Table Grapes during Postharvest Storage: A Review of the Mechanisms Implicated in the Beneficial Effects of Treatments Applied for Quality Retention
Abstract
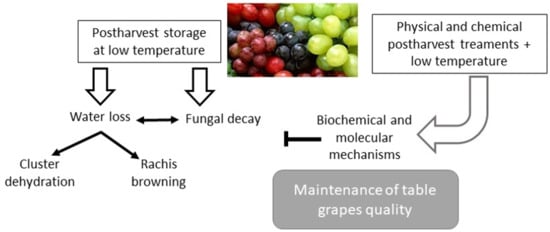
:1. Introduction
2. Mechanisms Associated with Effectiveness of the Postharvest Treatments Applied to Maintain Table Grape Quality
2.1. Effect on the Cell Wall
2.2. Effect on the Plasma Membrane
2.3. Effect on Oxidative Stress
2.4. Effect on Phenylpropanoid Metabolism
2.5. Molecular Basis of the Response of Table Grapes to Postharvest Treatments
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANR | Anthocyanidin reductase |
| AP2/ERF | APETALA2/ethylene response factor |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| CL | Cellulase |
| CHI | Chalcone isomerase |
| DFR | Dihydroflavonol 4-reductase |
| ERFs | Ethylene response factors |
| FLS | Flavanol synthase |
| F3H | Flavanone 3-hydroxylase |
| AOMT | Flavonoid 3′,5′-methyltransferase |
| GR | Glutathione reductase |
| OIV | International Organization of Vine and Wine |
| LAR | Leucoanthocyanidin reductase |
| LDOX | Leucoanthocyanin dioxygenase |
| MDA | Malondialdehyde |
| PRs | Pathogenesis related proteins |
| PL | Pectate lyase |
| PE | Pectinesterase |
| POD | Peroxidase |
| PAL | Phenylalanine ammonia lyase |
| PG | Polygalacturonase |
| PPO | Polyphenol oxidase |
| ROS | Reactive oxygen species |
| ° Brix | Soluble, Solid content |
| STS | Stilbene synthase |
| SOD | Superoxide dismutase |
| SAR | Systemic acquired resistance |
| TIL | Temperature-induced lipocalin |
| UFGT | UDP-glucose: flavonoid 3-O-glucosyltransferase |
| XTH | Xyloglucan endotransglycosylase/hydrolase |
| β-GAL | β-galactosidase |
References
- Robinson, S.P.; Davies, C. Molecular biology of grape berry ripening. Aust. J. Grape Wine Res. 2000, 6, 175–188. [Google Scholar] [CrossRef]
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New approaches for postharvest quality retention of table grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- Romanazzi, G.; Lichter, A.; Gabler, F.M.; Smilanick, J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 63, 141–147. [Google Scholar] [CrossRef]
- Sonker, N.; Pandey, A.K.; Singh, P. Strategies to control post-harvest diseases of table grape: A review. J. Wine Res. 2016, 27, 105–122. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Albersheim, P.; Darvill, A.; Roberts, K.; Sederoff, R.; Staehelin, A. Plant Cell Walls from Chemistry to Biology, 1st ed.; Garland Science: New York, NY, USA, 2010; pp. 1–430. [Google Scholar]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Properties of Wine Polysaccharides. In Pectins—Extraction, Purification, Characterization and Applications; Masuelli, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Payasi, A.; Mishra, N.N.; Chaves, A.L.S.; Singh, R. Biochemistry of fruit softening: An overview. Physiol. Mol. Biol. Plants 2009, 15, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, M.; Kobayashi, S. Expression of a xyloglucan endo-transglycosylase gene is closely related to grape berry softening. Plant Sci. 2002, 162, 621–628. [Google Scholar] [CrossRef]
- Correa, J.; Mamani, M.; Muñoz-Espinoza, C.; González-Agüero, M.; Defilippi, B.G.; Campos-Vargas, R.; Pinto, M.; Hinrichsen, P. New stable QTLs for berry firmness in table grapes. Am. J. Enol. Vitic. 2016, 67, 212–217. [Google Scholar] [CrossRef]
- Wong, D.C.J.; Lopez Gutierrez, R.; Dimopoulos, N.; Gambetta, G.A.; Castellarin, S.D. Combined physiological, transcriptome, and cis-regulatory element analyses indicate that key aspects of ripening, metabolism, and transcriptional program in grapes (Vitis vinifera L.) are differentially modulated accordingly to fruit size. BMC Genomics 2016, 17, e416. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, L.; Guo, Y.; Lin, H.; Liu, Z.; Li, K.; Guo, X. Transcriptome analysis of table grapes (Vitis vinifera L.) identified a gene network module associated with berry firmness. PLoS ONE 2020, 15, e0237526. [Google Scholar] [CrossRef] [PubMed]
- Ejsmentewicz, T.; Balic, I.; Sanhueza, D.; Barria, R.; Meneses, C.; Orellana, A.; Prieto, H.; Defilippi, B.G.; Campos-Vargas, R. Comparative study of two table grape varieties with contrasting texture during cold storage. Molecules 2015, 20, 3667–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Wu, Y.; Li, Y. Changes in firmness, cell wall composition and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Becatti, E.; Chkaiban, L.; Tonutti, P.; Forcato, C.; Bonghi, C.; Ranier, A.M. Short-term postharvest carbon dioxide treatments induce selective molecular and metabolic changes in grape berries. J. Agric. Food Chem. 2010, 58, 8012–8020. [Google Scholar] [CrossRef]
- Bang, J.; Lim, S.; Yi, G.; Lee, J.G.; Lee, E.J. Integrated transcriptomic-metabolomic analysis reveals cellular responses of harvested strawberry fruit subjected to short-term exposure to high levels of carbon dioxide. Postharvest Biol. Technol. 2019, 148, 120–131. [Google Scholar] [CrossRef]
- Besada, C.; Llorca, E.; Novillo, P.; Hernando, I.; Salvador, A. Short-term high CO2 treatment alleviates chilling injury of persimmon cv. Fuyu by preserving the parenchyma structure. Food Control. 2014, 51, 163–170. [Google Scholar] [CrossRef]
- Blanch, M.; Fernandez-Caballero, C.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Accumulation and distribution of potassium and its association with water balance in the skin of Cardinal table grapes during storage. Sci. Hortic. 2014, 175, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Hernandez, M.; Blanch, M.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. High CO2 alleviates cell ultrastructure damage in Autumn Royal table grapes by modulating fatty acid composition and membrane and cell oxidative status during long-term cold storage. Postharvest Biol. Technol. 2020, 160, 111037. [Google Scholar] [CrossRef]
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef] [Green Version]
- Örvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2000, 23, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.H.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and unspecific responses of plants to cold and drought stress. J. Biosci. 2007, 32, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, Y.; Li, Y. Effects of high O2 levels on post-harvest quality and shelf life of table grapes during long-term storage. Eur. Food Res. Technol. 2005, 221, 392–397. [Google Scholar] [CrossRef]
- Rosales, R.; Romero, I.; Fernandez-Caballero, C.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Low temperature and short-term high-CO2 treatment in postharvest storage of table grapes at two maturity stages: Effects on transcriptome profiling. Front. Plant Sci. 2016, 7, e1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champa, H.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Postharvest treatment of polyamines maintains quality and extends shelf-life of table grapes (Vitis vinifera L.) cv. Flame Seedless. Postharvest Biol. Technol. 2014, 91, 57–63. [Google Scholar] [CrossRef]
- Blanch, M.; Alvarez, I.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Involvement of fatty acids in the response to high CO2 and low temperature in harvested strawberries. Postharvest Biol. Technol. 2019, 147, 196–205. [Google Scholar] [CrossRef]
- Liang, S.; Kuang, J.; Ji, S.; Chen, Q.; Deng, W.; Min, T.; Shan, W.; Chen, J.; Lu, W. The membrane lipid metabolism in horticultural products suffering chilling injury. Food Qual. Saf. 2020, 4, 9–14. [Google Scholar] [CrossRef]
- Wan, S.B.; Wang, W.; Wen, P.F.; Chen, J.Y.; Kong, W.F.; Pan, Q.H.; Zhan, J.C.; Tian, L.; Liu, H.T.; Huang, W.D. Cloning of phospholipase D from grape berry and its expression under heat acclimation. J. Biochem. Mol. Biol. 2007, 40, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Frenette Charron, J.B.; Breton, G.; Badawi, M.; Sarhan, F. Molecular and structural analyses of a novel temperature stress-induced lipocalin from wheat and Arabidopsis. FEBS Lett. 2002, 517, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Yun, Z.; Jin, S.; Ding, Y.; Wang, Z.; Gao, H.; Pan, Z.; Xu, J.; Cheng, Y.; Deng, X. Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage. J. Exp. Bot. 2012, 63, 2873–2893. [Google Scholar] [CrossRef]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Lipid transfer proteins as components of the plant innate immune system: Structure, functions, and applications. Acta Nat. 2016, 8, 47–61. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Fernandez-Caballero, C.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Water status and quality improvement in high-CO2 treated table grapes. Food Chem. 2011, 128, 34–39. [Google Scholar] [CrossRef]
- Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Water distribution and ionic balance in response to high CO2 treatments in strawberries (Fragaria vesca L. cv. Mara de Bois). Postharvest Biol. Technol. 2012, 73, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Tucker, G.A. Introduction BT—Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 1–51. [Google Scholar]
- Crisosto, C.H.; Smilanick, J.L.; Dokoozlian, N.K.; Luvisi, D.A. Maintaining table grape post-harvest quality for long distant markets. In Proceedings of the International Symposium on Table Grape Production, Anaheim, California, 28–29 June 1994; Rantz, J.M., Ed.; pp. 195–199. [Google Scholar]
- Sanchez-Ballesta, M.T.; Jiménez, J.B.; Romero, I.; Orea, J.M.; Maldonado, R.; Ureña, Á.G.; Escribano, M.I.; Merodio, C. Effect of high CO2 pretreatment on quality, fungal decay and molecular regulation of stilbene phytoalexin biosynthesis in stored table grapes. Postharvest Biol. Technol. 2006, 42, 209–216. [Google Scholar] [CrossRef]
- Rosales, R.; Fernandez-Caballero, C.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Molecular analysis of the improvement in rachis quality by high CO2 levels in table grapes stored at low temperature. Postharvest Biol. Technol. 2013, 77, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Hernandez, M.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Deciphering the role of CBF/DREB transcription factors and dehydrins in maintaining the quality of table grapes cv. Autumn Royal treated with high CO2 levels and stored at 0 °C. Front. Plant Sci. 2017, 8, e1591. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Aguayo, E.; Artés, F. Alternative atmosphere treatments for keeping quality of “Autumn seedless” table grapes during long-term cold storage. Postharvest Biol. Technol. 2004, 31, 59–67. [Google Scholar] [CrossRef]
- Romero, I.; Domínguez, I.; Doménech-Carbó, A.; Gavara, R.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Effect of high levels of CO2 on the electrochemical behavior and the enzymatic and non-enzymatic antioxidant systems in black and white table grapes stored at 0 °C. J. Sci. Food Agric. 2019, 99, 6859–6867. [Google Scholar] [CrossRef]
- Bendel, P.; Zemah, H.; Kamenetsky, R.; Vergeldt, F.; Van As, H. Magnetization transfer and double-quantum filtered imaging as probes for motional restricted water in tulip bulbs. Magn. Reson. Imaging 2001, 19, 857–865. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Stushnoff, C. The state of water in acclimating vegetative buds from Malus and Amelanchier and its relationship to winter hardiness. Physiol. Plant. 1992, 86, 503–511. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G.; Koster, K.L. What is “unfreezable water”, how unfreezable is it and how much is there? Cryo Lett. 2002, 23, 157–166. [Google Scholar]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Baek, K.-H.; Skinner, D.Z. Production of reactive oxygen species by freezing stress and the protective roles of antioxidant enzymes in plants. J. Agric. Chem. Environ. 2012, 01, 34–40. [Google Scholar] [CrossRef]
- Pavez, L.; Hödar, C.; Olivares, F.; González, M.; Cambiazo, V. Effects of postharvest treatments on gene expression in Prunus persica fruit: Normal and altered ripening. Postharvest Biol. Technol. 2013, 75, 125–134. [Google Scholar] [CrossRef]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitão, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Reboredo, F.H.; Partelli, F.L.; et al. Protective response mechanisms to heat stress in interaction with high [CO2] conditions in coffea spp. Front. Plant Sci. 2016, 7, e947. [Google Scholar] [CrossRef] [Green Version]
- Blanch, M.; Rosales, R.; Goya, L.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. NADP-malic enzyme and glutathione reductase contribute to glutathione regeneration in Fragaria vesca fruit treated with protective high CO2 concentrations. Postharvest Biol. Technol. 2013, 86, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Wu, Z.; Li, H.; Wang, Y.; Liu, F.; Cai, H.; Newlove, A.A.; Wang, Y. Biochemical and proteomic analysis of “Kyoho” grape (Vitis labruscana) berries during cold storage. Postharvest Biol. Technol. 2014, 88, 79–87. [Google Scholar] [CrossRef]
- Ni, Z.J.; Hu, K.D.; Song, C.B.; Ma, R.H.; Li, Z.R.; Zheng, J.L.; Fu, L.H.; Wei, Z.J.; Zhang, H. Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid. Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud, E.; Ivanova, A.; Gordon, C.S.; Whelan, J.; Considine, M.J. Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ. 2012, 35, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, L.; Wang, Q.; Zuo, J. Low temperature conditioning combined with methyl jasmonate can reduce chilling injury in bell pepper. Sci. Hortic. 2019, 243, 434–439. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Xie, B.; Hu, S.; Zheng, Y.; Jin, P. Effects of exogenous calcium and calcium chelant on cold tolerance of postharvest loquat fruit. Sci. Hortic. 2020, 269, 109391. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Macheix, J.-J.; Fleuriet, A.; Billot, J. The main phenolics of fruits. In Fruit Phenolics, 1st ed.; Macheix, J.-J., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 1–116. [Google Scholar]
- Šamec, D.; Piljac-Žegarac, J. Postharvest stability of antioxidant compounds in hawthorn and cornelian cherries at room and refrigerator temperatures—Comparison with blackberries, white and red grapes. Sci. Hortic. 2011, 131, 15–21. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Tomás-Barberán, F.A.; Artés, F. Modified atmosphere packaging preserves quality of SO2-free “Superior seedless” table grapes. Postharvest Biol. Technol. 2006, 39, 146–154. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: A new “Functional” fruit? J. Agric. Food Chem. 2001, 49, 5052–5058. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Fernandez Caballero, C.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Influence of the stage of ripeness on phenolic metabolism and antioxidant activity in table grapes exposed to different CO2 treatments. Postharvest Biol. Technol. 2009, 54, 118–121. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Lichter, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Alchanatis, V.; Ostrovsky, V.; Lurie, S. Physical and visual properties of grape rachis as affected by water vapor pressure deficit. Postharvest Biol. Technol. 2011, 59, 25–33. [Google Scholar] [CrossRef]
- Carvajal-Millán, E.; Carvallo, T.; Orozco, J.A.; Martínez, M.A.; Tapia, I.; Guerrero, V.M.; Rascón-Chu, A.; Llamas, J.; Gardea, A.A. Polyphenol oxidase activity, color changes, and dehydration in table grape rachis during development and storage as affected by N-(2-chloro-4-pyridyl)-N-phenylurea. J. Agric. Food Chem. 2001, 49, 946–951. [Google Scholar] [CrossRef]
- Kaur, S.; Arora, N.K.; Gill, K.B.S.; Sharma, S.; Gill, M.I.S. Hexanal formulation reduces rachis browning and postharvest losses in table grapes cv. ‘Flame Seedless’. Sci. Hortic. 2019, 248, 265–273. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Naikoo, M.I. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Naikoo, M.I., Dar, M.I., Raghib, F., Jaleel, H., Ahmad, B., Raina, A., Khan, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovina, A.S.; Kiselev, K. V Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, e130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Agbayani, R.; Jackson, M.C.; Tang, C.S.; Moore, P.H. Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta 2004, 220, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Schwekendiek, A.; Spring, O.; Heyerick, A.; Pickel, B.; Pitsch, N.T.; Peschke, F.; de Keukeleire, D.; Weber, G. Constitutive expression of a grapevine stilbene synthase gene in transgenic hop (Humulus lupulus L.) yields resveratrol and its derivatives in substantial quantities. J. Agric. Food Chem. 2007, 55, 7002–7009. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, S.; Singer, S.D.; Yin, X.; Yang, J.; Wang, Y.; Wang, X. Expression of the grape VqSTS21 gene in Arabidopsis confers resistance to osmotic stress and biotrophic pathogens but not Botrytis cinerea. Front. Plant Sci. 2016, 7, e1379. [Google Scholar] [CrossRef] [Green Version]
- Kiselev, K.V.; Aleynova, O.A. Influence of overexpression of stilbene synthase VaSTS7 gene on resveratrol production in transgenic cell cultures of grape Vitis amurensis Rupr. Appl. Biochem. Microbiol. 2016, 52, 56–60. [Google Scholar] [CrossRef]
- Carlos-Hilario, L.; Shimshock, R.; Ng, C.; Bingham, J.-P.; Christopher, D.A. Screening Carica papaya native promoters driving stilbene synthase expression in Arabidopsis thaliana for resveratrol glucoside (piceid) synthesis. Plant Biotechnol. Rep. 2015, 9, 307–317. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef]
- Freitas, P.M.; López-Gálvez, F.; Tudela, J.A.; Gil, M.I.; Allende, A. Postharvest treatment of table grapes with ultraviolet-C and chitosan coating preserves quality and increases stilbene content. Postharvest Biol. Technol. 2015, 105, 51–57. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Choi, S.J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]
- Hasan, M.M.; Baek, K.-H. Induction of resveratrol biosynthesis in grape skins and leaves by ultrasonication treatment. Korean J. Hortic. Sci. Technol. 2013, 31, 496–502. [Google Scholar] [CrossRef]
- Maoz, I.; De Rosso, M.; Kaplunov, T.; Vedova, A.D.; Sela, N.; Flamini, R.; Lewinsohn, E.; Lichter, A. Metabolomic and transcriptomic changes underlying cold and anaerobic stresses after storage of table grapes. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Ballesta, M.T.; Alvarez, I.; Escribano, M.I.; Merodio, C.; Romero, I. Effect of high CO2 levels and low temperature on stilbene biosynthesis pathway gene expression and stilbenes production in white, red and black table grape cultivars during postharvest storage. Plant Physiol. Biochem. 2020, 151, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef]
- Crupi, P.; Pichierri, A.; Basile, T.; Antonacci, D. Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem. 2013, 141, 802–808. [Google Scholar] [CrossRef]
- Romero, I.; Domínguez, I.; Morales-Diaz, N.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Regulation of flavonoid biosynthesis pathway by a single or dual short-term CO2 treatment in black table grapes stored at low temperature. Plant Physiol. Biochem. 2020, 156, 30–38. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Romero, I.; Jiménez, J.B.; Orea, J.M.; González-Ureña, Á.; Escribano, M.I.; Merodio, C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol. Technol. 2007, 46, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Jia, H.; Wu, W.; Wang, X.; Fang, J.; Wang, C. Functional conservation analysis and expression modes of grape anthocyanin synthesis genes responsive to low temperature stress. Gene 2015, 574, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, L.; Belwal, T.; Zhang, X.; Lu, H.; Chen, C.; Li, L. Exogenous melatonin and abscisic acid expedite the flavonoids biosynthesis in grape berry of Vitis vinifera cv. Kyoho. Molecules 2020, 25, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Sato, A. Postharvest light irradiation and appropriate temperature treatment increase anthocyanin accumulation in grape berry skin. Postharvest Biol. Technol. 2019, 147, 89–99. [Google Scholar] [CrossRef]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [Green Version]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genom. Data 2017, 14, 56–62. [Google Scholar] [CrossRef]
- Lijavetzky, D.; Carbonell-Bejerano, P.; Grimplet, J.; Bravo, G.; Flores, P.; Fenoll, J.; Hellín, P.; Oliveros, J.C.; Martínez-Zapater, J.M. Berry flesh and skin ripening features in Vitis vinifera as assessed by transcriptional profiling. PLoS ONE 2012, 7, e39547. [Google Scholar] [CrossRef]
- Xiao, H.; Siddiqua, M.; Braybrook, S.; Nassuth, A. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ. 2006, 29, 1410–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhu, W.; Fang, L.; Sun, X.; Su, L.; Liang, Z.; Wang, N.; Londo, J.P.; Li, S.; Xin, H. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera. BMC Plant Biol. 2014, 14, e103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licausi, F.; Giorgi, F.M.; Zenoni, S.; Osti, F.; Pezzotti, M.; Perata, P. Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis vinifera. BMC Genomics 2010, 11, e719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londo, J.P.; Kovaleski, A.P.; Lillis, J.A. Divergence in the transcriptional landscape between low temperature and freeze shock in cultivated grapevine (Vitis vinifera). Hortic. Res. 2018, 5, e10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019, 280, 132–142. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Tindjau, R.; Wong, D.C.J.; Matzat, T.; Haslam, T.; Song, C.; Gambetta, G.A.; Kunst, L.; Castellarin, S.D. Drought stress modulates cuticular wax composition of the grape berry. J. Exp. Bot. 2020, 71, 3126–3141. [Google Scholar] [CrossRef]
- Romero, I.; Vazquez-Hernandez, M.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Expression profiles and DNA-binding affinity of five ERF genes in bunches of Vitis vinifera cv. Cardinal treated with high levels of CO2 at low temperature. Front. Plant Sci. 2016, 7, e1748. [Google Scholar] [CrossRef]
- Romero, I.; Alegria-Carrasco, E.; Gonzalez de Pradena, A.; Vazquez-Hernandez, M.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. WRKY transcription factors in the response of table grapes (cv. Autumn Royal) to high CO2 levels and low temperature. Postharvest Biol. Technol. 2019, 150, 42–51. [Google Scholar] [CrossRef]
- Yin, X.; Allan, A.C.; Xu, Q.; Burdon, J.; Dejnoprat, S.; Chen, K.; Ferguson, I.B. Differential expression of kiwifruit ERF genes in response to postharvest abiotic stress. Postharvest Biol. Technol. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Mao, J.; Zou, Y.; Fu, D.; Chen, W.; Lu, W. Isolation and characterization of ethylene response factor family genes during development, ethylene regulation and stress treatments in papaya fruit. Plant Physiol. Biochem. 2013, 70, 81–92. [Google Scholar] [CrossRef]
- Busam, G.; Kassemeyer, H.H.; Matern, U. Differential expression of chitinases in Vitis vinifera L. responding to systemic acquired resistance activators or fungal challenge. Plant Physiol. 1997, 115, 1029–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.P.; Jacobs, A.K.; Dry, I.B. A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol. 1997, 114, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoki, S.; Suzuki, S. Pathogenesis-Related Proteins in Grape. In Grape and wine Biotechnology, 1st ed.; Morata, A., Loira, I., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 43–59. [Google Scholar]
- Nookaraju, A.; Agrawal, D.C. Enhanced tolerance of transgenic grapevines expressing chitinase and β-1,3-glucanase genes to downy mildew. Plant Cell Tissue Organ. Cult. 2012, 111, 15–28. [Google Scholar] [CrossRef]
- Dhekney, S.A.; Li, Z.T.; Gray, D.J. Grapevines engineered to express cisgenic Vitis vinifera thaumatin-like protein exhibit fungal disease resistance. In Vitro Cell Dev. Biol. Plant 2011, 47, 458–466. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; González-Aguilar, G.A.; Martínez-Téllez, M.Á. Quality and PR gene expression of table grapes treated with ozone and sulfur dioxide to control fungal decay. J. Sci. Food Agric. 2016, 96, 2018–2024. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Bo, Z.; Chen, S.; Wang, Z.; Zheng, Y.; Fang, Y. Regulation of redox status contributes to priming defense against Botrytis cinerea in grape berries treated with β-aminobutyric acid. Sci. Hortic. 2019, 244, 352–364. [Google Scholar] [CrossRef]
- Xiao, H.; Nassuth, A. Stress- and development-induced expression of spliced and unspliced transcripts from two highly similar dehydrin 1 genes in V. riparia and V. vinifera. Plant Cell Rep. 2006, 25, 968–977. [Google Scholar] [CrossRef]
- Fernandez-Caballero, C.; Rosales, R.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Unraveling the roles of CBF1, CBF4 and dehydrin 1 genes in the response of table grapes to high CO2 levels and low temperature. J. Plant Physiol. 2012, 169, 744–748. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.D.; Wang, Y. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 2012, 12, e140. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.; Vazquez-Hernandez, M.; Rosales, R.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. Differential regulation of dehydrin expression and trehalose levels in Cardinal table grape skin by low temperature and high CO2. J. Plant Physiol. 2015, 179, 1–11. [Google Scholar] [CrossRef]
- Rosales, R.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. The crucial role of Φ- And K-segments in the in vitro functionality of Vitis vinifera dehydrin DHN1a. Phytochemistry 2014, 108, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zenoni, S.; Ferrarini, A.; Giacomelli, E.; Xumerle, L.; Fasoli, M.; Malerba, G.; Bellin, D.; Pezzotti, M.; Delledonne, M. Characterization of transcriptional complexity during berry development in Vitis vinifera using RNA-Seq. Plant Physiol. 2010, 152, 1787–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweetman, C.; Wong, D.C.; Ford, C.M.; Drew, D.P. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics 2012, 13, e691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.-T.; Wang, J.-F.; Cramer, G.; Dai, Z.-W.; Duan, W.; Xu, H.-G.; Wu, B.-H.; Fan, P.-G.; Wang, L.-J.; Li, S.-H. Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol. 2012, 12, e174. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Jiao, C.; Singer, S.D.; Gao, M.; Xu, X.; Zhou, Y.; Li, Z.; Fei, Z.; Wang, Y.; Wang, X. Gibberellin-induced changes in the transcriptome of grapevine (Vitis labrusca × V. vinifera) cv. Kyoho flowers. BMC Genom. 2015, 16, e128. [Google Scholar] [CrossRef] [Green Version]
- Domingos, S.; Fino, J.; Paulo, O.S.; Oliveira, C.M.; Goulao, L.F. Molecular candidates for early-stage flower-to-fruit transition in stenospermocarpic table grape (Vitis vinifera L.) inflorescences ascribed by differential transcriptome and metabolome profiles. Plant Sci. 2016, 244, 40–56. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.S.; Zhang, C.; Kurjogi, M.M.; Pervaiz, T.; Zheng, T.; Zhang, C.; Lide, C.; Shangguan, L.; Fang, J. Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci. Rep. 2017, 7, e13134. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Gaonkar, T.; Upadhyay, A.K.; Jogaiah, S.; Shinde, M.P.; Kadoo, N.Y.; Gupta, V.S. Global transcriptome analysis of grapevine (Vitis vinifera L.) leaves under salt stress reveals differential response at early and late stages of stress in table grape cv. Thompson Seedless. Plant Physiol. Biochem. 2018, 129, 168–179. [Google Scholar] [CrossRef]
- Savoi, S.; Herrera, J.C.; Forneck, A.; Griesser, M. Transcriptomics of the grape berry shrivel ripening disorder. Plant Mol. Biol. 2019, 100, 285–301. [Google Scholar] [CrossRef] [Green Version]
- Balic, I.; Vizoso, P.; Nilo-Poyanco, R.; Sanhueza, D.; Olmedo, P.; Sepúlveda, P.; Arriagada, C.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Transcriptome analysis during ripening of table grape berry cv. Thompson Seedless. PLoS ONE 2018, 13, e0190087. [Google Scholar] [CrossRef]
- Wei, L.; Cao, Y.; Cheng, J.; Xiang, J.; Shen, B.; Wu, J. Comparative transcriptome analyses of a table grape ‘Summer Black’ and its early-ripening mutant ‘Tiangong Moyu’ identify candidate genes potentially involved in berry development and ripening. J. Plant Interact. 2020, 15, 213–222. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Bian, F.; Sun, H.; Zhai, H.; Yao, Y. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 2017, 8, e1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, M.; García-Rojas, M.; Muñoz-Espinoza, C.; Carrasco-Valenzuela, T.; Defilippi, B.; González-Agüero, M.; Meneses, C.; Infante, R.; Hinrichsen, P. Transcriptomic study of pedicels from GA(3)-treated table grape genotypes with different susceptibility to berry drop reveals responses elicited in cell wall yield, primary growth and phenylpropanoids synthesis. BMC Plant Biol. 2020, 20, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Fang, J.; Jia, H. Integrative transcriptomics and metabolomics data exploring the effect of chitosan on postharvest grape resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 2016, 6, 32289. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-free fenetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Nakajima, I.; Ban, Y.; Azuma, A.; Onoue, N.; Moriguchi, T.; Yamamoto, T.; Toki, S.; Endo, M. CRISPR/Cas9-mediated targeted mutagenesis in grape. PLoS ONE 2017, 12, e0177966. [Google Scholar] [CrossRef] [Green Version]
- Giacomelli, L.; Zeilmaker, T.; Malnoy, M.; Rouppe van der Voort, J.; Moser, C. Generation of mildew-resistant grapevine clones via genome editing. In Proceedings of the XII International Conference on Grapevine Breeding and Genetics, Bordeaux, France, 15–20 July 2019; Delrot, S., Ollat, N., Gallusci, P., Eds.; Acta Horticulturae: Leuven, Belgium, 2019; Volume 1248, pp. 195–200. [Google Scholar]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-Y.; Jiao, Y.-T.; Wang, Y.-T.; Zhang, N.; Wang, B.-B.; Liu, R.-Q.; Yin, X.; Xu, Y.; Liu, G.-T. CRISPR/Cas9-mediated VvPR4b editing decreases downy mildew resistance in grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7, e149. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, I.; Vazquez-Hernandez, M.; Maestro-Gaitan, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Table Grapes during Postharvest Storage: A Review of the Mechanisms Implicated in the Beneficial Effects of Treatments Applied for Quality Retention. Int. J. Mol. Sci. 2020, 21, 9320. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239320
Romero I, Vazquez-Hernandez M, Maestro-Gaitan I, Escribano MI, Merodio C, Sanchez-Ballesta MT. Table Grapes during Postharvest Storage: A Review of the Mechanisms Implicated in the Beneficial Effects of Treatments Applied for Quality Retention. International Journal of Molecular Sciences. 2020; 21(23):9320. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239320
Chicago/Turabian StyleRomero, Irene, Maria Vazquez-Hernandez, Isaac Maestro-Gaitan, Maria Isabel Escribano, Carmen Merodio, and Maria Teresa Sanchez-Ballesta. 2020. "Table Grapes during Postharvest Storage: A Review of the Mechanisms Implicated in the Beneficial Effects of Treatments Applied for Quality Retention" International Journal of Molecular Sciences 21, no. 23: 9320. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239320