wray, V.; Nimtzt, M.; Strack, D. Betacyanins from bracts of Bougainvillea glabra. Phytochemistry 1994, 37, 761–767. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Xing, J.; Sun, M.; Corke, H. Rapid identification of betacyanins from Amaranthus tricolor, Gomphrena globosa, and Hylocereus polyrhizus by matrix-assisted laser desorption/ ionization quadrupole ion trap time-of-flight mass spectrometry (MALDI-QIT-TOF MS). J. Agric. Food Chem. 2006, 54, 6520–6526. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Structural characterization of phenolics and betacyanins in Gomphrena globosa by high-performance liquid chromatography-diode array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3441–3446. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef]
- Wybraniec, S.; Mizrahi, Y. Generation of decarboxylated and dehydrogenated betacyanins in thermally treated purified fruit extract from purple pitaya (Hylocereus polyrhizus) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 6704–6712. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Di Genova, B.M.; Dörr, F.A.; Pinto, E.; Bastos, E.L. Effect of dielectric microwave heating on the color and antiradical capacity of betanin. J. Food Eng. 2013, 118, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
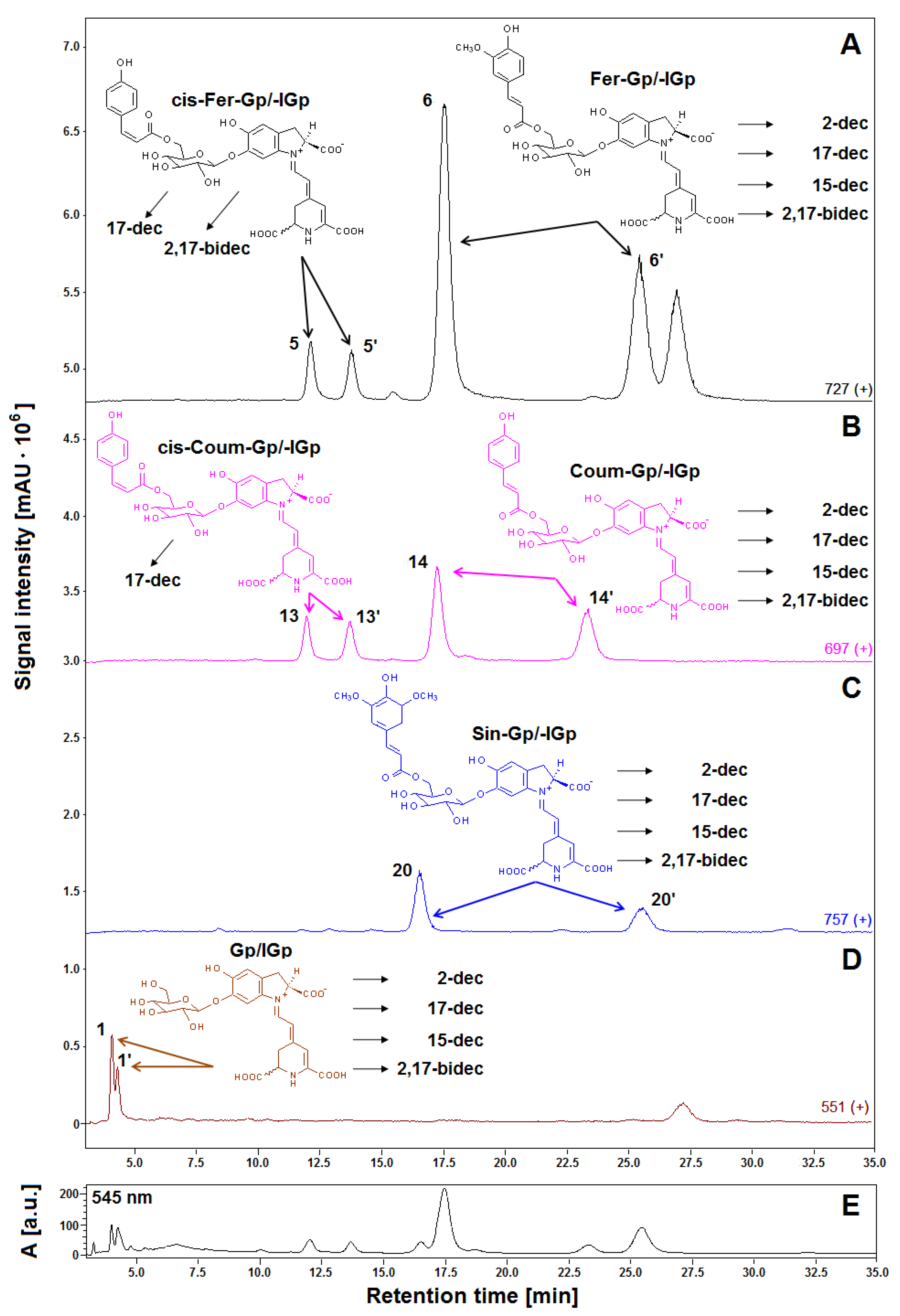
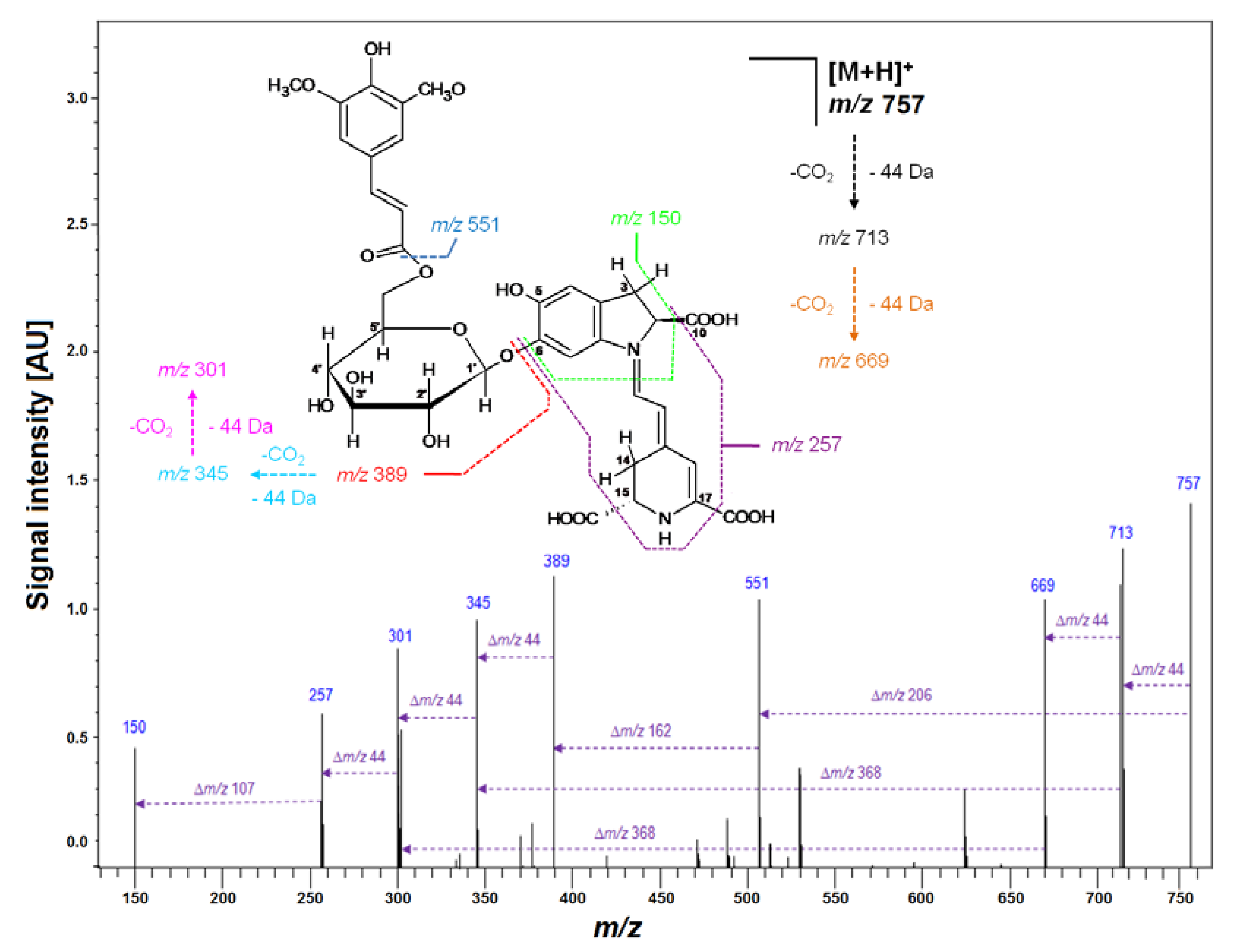



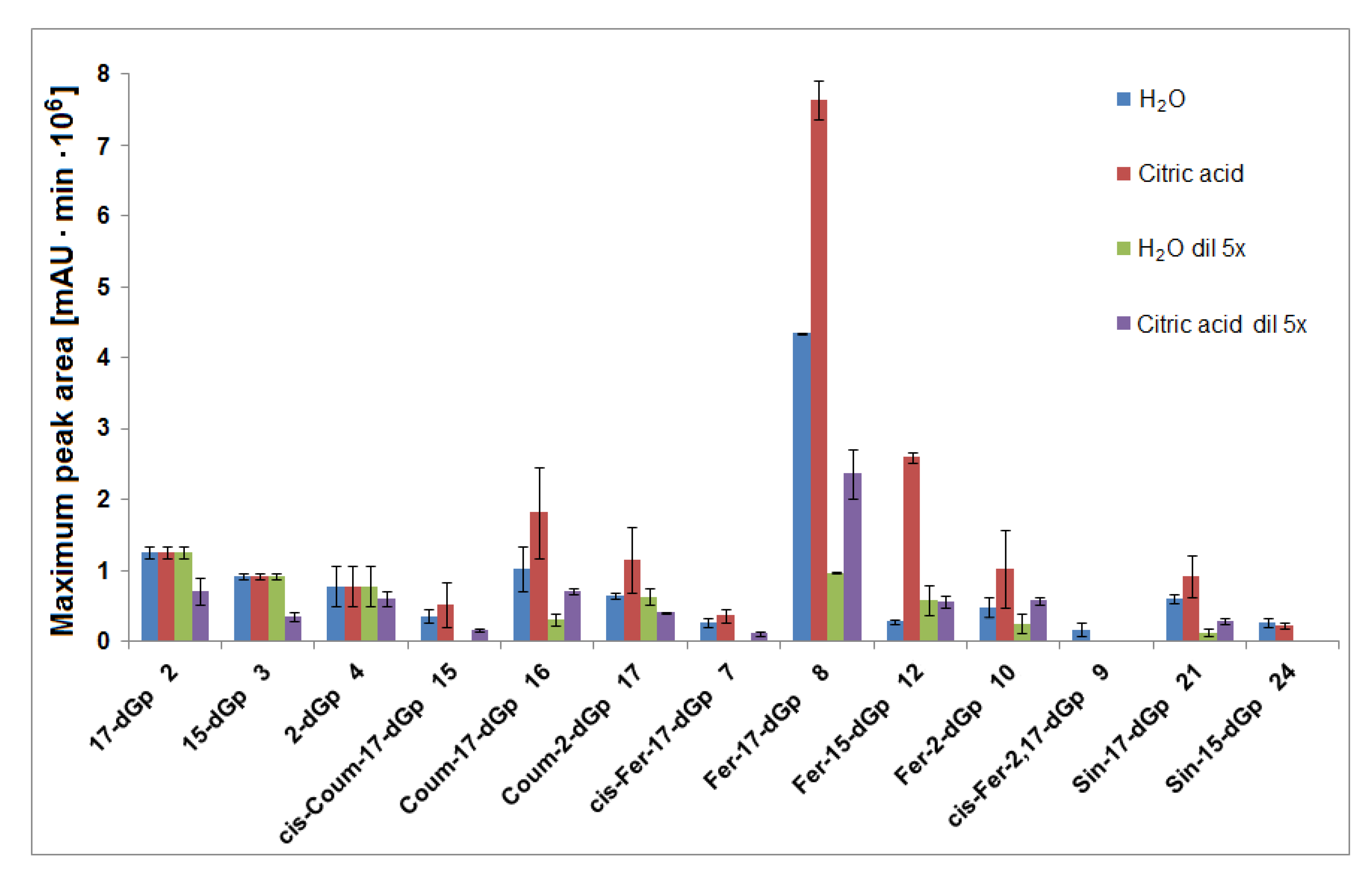
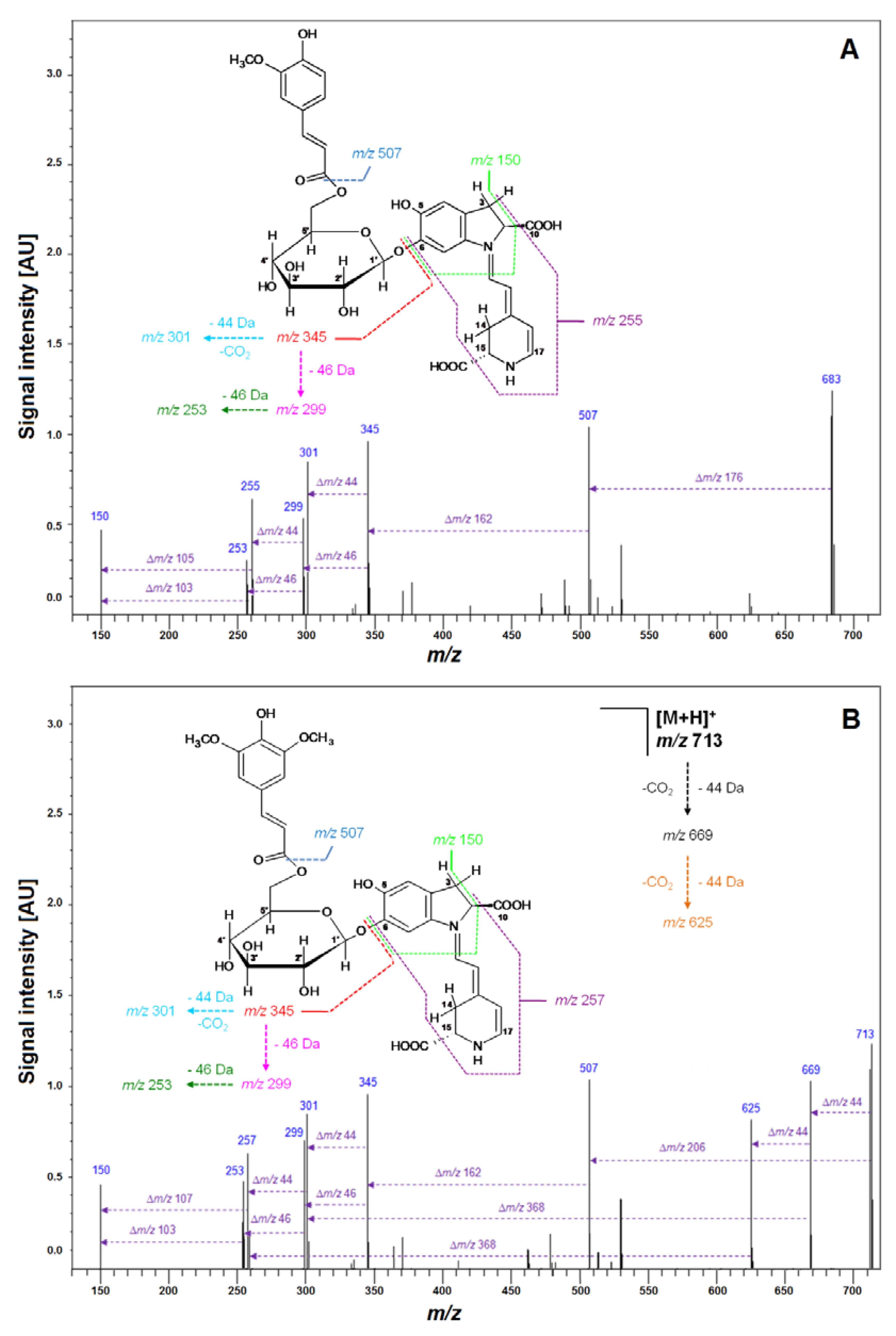


| No. | Compound | Abbreviation | Rt [min] | λmax [nm] | m/z [M + H]+ |
|---|---|---|---|---|---|
| 1 | gomphrenin | Gp | 4.0 | 538 | 551 |
| 1′ | isogomphrenin | IGp | 4.2 | 538 | 551 |
| 2 | 17-decarboxy-gomphrenin | 17-dGp | 4.1 | 507 | 507 |
| 2′ | 17-decarboxy-isogomphrenin | 17-dIGp | 4.4 | 507 | 507 |
| 3 | 15-decarboxy-gomphrenin | 15-dGp | 4.8 | 530 | 507 |
| 4/4′ | 2-decarboxy-gomphrenin | 2-dGp | 5.3 | 533 | 507 |
| 5 | cis-feruloyl-gomphrenin | cis-Fer-Gp | 11.4 | 545 | 727 |
| 5′ | cis-feruloyl-isogomphrenin | cis-Fer-IGp | 12.9 | 545 | 727 |
| 6 | feruloyl-gomphrenin | Fer-Gp | 16.3 | 545 | 727 |
| 6′ | feruloyl-isogomphrenin | Fer-IGp | 23.5 | 545 | 727 |
| 7 | cis-feruloyl-17-decarboxy-gomphrenin | cis-Fer-17-dGp | 12.9 | 515 | 683 |
| 7ʹ | cis-feruloyl-17-decarboxy-isogomphrenin | cis-Fer-17-dIGp | 15.2 | 515 | 683 |
| 8 | feruloyl-17-decarboxy-gomphrenin | Fer-17-dGp | 17.5 | 515 | 683 |
| 9 | cis-feruloyl-2,17-bidecarboxy-gomphrenin | cis-Fer-2,17-dGp | 21.4 | 509 | 639 |
| 10 | feruloyl-2-decarboxy-gomphrenin | Fer-2-dGp | 21.6 | 537 | 683 |
| 8′ | feruloyl-17-decarboxy-isogomphrenin | Fer-17-dIGp | 24.0 | 515 | 683 |
| 11 | feruloyl-2,17-bidecarboxy-gomphrenin | Fer-2,17-dGp | 24.1 | 520 | 639 |
| 10′ | feruloyl-2-decarboxy-isogomphrenin | Fer-2-dIGp | 27.9 | 537 | 683 |
| 12 | feruloyl-15-decarboxy-gomphrenin | Fer-15-dGp | 30.4 | 530 | 683 |
| 11′ | feruloyl-2,17-bidecarboxy-isogomphrenin | Fer-2,17-dIGp | 31.0 | 520 | 639 |
| 13 | cis-coumaroyl-gomphrenin | cis-Coum-Gp | 11.2 | 545 | 697 |
| 13′ | cis-coumaroyl-isogomphrenin | cis-Coum-IGp | 12.8 | 545 | 697 |
| 14 | coumaroyl-gomphrenin | Coum-Gp | 16.1 | 545 | 697 |
| 14′ | coumaroyl-isogomphrenin | Coum-IGp | 21.5 | 545 | 697 |
| 15 | cis-coumaroyl-17-decarboxy-gomphrenin | cis-Coum-17-dGp | 12.9 | 512 | 653 |
| 15′ | cis-coumaroyl-17-decarboxy-isogomphrenin | cis-Coum-17-dIGp | 15.0 | 512 | 653 |
| 16 | coumaroyl-17-decarboxy-gomphrenin | Coum-17-dGp | 17.4 | 512 | 653 |
| 17 | coumaroyl-2-decarboxy-gomphrenin | Coum-2-dGp | 22.5 | 539 | 653 |
| 16′ | coumaroyl-17-decarboxy-isogomphrenin | Coum-17-dIGp | 22.8 | 512 | 653 |
| 18 | coumaroyl-2,17-bidecarboxy-gomphrenin | Coum-2,17-dGp | 26.1 | 515 | 609 |
| 17′ | coumaroyl-2-decarboxy-isogomphrenin | Coum-2-dIGp | 28.8 | 539 | 653 |
| 19 | coumaroyl-15-decarboxy-gomphrenin | Coum-15-dGp | 31.0 | 532 | 653 |
| 18′ | coumaroyl-2,17-bidecarboxy-isogomphrenin | Coum-2,17-dIGp | 31.9 | 515 | 609 |
| 20 | sinapoyl-gomphrenin | Sin-Gp | 15.4 | 545 | 757 |
| 20′ | sinapoyl-isogomphrenin | Sin-IGp | 23.6 | 545 | 757 |
| 21 | sinapoyl-17-decarboxy-gomphrenin | Sin-17-dGp | 17.1 | 517 | 713 |
| 22 | sinapoyl-2-decarboxy-gomphrenin | Sin-2-dGp | 19.3 | 536 | 713 |
| 23 | sinapoyl-2,17-bidecarboxy-gomphrenin a | Sin-2,17-dGp | 21.6 | - b | 669 |
| 23′ | sinapoyl-2,17-bidecarboxy-isogomphrenin a | Sin-2,17-dIGp | 22.4 | - b | 669 |
| 21′ | sinapoyl-17-decarboxy-isogomphrenin | Sin-17-dIGp | 24.0 | 517 | 713 |
| 22′ | sinapoyl-2-decarboxy-isogomphrenin | Sin-2-dIGp | 26.2 | 536 | 713 |
| 24 | sinapoyl-15-decarboxy-gomphrenin a | Sin-15-dGp | 29.7 | - b | 713 |
| No. | Compound | Molecular Formula | [M + H]+ Observed | [M + H]+ Predicted | Error [mDa] | Error [ppm] | Principal MS2 Ions |
|---|---|---|---|---|---|---|---|
| 5 | cis-Fer-Gp | C34H35N2O16 | 727.1947 | 727.1981 | −3.4 | −4.68 | 551; 389; 345 |
| 6 | Fer-Gp | C34H35N2O16 | 727.1970 | 727.1981 | −1.1 | −1.51 | 551; 389; 345 |
| 7 | cis-Fer-17-dGp | C33H35N2O14 | 683.2068 | 683.2083 | −1.5 | −2.20 | 507; 345 |
| 8 | Fer-17-dGp | C33H35N2O14 | 683.2081 | 683.2083 | −0.2 | −0.29 | 507; 345; 301 |
| 10 | Fer-2-dGp | C33H35N2O14 | 683.2075 | 683.2083 | −0.8 | −1.17 | 507; 345 |
| 11 | Fer-2,17-dGp | C32H35N2O12 | 639.2174 | 639.2185 | −1.1 | −1.72 | 463; 301, 257 |
| 12 | Fer-15-dGp | C33H35N2O14 | 683.2063 | 683.2083 | −2.0 | −2.93 | 507; 345; 301 |
| 13 | cis-Coum-Gp | C33H33N2O15 | 697.1886 | 697.1875 | 1.1 | 1.58 | 551; 389 |
| 14 | Coum-Gp | C33H33N2O15 | 697.1887 | 697.1875 | 1.2 | 1.72 | 551; 389; 345 |
| 15 | cis-Coum-17-dGp | C32H33N2O13 | 653.1960 | 653.1977 | −1.7 | −2.60 | 507; 345 |
| 16 | Coum-17-dGp | C32H33N2O13 | 653.1990 | 653.1977 | 1.3 | 1.99 | 507; 345; 301 |
| 18 | Coum-2,17-dGp | C31H33N2O11 | 609.2085 | 609.2079 | 0.6 | 0.98 | 463; 301; 257 |
| 20 | Sin-Gp | C35H37N2O17 | 757.2117 | 757.2087 | 3.0 | 3.96 | 551; 389; 345; 301 |
| 21 | Sin-17-dGp | C34H37N2O15 | 713.2205 | 713.2188 | 1.7 | 2.38 | 507; 345; 301 |
| 22 | Sin-2-dGp | C34H37N2O15 | 713.2156 | 713.2188 | −3.2 | −4.49 | 507; 345; 301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobnicka, N.; Sutor, K.; Kumorkiewicz-Jamro, A.; Spórna-Kucab, A.; Antonik, M.; Dziedzic, E.; Świergosz, T.; Ortyl, J.; Wybraniec, S. Phytochemical Molecules from the Decarboxylation of Gomphrenins in Violet Gomphrena globosa L.—Floral Infusions from Functional Food. Int. J. Mol. Sci. 2020, 21, 8834. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228834
Drobnicka N, Sutor K, Kumorkiewicz-Jamro A, Spórna-Kucab A, Antonik M, Dziedzic E, Świergosz T, Ortyl J, Wybraniec S. Phytochemical Molecules from the Decarboxylation of Gomphrenins in Violet Gomphrena globosa L.—Floral Infusions from Functional Food. International Journal of Molecular Sciences. 2020; 21(22):8834. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228834
Chicago/Turabian StyleDrobnicka, Natalia, Katarzyna Sutor, Agnieszka Kumorkiewicz-Jamro, Aneta Spórna-Kucab, Michał Antonik, Ewa Dziedzic, Tomasz Świergosz, Joanna Ortyl, and Sławomir Wybraniec. 2020. "Phytochemical Molecules from the Decarboxylation of Gomphrenins in Violet Gomphrena globosa L.—Floral Infusions from Functional Food" International Journal of Molecular Sciences 21, no. 22: 8834. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228834






