Adaptive Immune Responses in Human Atherosclerosis
Abstract
:1. Introduction
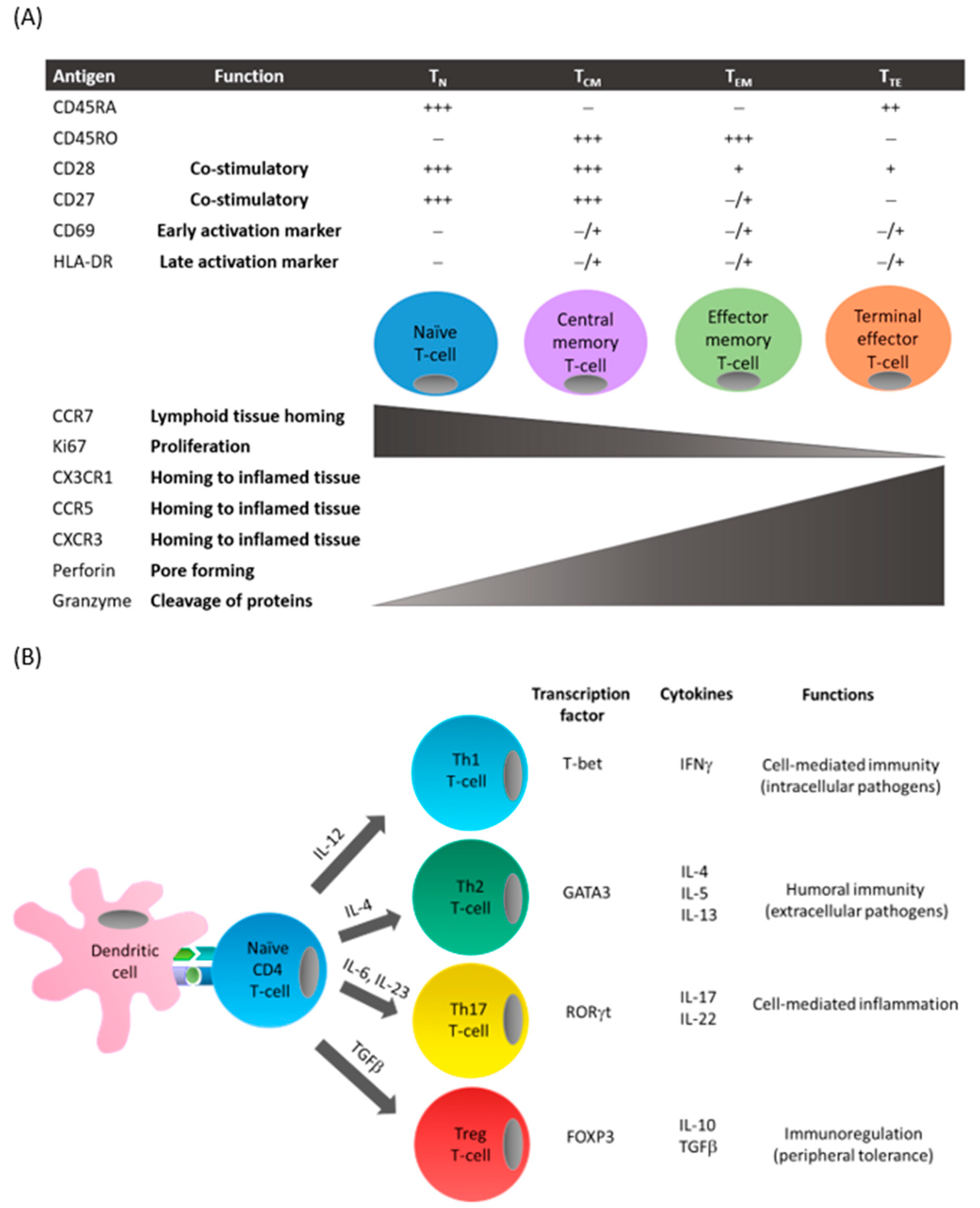
2. Development of T-Cell Subsets
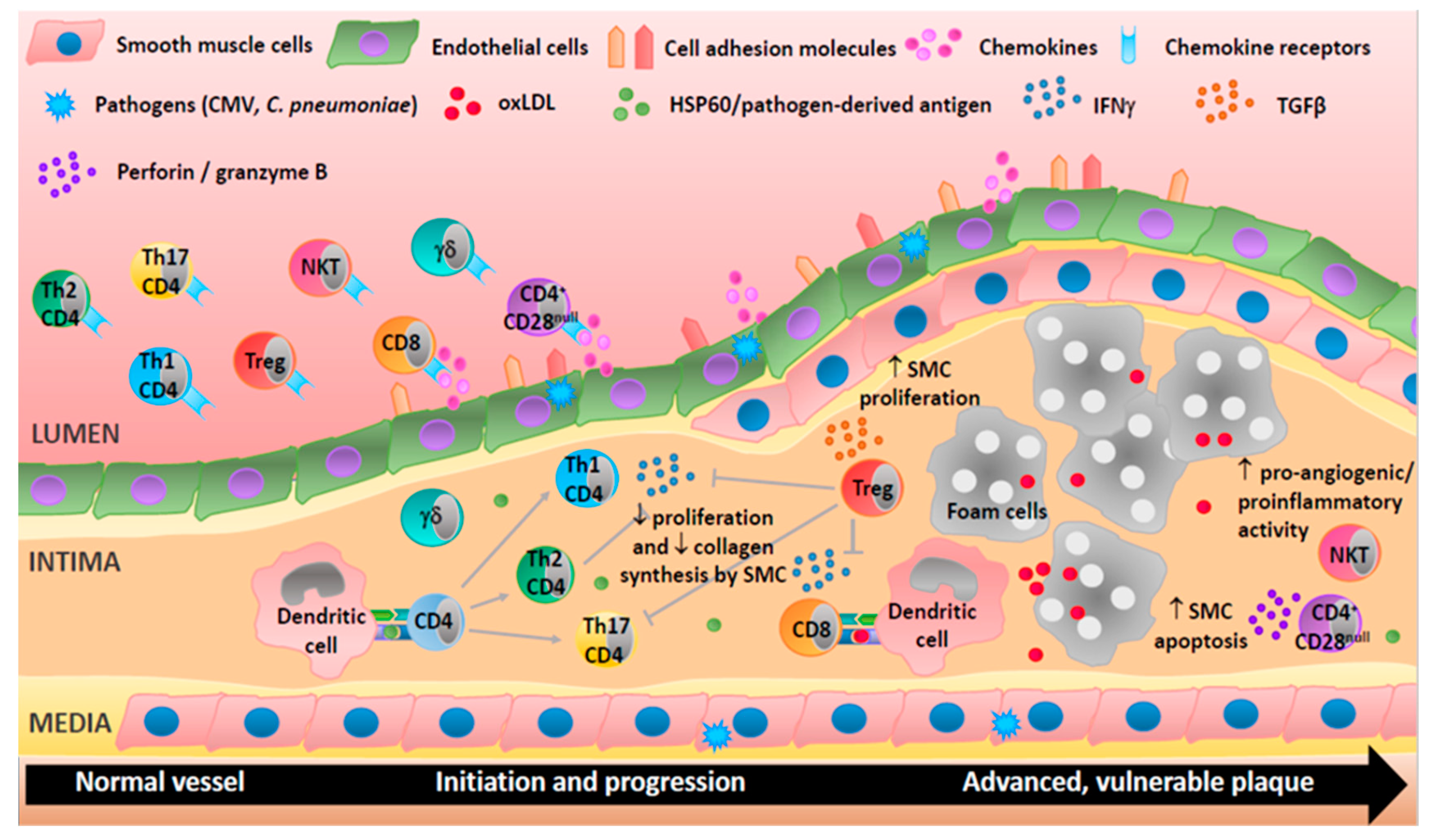
3. T-Cells in Atherosclerotic Plaques
3.1. CD3+ T-Cells
3.2. CD4+ T-Cells
3.3. CD8+ T-Cells
3.4. Treg Cells
3.5. γδ T-Cells
3.6. NKT Cells
4. B-Cells in Atherosclerotic Lesions
5. Activation of T-Cells in Atherosclerotic Plaques
6. Role of Pathogens in the Pathogenesis of Atherosclerosis
7. Clinical Utility of Profiling Circulating Cells of the Adaptive Immune System to Monitor Atherosclerosis
7.1. CD4+ T-Cells
7.2. CD8+ T-Cells
7.3. Treg Cells
7.4. B-Cells and NKT Cells
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| BPIFB4 | Bactericidal/permeability-increasing fold-containing family B member 4 |
| CAD | Coronary artery disease |
| CMV | Cytomegalovirus |
| CV | Cardiovascular |
| DNA | Deoxyribonucleic acid |
| EBV | Epstein–Barr virus |
| FOXP3 | Forkhead box 3 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HIV | Human immunodeficiency virus |
| Hs-CRP | High-sensitivity C-reactive protein |
| HSP | Heat shock protein |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| IMT | Intima–media thickness |
| IP-10 | IFN-inducible protein 10 |
| I-TAC | IFN-inducible T-cell α chemoattractant |
| LDL | Low-density lipoproteins |
| LVEF | Left ventricular ejection fraction |
| MHC | Major histocompatibility complex |
| MI | Myocardial infarction |
| MIG | Monokine induced by IFNγ |
| NKT | Natural killer T-cells |
| NSTE | Non-ST elevation |
| oxLDL | Oxidized low-density lipoproteins |
| PBMC | Peripheral blood mononuclear cells |
| PCI | Percutaneous coronary intervention |
| PCR | Polymerase chain reaction |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PD-1 | Programmed cell death 1 |
| PWV | Pulse wave velocity |
| RNA | Ribonucleic acid |
| SAP | Stable angina pectoris |
| STE | ST elevation |
| TCR | T-cell receptor |
| TGFβ | Transforming growth factor beta |
| Th | T helper |
| TNFα | Tumor necrosis factor alpha |
| Treg | Regulatory T-cells |
| TCM | Central memory T-cells |
| TEM | Effector memory T-cells |
| TN | Naïve T-cells |
| TTE | Terminal effector T-cells |
| UA | Unstable angina |
References
- Wong, B.W.; Meredith, A.; Lin, D.; McManus, B.M. The Biological Role of Inflammation in Atherosclerosis. Can. J. Cardiol. 2012, 28, 631–641. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The role of cytokines in the development of atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.; Ludewick, H.P.; Misra, A.; Lee, S.; Dwivedi, G. Macrophages and T cells in atherosclerosis: A translational perspective. Am. J. Physiol. Circ. Physiol. 2019, 317, H375–H386. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005, 6, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G.K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arter. Off. J. Am. Heart Assoc. Inc. 1986, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kortelainen, M.-L.; Porvari, K. Adventitial macrophage and lymphocyte accumulation accompanying early stages of human coronary atherogenesis. Cardiovasc. Pathol. 2014, 23, 193–197. [Google Scholar] [CrossRef]
- Van Dijk, R.A.; Duinisveld, A.J.F.; Schaapherder, A.F.; Mulder-Stapel, A.; Hamming, J.F.; Kuiper, J.; De Boer, O.J.; Van Der Wal, A.C.; Kolodgie, F.D.; Virmani, R.; et al. A Change in Inflammatory Footprint Precedes Plaque Instability: A Systematic Evaluation of Cellular Aspects of the Adaptive Immune Response in Human Atherosclerosis. J. Am. Heart Assoc. 2015, 4, e001403. [Google Scholar] [CrossRef] [Green Version]
- Rohm, I.; Atiskova, Y.; Drobnik, S.; Fritzenwanger, M.; Kretzschmar, D.; Pistulli, R.; Zanow, J.; Krönert, T.; Mall, G.; Figulla, H.R.; et al. Decreased Regulatory T Cells in Vulnerable Atherosclerotic Lesions: Imbalance between Pro- and Anti-Inflammatory Cells in Atherosclerosis. Mediat. Inflamm. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- De Boer, O.J.; Hirsch, F.; Van Der Wal, A.C.; Van Der Loos, C.M.; Das, P.K.; Becker, A.E. Costimulatory molecules in human atherosclerotic plaques: An indication of antigen specific T lymphocyte activation. Atherosclerosis 1997, 133, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Stemme, S.; Holm, J.; Hansson, G.K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arter. Thromb. A J. Vasc. Biol. 1992, 12, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Feng, X.; Cai, W.; Liu, T.; Liang, Z.; Sun, Y.; Yan, C.; Han, Y. Chemokine CX3CL1 and its receptor CX3CR1 are associated with human atherosclerotic lesion volnerability. Thromb. Res. 2015, 135, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, E.; Mauriello, A.; Partenzi, A.; Anemona, L.; Spagnoli, L.G. Flow cytometry analysis of atherosclerotic plaque cells from human carotids: A validation study. Cytometry 2000, 39, 158–165. [Google Scholar] [CrossRef]
- Lebedeva, A.; Vorobyeva, D.; Vagida, M.; Ivanova, O.; Felker, E.; Fitzgerald, W.; Danilova, N.; Gontarenko, V.; Shpektor, A.; Vasilieva, E.; et al. Ex vivo culture of human atherosclerotic plaques: A model to study immune cells in atherogenesis. Atherosclerosis 2017, 267, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Carrizzo, A.; Ferrario, A.; Maciag, A.; Cattaneo, M.; Spinelli, C.C.; Montella, F.; Damato, A.; Ciaglia, E.; Puca, A.A. A Model of Evolutionary Selection: The Cardiovascular Protective Function of the Longevity Associated Variant of BPIFB4. Int. J. Mol. Sci. 2018, 19, 3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossena, M.; Ferrario, A.; Lopardo, V.; Ciaglia, E.; Puca, A.A. New Insights for BPIFB4 in Cardiovascular Therapy. Int. J. Mol. Sci. 2020, 21, 7163. [Google Scholar] [CrossRef]
- Ciaglia, E.; Montella, F.; Lopardo, V.; Scala, P.; Ferrario, A.; Cattaneo, M.; Carrizzo, A.; Malovini, A.; Madeddu, P.; Vecchione, C.; et al. Circulating BPIFB4 Levels Associate With and Influence the Abundance of Reparative Monocytes and Macrophages in Long Living Individuals. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Ciaglia, E.; Montella, F.; Maciag, A.; Scala, P.; Ferrario, A.; Banco, C.; Carrizzo, A.; Spinelli, C.C.; Cattaneo, M.; De Candia, P.; et al. Longevity-Associated Variant of BPIFB4 Mitigates Monocyte-Mediated Acquired Immune Response. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2019, 74, S38–S44. [Google Scholar] [CrossRef]
- Grivel, J.-C.; Ivanova, O.; Pinegina, N.; Blank, P.S.; Shpektor, A.; Margolis, L.B.; Vasilieva, E. Activation of T Lymphocytes in Atherosclerotic Plaques. Arter. Thromb. Vasc. Biol. 2011, 31, 2929–2937. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.-A.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J.; Ulfgren, A.-K.; Nyberg, P.; Hedin, U.; Swedenborg, J.; Andersson, U.; Hansson, G.K. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999, 145, 33–43. [Google Scholar] [CrossRef]
- De Boer, O.J.; Van Der Wal, A.C.; Verhagen, C.E.; Becker, A.E. Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J. Pathol. 1999, 188, 174–179. [Google Scholar] [CrossRef]
- Oliveira, R.T.D.; Silva, R.M.; Teo, F.H.; Mineiro, M.F.; Ferreira, M.C.; Altemani, A.; Mamoni, R.L.; Menezes, F.H.; Blotta, M.H.S.L. Detection of TCD4+ subsets in human carotid atheroma. Cytokine 2013, 62, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Sauty, A.; Iarossi, A.S.; Sukhova, G.K.; Neote, K.; Libby, P.; Luster, A.D. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Investig. 1999, 104, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Mallat, Z.; Corbaz, A.; Scoazec, A.; Besnard, S.; Leseèche, G.; Chvatchko, Y.; Tedgui, A. Expression of Interleukin-18 in Human Atherosclerotic Plaques and Relation to Plaque Instability. Circulation 2001, 104, 1598–1603. [Google Scholar] [CrossRef] [Green Version]
- Pigarevskii, P.V.; Maltseva, S.V.; Snegova, V.; Davydova, N.G. Role of Interleukin-18 in Destabilization of the Atherosclerotic Plaque in Humans. Bull. Exp. Biol. Med. 2014, 157, 821–824. [Google Scholar] [CrossRef]
- Uyemura, K.; Demer, L.L.; Castle, S.C.; Jullien, D.; Berliner, J.A.; Gately, M.K.; Warrier, R.R.; Pham, N.; Fogelman, A.M.; Modlin, R.L. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J. Clin. Investig. 1996, 97, 2130–2138. [Google Scholar] [CrossRef]
- Voloshyna, I.; Littlefield, M.J.; Reiss, A.B. Atherosclerosis and interferon-gamma: New insights and therapeutic targets. Trends Cardiovasc. Med. 2014, 24, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.L.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Yu, X.; Ding, Y.-J.; Fu, Q.-Q.; Xie, J.-J.; Tang, T.-T.; Yao, R.; Chen, Y.; Liao, Y.-H. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008, 127, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Zeng, Q.T. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 2006, 17, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Wang, X.Q.; Du, R.Z.; Zhang, K.N.; Liu, X.G.; Ma, D.X.; Yu, S.; Su, G.H.; Li, Z.H.; et al. Elevated frequencies of circulating Th22 cell in addition to Th17 cell and Th17/Th1 cell in patients with acute coronary syndrome. PLoS ONE 2013, 8, e71466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, F.; Pan, H.; Zhao, Y.; Wang, S.; Sun, S.; Li, J.; Hu, X.; Wang, L. Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis 2012, 221, 232–241. [Google Scholar] [CrossRef]
- Liuzzo, G.; Goronzy, J.J.; Yang, H.; Kopecky, S.L.; Holmes, D.R.; Frye, R.L.; Weyand, C.M. Monoclonal T-Cell Proliferation and Plaque Instability in Acute Coronary Syndromes. Circulation 2000, 101, 2883–2888. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, I.E.; Baruah, P.; Finlayson, C.J.; Loftus, I.M.; Antunes, R.F.; Lim, P.; Bunce, N.; Kaski, J.C. High Levels of Costimulatory Receptors OX40 and 4-1BB Characterize CD4+CD28null T Cells in Patients With Acute Coronary Syndrome. Circ. Res. 2012, 110, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, I.E. The life (and death) of CD4+CD28null T cells in inflammatory diseases. Immunology 2015, 146, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Paul, V.S.V.; Paul, C.M.P.; Kuruvilla, S. Quantification of Various Inflammatory Cells in Advanced Atherosclerotic Plaques. J. Clin. Diagn. Res. 2016, 10, EC35–EC38. [Google Scholar] [CrossRef]
- Van Duijn, J.; Kritikou, E.; Benne, N.; Van Der Heijden, T.; Van Puijvelde, G.H.; Kröner, M.J.; Schaftenaar, F.H.; Foks, A.; Wezel, A.; Smeets, H.; et al. CD8+ T-cells contribute to lesion stabilization in advanced atherosclerosis by limiting macrophage content and CD4+ T-cell responses. Cardiovasc. Res. 2018, 115, 729–738. [Google Scholar] [CrossRef]
- Hendel, A.; Cooper, D.; Abraham, T.; Zhao, H.; Allard, M.F.; Granville, D.J. Proteinase inhibitor 9 is reduced in human atherosclerotic lesion development. Cardiovasc. Pathol. 2012, 21, 28–38. [Google Scholar] [CrossRef]
- Choy, J.C.; McDonald, P.C.; Suarez, A.C.; Hung, V.H.Y.; Wilson, J.E.; McManus, B.M.; Granville, D.J. Granzyme B in Atherosclerosis and Transplant Vascular Disease: Association with Cell Death and Atherosclerotic Disease Severity. Mod. Pathol. 2003, 16, 460–470. [Google Scholar] [CrossRef]
- De Boer, O.J.; Van Der Meer, J.J.; Teeling, P.; Van Der Loos, C.M.; Van Der Wal, A.C. Low Numbers of FOXP3 Positive Regulatory T Cells Are Present in all Developmental Stages of Human Atherosclerotic Lesions. PLoS ONE 2007, 2, e779. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Chung, S.; White, G.; Bao, S.; Celermajer, D. The “atheroprotective” mediators apolipoproteinA-I and Foxp3 are over-abundant in unstable carotid plaques. Int. J. Cardiol. 2010, 145, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Dietel, B.; Cicha, I.; Voskens, C.J.; Verhoeven, E.; Achenbach, S.; Garlichs, C.D. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis 2013, 230, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Toma, I.; McCaffrey, T.A. Transforming growth factor-β and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012, 347, 155–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foks, A.C.; Lichtman, A.H.; Kuiper, J. Treating Atherosclerosis with Regulatory T Cells. Arter. Thromb. Vasc. Biol. 2015, 35, 280–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, H.-X.; Guo, B.-B.; Liu, Q.; Li, Y.-K.; Yang, Z.; Feng, W.-J.; Mo, Z.-C. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol. Sin. 2018, 39, 1249–1258. [Google Scholar] [CrossRef]
- Lawand, M.; Déchanet-Merville, J.; Dieu-Nosjean, M.-C. Key Features of Gamma-Delta T-Cell Subsets in Human Diseases and Their Immunotherapeutic Implications. Front. Immunol. 2017, 8, 761. [Google Scholar] [CrossRef] [Green Version]
- Vu, D.M.; Tai, A.; Tatro, J.B.; Karas, R.H.; Huber, B.T.; Beasley, D. gammadeltaT cells are prevalent in the proximal aorta and drive nascent atherosclerotic lesion progression and neutrophilia in hypercholesterolemic mice. PLoS ONE 2014, 9, e109416. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Wu, R.; Hedrick, C.C. Gammadelta (γδ) T lymphocytes do not impact the development of early atherosclerosis. Atherosclerosis 2014, 234, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Kleindienst, R.; Xu, Q.; Willeit, J.; Waldenberger, F.R.; Weimann, S.; Wick, G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am. J. Pathol. 1993, 142, 1927–1937. [Google Scholar] [PubMed]
- Millonig, G.; Malcom, G.T.; Wick, G. Early inflammato.ry-immunological lesions in juvenile atherosclerosis from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY)-study. Atherosclerosis 2002, 160, 441–448. [Google Scholar] [CrossRef]
- Van Puijvelde, G.H.; Kuiper, J. NKT cells in cardiovascular diseases. Eur. J. Pharmacol. 2017, 816, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, M.; Ammi, R.; Van Brussel, I.; Roth, L.; De Winter, B.Y.; Vercauteren, S.R.; Hendriks, J.M.; Lauwers, P.; Van Schil, P.E.; De Meyer, G.R.Y.; et al. Linking CD11b+ Dendritic Cells and Natural Killer T Cells to Plaque Inflammation in Atherosclerosis. Mediat. Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobryshev, Y.V.; Lord, R.S. Co-accumulation of Dendritic Cells and Natural Killer T Cells within Rupture-prone Regions in Human Atherosclerotic Plaques. J. Histochem. Cytochem. 2005, 53, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakakis, E.; Cavallari, M.; Andert, J.; Philippova, M.; Koella, C.; Bochkov, V.; Erne, P.; Wilson, S.B.; Mori, L.; Biedermann, B.C.; et al. Invariant natural killer T cells: Linking inflammation and neovascularization in human atherosclerosis. Eur. J. Immunol. 2010, 40, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.; Pejnovic, N.; Hamilton, H.; Liew, T.V.; Popadić, S.; Poggi, A.; Khan, S.M. Atherosclerotic Abdominal Aortic Aneurysm and the Interaction Between Autologous Human Plaque-Derived Vascular Smooth Muscle Cells, Type 1 NKT, and Helper T Cells. Circ. Res. 2005, 96, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Huan, T.; Zhang, B.; Wang, Z.; Joehanes, R.; Zhu, J.; Johnson, A.D.; Ying, S.; Munson, P.J.; Raghavachari, N.; Wang, R.; et al. A Systems Biology Framework Identifies Molecular Underpinnings of Coronary Heart Disease. Arter. Thromb. Vasc. Biol. 2013, 33, 1427–1434. [Google Scholar] [CrossRef] [Green Version]
- Hamze, M.; Desmetz, C.; Berthe, M.L.; Roger, P.; Boulle, N.; Brancherau, P.; Picard, E.; Guzman, C.; Tolza, C.; Guglielmi, P. Characterization of Resident B Cells of Vascular Walls in Human Atherosclerotic Patients. J. Immunol. 2013, 191, 3006–3016. [Google Scholar] [CrossRef] [Green Version]
- De Palma, R.; Del Galdo, F.; Abbate, G.; Chiariello, M.; Calabrò, R.; Forte, L.; Cimmino, G.; Papa, M.F.; Russo, M.G.; Ambrosio, G.; et al. Patients with Acute Coronary Syndrome Show Oligoclonal T-Cell Recruitment Within Unstable Plaque. Circulation 2006, 113, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Benagiano, M.; D’Elios, M.M.; Amedei, A.; Azzurri, A.; Van Der Zee, R.; Ciervo, A.; Rombolà, G.; Romagnani, S.; Cassone, A.; Del Prete, G. Human 60-kDa Heat Shock Protein Is a Target Autoantigen of T Cells Derived from Atherosclerotic Plaques. J. Immunol. 2005, 174, 6509–6517. [Google Scholar] [CrossRef] [Green Version]
- Rossmann, A.; Henderson, B.; Heidecker, B.; Seiler, R.; Fraedrich, G.; Singh, M.; Parson, W.; Keller, M.; Grubeck-Loebenstein, B.; Wick, G. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp. Gerontol. 2008, 43, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Steuer, J.; Gillgren, P.; Hayderi, A.; Liu, A.; Frostegård, J. Induction of Dendritic Cell–Mediated Activation of T Cells From Atherosclerotic Plaques by Human Heat Shock Protein 60. J. Am. Hear. Assoc. 2017, 6, e006778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc. Pharmacol. 2016, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stemme, S.; Faber, B.; Holm, J.; Wiklund, O.; Witztum, J.L.; Hansson, G.K. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 1995, 92, 3893–3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Frostegård, J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J. Intern. Med. 2018, 284, 193–210. [Google Scholar] [CrossRef]
- Frostegård, J.; Zhang, Y.; Sun, J.; Yan, K.; Liu, A. Oxidized Low-Density Lipoprotein (OxLDL)–Treated Dendritic Cells Promote Activation of T Cells in Human Atherosclerotic Plaque and Blood, Which Is Repressed by Statins: MicroRNA let-7c Is Integral to the Effect. J. Am. Hear. Assoc. 2016, 5, e003976. [Google Scholar] [CrossRef] [Green Version]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Lv, Y.; Han, F.-F.; Gong, L.-L.; Liu, H.; Ma, J.; Yu, W.-Y.; Wan, Z.-R.; Jia, Y.-J.; Zhang, W.; Shi, M.; et al. Human cytomegalovirus infection and vascular disease risk: A meta-analysis. Virus Res. 2017, 227, 124–134. [Google Scholar] [CrossRef]
- Lichtner, M.; Cicconi, P.; Vita, S.; Cozzi-Lepri, A.; Galli, M.; Caputo, S.L.; Saracino, A.; De Luca, A.; Moioli, M.; Maggiolo, F.; et al. Cytomegalovirus Coinfection Is Associated With an Increased Risk of Severe Non–AIDS-Defining Events in a Large Cohort of HIV-Infected Patients. J. Infect. Dis. 2015, 211, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Courivaud, C.; Bamoulid, J.; Chalopin, J.-M.; Gaiffe, E.; Tiberghien, P.; Saas, P.; Ducloux, D. Cytomegalovirus Exposure and Cardiovascular Disease in Kidney Transplant Recipients. J. Infect. Dis. 2013, 207, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyanka, S.; Kaarthikeyan, G.; Nadathur, J.D.; Mohanraj, A.; Kavarthapu, A. Detection of cytomegalovirus, Epstein–Barr virus, and Torque Teno virus in subgingival and atheromatous plaques of cardiac patients with chronic periodontitis. J. Indian Soc. Periodontol. 2017, 21, 456–460. [Google Scholar] [PubMed]
- Heybar, H.; Alavi, S.M.; Nejad, M.F.; Latifi, M. Cytomegalovirus Infection and Atherosclerosis in Candidate of Coronary Artery Bypass Graft. Jundishapur J. Microbiol. 2015, 8, e15476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Mao, Y.; Dong, B.; Guan, W.; Shi, J.; Wang, S. Detection of specific Chlamydia pneumoniae and cytomegalovirus antigens in human carotid atherosclerotic plaque in a Chinese population. Oncotarget 2017, 8, 55435–55442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, A.; Erdoğan, M.B.; Ekşi, F.; Yamak, B. Demonstration of Chlamydophila pneumoniae, Mycoplasma pneumoniae, Cytomegalovirus, and Epstein-Barr virus in atherosclerotic coronary arteries, nonrheumatic calcific aortic and rheumatic stenotic mitral valves by polymerase chain reaction. Anadolu Kardiyol. Dergisi/Anatol. J. Cardiol. 2011, 11, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xenaki, E.; Hassoulas, J.; Apostolakis, S.; Sourvinos, G.; Spandidos, D.A. Detection of Cytomegalovirus in Atherosclerotic Plaques and Nonatherosclerotic Arteries. Angiology 2009, 60, 504–508. [Google Scholar] [CrossRef]
- Pampou, S.Y.; Gnedoy, S.N.; Bystrevskaya, V.B.; Smirnov, V.N.; Chazov, E.I.; Melnick, J.L.; DeBakey, M.E. Cytomegalovirus genome and the immediate-early antigen in cells of different layers of human aorta. Virchows Arch. 2000, 436, 539–552. [Google Scholar] [CrossRef]
- Yi, L.; Wang, D.-X.; Feng, Z.-J. Detection of Human Cytomegalovirus in Atherosclerotic Carotid Arteries in Humans. J. Formos. Med. Assoc. 2008, 107, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Yaiw, K.-C.; Ovchinnikova, O.; Taher, C.; Mohammad, A.-A.; Davoudi, B.; Shlyakhto, E.V.; Rotar, O.P.; Konradi, A.; Wilhelmi, V.; Rahbar, A.; et al. High prevalence of human cytomegalovirus in carotid atherosclerotic plaques obtained from Russian patients undergoing carotid endarterectomy. Herpesviridae 2013, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Nikitskaya, E.; Lebedeva, A.; Ivanova, O.; Maryukhnich, E.; Shpektor, A.; Grivel, J.; Margolis, L.; Vasilieva, E. Cytomegalovirus-Productive Infection Is Associated With Acute Coronary Syndrome. J. Am. Heart Assoc. 2016, 5, e003759. [Google Scholar] [CrossRef] [Green Version]
- Van De Berg, P.J.E.J.; Yong, S.-L.; Remmerswaal, E.B.M.; Van Lier, R.A.W.; Berge, I.J.M.T. Cytomegalovirus-Induced Effector T Cells Cause Endothelial Cell Damage. Clin. Vaccine Immunol. 2012, 19, 772–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacre, K.; Hunt, P.W.; Hsue, P.Y.; Maidji, E.; Martin, J.N.; Deeks, S.G.; Autran, B.; McCune, J.M. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 2012, 26, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadi, M.; Fazel, M.; Saadat, S.H.; Naseri, M.H.; Ghasemi, M.; Dabiri, H.; Aryan, R.S.; Esfahani, A.; Ahmadi, A.; Kazemi-Saleh, D.; et al. Cytomegalovirus Localization In Atherosclerotic Plaques Is Associated With Acute Coronary Syndromes: Report Of 105 Patients. Methodist DeBakey Cardiovasc. J. 2012, 8, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Moroi, M.; Yamamoto, M.; Kubota, T.; Ono, T.; Funatsu, A.; Komatsu, H.; Tsuji, T.; Hara, H.; Hara, H.; et al. Presence and Severity of Chlamydia pneumoniae and Cytomegalovirus Infection in Coronary Plaques Are Associated With Acute Coronary Syndromes. Int. Heart J. 2006, 47, 511–519. [Google Scholar] [CrossRef] [Green Version]
- De Boer, O.J.; Teeling, P.; Idu, M.M.; Becker, A.E.; Van Der Wal, A.C. Epstein Barr virus specific T-cells generated from unstable human atherosclerotic lesions: Implications for plaque inflammation. Atherosclerosis 2006, 184, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, A. Cardioprotective effect of influenza and pneumococcal vaccination in patients with cardiovascular diseases. Vaccine 2018, 36, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.T.; Van Der Meer, J.J.; Teeling, P.; Van Der Sluijs, K.; Idu, M.M.; Rimmelzwaan, G.F.; Levi, M.; Van Der Wal, A.C.; De Boer, O.J. Selective Expansion of Influenza A Virus-Specific T Cells in Symptomatic Human Carotid Artery Atherosclerotic Plaques. Stroke 2008, 39, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Mosorin, M.; Surcel, H.-M.; Laurila, A.; Lehtinen, M.; Karttunen, R.; Juvonen, J.; Paavonen, J.; Morrison, R.P.; Saikku, P.; Juvonen, T. Detection ofChlamydia pneumoniae–Reactive T Lymphocytes in Human Atherosclerotic Plaques of Carotid Artery. Arter. Thromb. Vasc. Biol. 2000, 20, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- De Boer, O.J.; Van Der Wal, A.C.; Houtkamp, M.A.; Ossewaarde, J.M.; Teeling, P.; Becker, A.E. Unstable atherosclerotic plaques contain T-cells that respond to Chlamydia pneumoniae. Cardiovasc. Res. 2000, 48, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Nadareishvili, Z.G.; Koziol, D.E.; Szekely, B.; Ruetzler, C.; Labiche, R.; McCarron, R.; DeGraba, T.J. Increased CD8+T Cells Associated With Chlamydia pneumoniae in Symptomatic Carotid Plaque. Stroke 2001, 32, 1966–1972. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Cianflone, D.; Vecchio, V.; Banfi, M.; Vermi, A.C.; de Metrio, M.; Grigore, L.; Pellegatta, F.; Pirillo, A.; Garlaschelli, K.; et al. Effector Memory T cells Are Associated with Atherosclerosis in Humans and Animal Models. J. Am. Heart Assoc. 2012, 1, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Moro-García, M.A.; Iglesias, F.L.; Avanzas, P.; Echeverría, A.; López-Larrea, C.; de la Tassa, C.M.; Alonso-Arias, R. Disease complexity in acute coronary syndrome is related to the patient’s immunological status. Int. J. Cardiol. 2015, 189, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, Y.; Cheng, M.; Ji, Y.; Yang, X.; Liu, P.; Jia, S.; Yuan, Z.-Y. Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis 2011, 217, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zong, W.; Zhang, M.; Tu, Y.; Zhou, Q.; Ni, M.; Li, Z.; Liu, H.; Zhang, J. Increased Ratio of Circulating T-Helper 1 to T-Helper 2 Cells and Severity of Coronary Artery Disease in Patients with Acute Myocardial Infarction: A Prospective Observational Study. Med. Sci. Monit. 2019, 25, 6034–6042. [Google Scholar] [CrossRef]
- Emoto, T.; Sasaki, N.; Yamashita, T.; Kasahara, K.; Yodoi, K.; Sasaki, Y.; Matsumoto, T.; Mizoguchi, T.; Hirata, K.-I. Regulatory/effector T-cell ratio is reduced in coronary artery disease. Circ. J. 2014, 78, 2935–2941. [Google Scholar] [CrossRef] [Green Version]
- Bergstrom, I.; Backteman, K.; Lundberg, A.; Ernerudh, J.; Jonasson, L. Persistent accumulation of interferon-gamma-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis 2012, 224, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Kolbus, D.; Ljungcrantz, I.; Andersson, L.; Hedblad, B.; Fredrikson, G.N.; Björkbacka, H.; Nilsson, J. Association between CD8+ T-cell subsets and cardiovascular disease. J. Intern. Med. 2013, 274, 41–51. [Google Scholar] [CrossRef]
- Zidar, D.A.; Mudd, J.C.; Juchnowski, S.; Lopes, J.P.; Sparks, S.; Park, S.S.; Ishikawa, M.; Osborne, R.; Washam, J.B.; Chan, C.; et al. Altered Maturation Status and Possible Immune Exhaustion of CD8 T Lymphocytes in the Peripheral Blood of Patients Presenting With Acute Coronary Syndromes. Arter. Thromb. Vasc. Biol. 2016, 36, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Podolec, J.; Niewiara, L.; Skiba, D.S.; Siedlinski, M.; Baran, J.; Komar, M.; Guzik, B.; Kablak-Ziembicka, A.; Kopeć, G.; Guzik, T.; et al. Higher levels of circulating naïve CD8+CD45RA+ cells are associated with lower extent of coronary atherosclerosis and vascular dysfunction. Int. J. Cardiol. 2018, 259, 26–30. [Google Scholar] [CrossRef]
- Ammirati, E.; Cianflone, D.; Banfi, M.; Vecchio, V.; Palini, A.; De Metrio, M.; Marenzi, G.; Panciroli, C.; Tumminello, G.; Anzuini, A.; et al. Circulating CD4 + CD25 hi CD127 lo Regulatory T-Cell Levels Do Not Reflect the Extent or Severity of Carotid and Coronary Atherosclerosis. Arter. Thromb. Vasc. Biol. 2010, 30, 1832–1841. [Google Scholar] [CrossRef] [Green Version]
- Hasib, L.; Lundberg, A.K.; Zachrisson, H.; Ernerudh, J.; Jonasson, L. Functional and homeostatic defects of regulatory T cells in patients with coronary artery disease. J. Intern. Med. 2015, 279, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, L.-J.; Wu, J.-X. Changes of circulating CD4+CD25+CD127low regulatory T cells in patients with acute coronary syndrome and its significance. Genet. Mol. Res. 2015, 14, 15930–15936. [Google Scholar] [CrossRef] [PubMed]
- Potekhina, A.V.; Pylaeva, E.; Provatorov, S.; Ruleva, N.; Masenko, V.; Noeva, E.; Krasnikova, T.; Arefieva, T. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis 2015, 238, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zheng, Y.; Yuan, X.; Ma, Y.; Xie, G.; Wang, W.; Chen, H.; Shen, L. Decreased frequencies and impaired functions of the CD31+subpopulation in Tregcells associated with decreased FoxP3 expression and enhanced Tregcell defects in patients with coronary heart disease. Clin. Exp. Immunol. 2016, 187, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Backteman, K.; Andersson, C.; Dahlin, L.-G.; Ernerudh, J.; Jonasson, L. Lymphocyte Subpopulations in Lymph Nodes and Peripheral Blood: A Comparison between Patients with Stable Angina and Acute Coronary Syndrome. PLoS ONE 2012, 7, e32691. [Google Scholar] [CrossRef] [Green Version]
- Meeuwsen, J.A.L.; Van Duijvenvoorde, A.; Gohar, A.; Kozma, M.O.; Van De Weg, S.M.; Gijsberts, C.M.; Haitjema, S.; Björkbacka, H.; Fredrikson, G.N.; De Borst, G.J.; et al. High Levels of (Un)Switched Memory B Cells Are Associated With Better Outcome in Patients With Advanced Atherosclerotic Disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Mantani, P.T.; Ljungcrantz, I.; Andersson, L.; Alm, R.; Hedblad, B.; Björkbacka, H.; Nilsson, J.-Å.; Fredrikson, G.N. Circulating CD40 + and CD86 + B Cell Subsets Demonstrate Opposing Associations With Risk of Stroke. Arter. Thromb. Vasc. Biol. 2014, 34, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.; Dobrovolny, J.; Tousek, P.; Kočka, V.; Teringova, E.; Nováková, L.; Widimský, P. Potential role of invariant natural killer T cells in outcomes of acute myocardial infarction. Int. J. Cardiol. 2015, 187, 663–665. [Google Scholar] [CrossRef]
- Pera, A.; Caserta, S.; Albanese, F.; Blowers, P.; Morrow, G.; Terrazzini, N.; Smith, H.E.; Rajkumar, C.; Reus, B.; Msonda, J.R.; et al. CD28null pro-atherogenic CD4 T-cells explain the link between CMV infection and an increased risk of cardiovascular death. Theranostics 2018, 8, 4509–4519. [Google Scholar] [CrossRef]
- Athanassopoulos, P.; Vaessen, L.M.B.; Balk, A.H.M.M.; Weimar, W.; Sharma, H.S.; Bogers, A.J.J.C. Altered Chemokine Receptor Profile on Circulating Leukocytes in Human Heart Failure. Cell Biophys. 2006, 44, 083–102. [Google Scholar] [CrossRef]
- Kyaw, T.; Tipping, P.; Toh, B.-H.; Bobik, A. Killer cells in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Yu, H.T.; Kim, N.-H.; Jang, J.; Kim, H.Y.; Kang, I.; Kim, H.C.; Park, S.; Lee, W.-W. Expansion of CD8+ T cells lacking the IL-6 receptor α chain in patients with coronary artery diseases (CAD). Atherosclerosis 2016, 249, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ghio, M.; Fabbi, P.; Contini, P.; Fedele, M.; Brunelli, C.; Indiveri, F.; Barsotti, A. OxLDL- and HSP-60 antigen-specific CD8+ T lymphocytes are detectable in the peripheral blood of patients suffering from coronary artery disease. Clin. Exp. Med. 2013, 13, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, K.; Li, J.; Dong, M.; Yang, J.; An, G.; Qin, W.; Gao, F.; Zhang, C.; Zhang, Y. Statins Induce the Accumulation of Regulatory T Cells in Atherosclerotic Plaque. Mol. Med. 2012, 18, 598–605. [Google Scholar] [CrossRef]
| Immune Cell/s | Patient Group | Findings | Ref |
|---|---|---|---|
| CD4+CD28null | CAD, Controls |
| [13] |
| CD4+ T-cells (Th1, Th17) | Acute MI (n = 26), UA (n = 16), SA (n = 16), Controls (n = 16) |
| [29] |
| NKT cells | Asymptomatic atherosclerosis patients (n = 10), Symptomatic atherosclerosis patients (n = 10), Controls (n = 10) |
| [52] |
| CD4+ T-cells | Chronic SAP (n = 30), Acute MI (n = 60), Controls (n = 40) |
| [91] |
| CD4+ T-cells, CD8+ T-cells, B-cells | Nonobstructive CAD (n = 21), ACS (n = 52), Controls (n = 50) |
| [92] |
| CD4+ T-cells (Th1, Th2, Th17, Tregs) | Acute MI (n = 19), UA (n = 25), SA (n = 20), Controls (n = 24) |
| [93] |
| CD4+ T-cells (Th1, Th2) | Stable CAD (n = 35), STE (n = 30), NSTE (n = 35), Controls (n = 33) |
| [94] |
| Tregs | CAD (SAP and previous MI) (n = 73), Controls (n = 64) |
| [95] |
| CD8+ T-cells | SAP (n = 34), ACS (n = 30), Controls (n = 36) |
| [96] |
| CD8+ T-cells | Subjects with a coronary event (n = 84), stroke (n = 54), or no event (n = 549) during the 15-year follow-up |
| [97] |
| CD8+ T-cells | Stable CAD (n = 66), ACS (n = 34) |
| [98] |
| CD8+ T-cells | Patients with nonsignificant lesions (n = 41), Patients with severe lesions (n = 37), Controls (n = 36) |
| [99] |
| Tregs | Chronic SAP (n = 36), NSTE ACS (n = 50), ST acute MI (n = 39), Controls (n = 75) |
| [100] |
| Tregs | ACS (n = 26), Post-ACS (n = 57, 24 STE, and 33 NSTE), Controls (n = 41) |
| [101] |
| Tregs | ACS (n = 48), SAP (n = 24), Controls (n = 24) |
| [102] |
| Tregs | PCI with no disease progression (n = 32), PCI with new stenosis (n = 24), Patients with three-vessel coronary disease (n = 34), No atherosclerosis (n = 27) |
| [103] |
| Tregs | SAP (n = 34), ACS (n = 37), Controls (n = 35) |
| [104] |
| CD4+ T-cells, CD8+ T-cells, B-cells, Tregs | SAP (n = 13), ACS (n = 13) |
| [105] |
| B-cells | Patients with advanced atherosclerosis who did not experience a secondary CV event during 3-year follow-up (n = 118) and those who did (n = 54) |
| [106] |
| B-cells | Individuals (n = 700) with a coronary event (n = 84), stroke (n = 66), or no event (n = 549) during 15-year follow-up |
| [107] |
| NKT cells | STE acute MI (PCI and follow-up) (n = 52) |
| [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Bartlett, B.; Dwivedi, G. Adaptive Immune Responses in Human Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 9322. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239322
Lee S, Bartlett B, Dwivedi G. Adaptive Immune Responses in Human Atherosclerosis. International Journal of Molecular Sciences. 2020; 21(23):9322. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239322
Chicago/Turabian StyleLee, Silvia, Benjamin Bartlett, and Girish Dwivedi. 2020. "Adaptive Immune Responses in Human Atherosclerosis" International Journal of Molecular Sciences 21, no. 23: 9322. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239322




