Differential Distribution of RBPMS in Pig, Rat, and Human Retina after Damage
Abstract
:1. Introduction
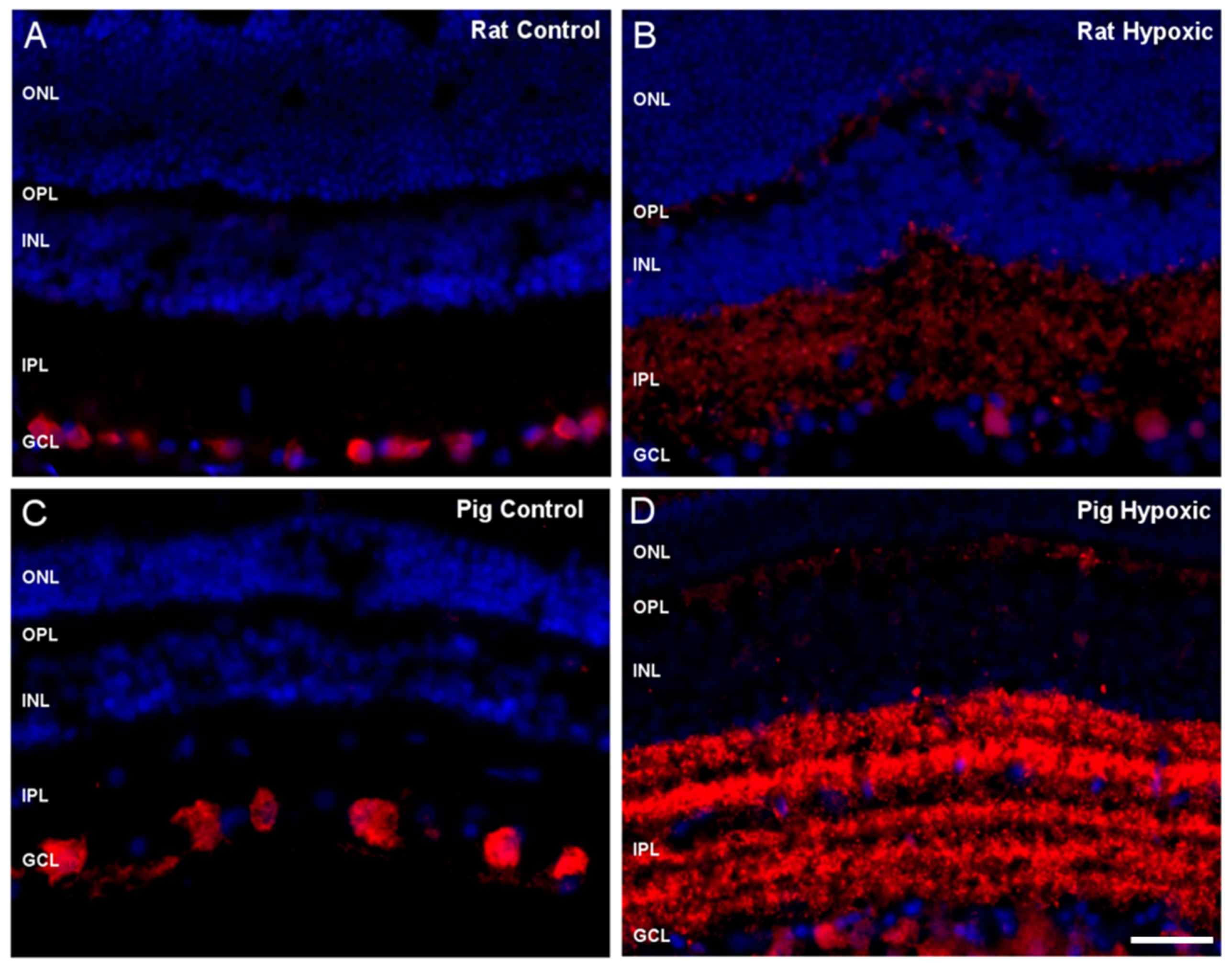
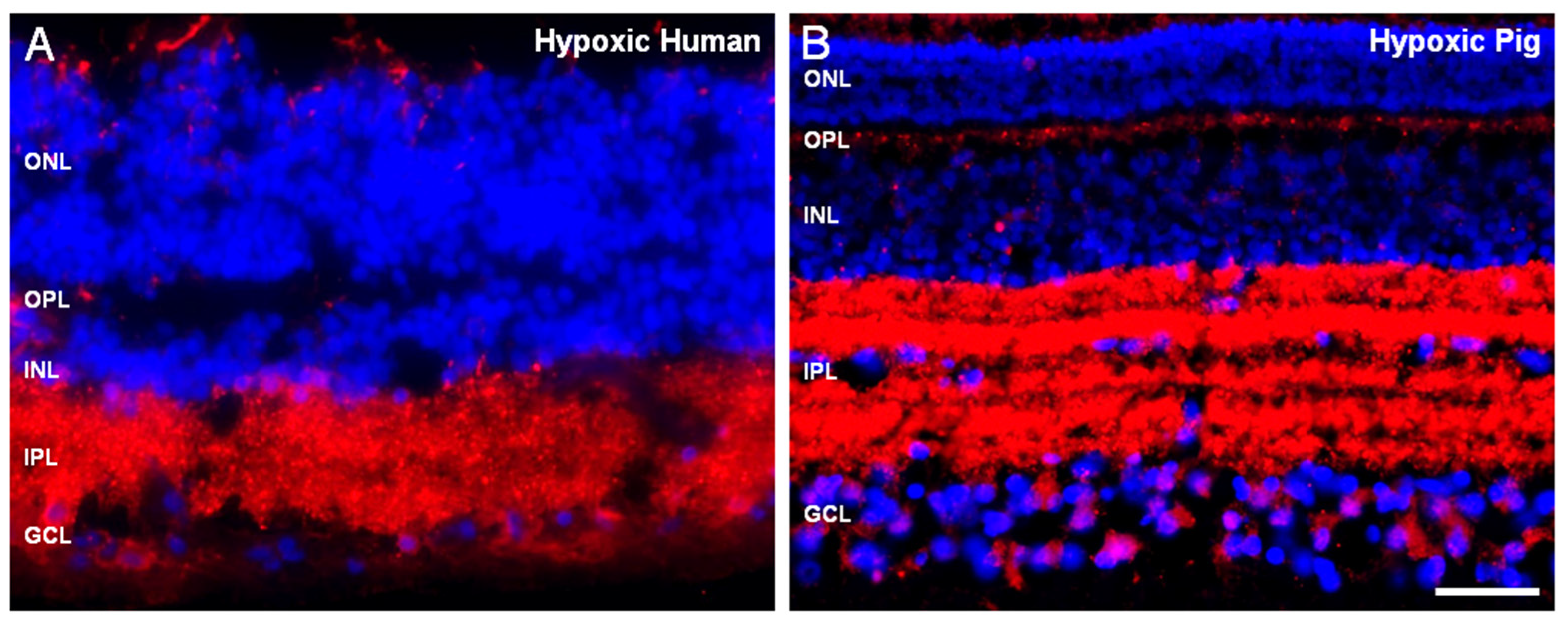
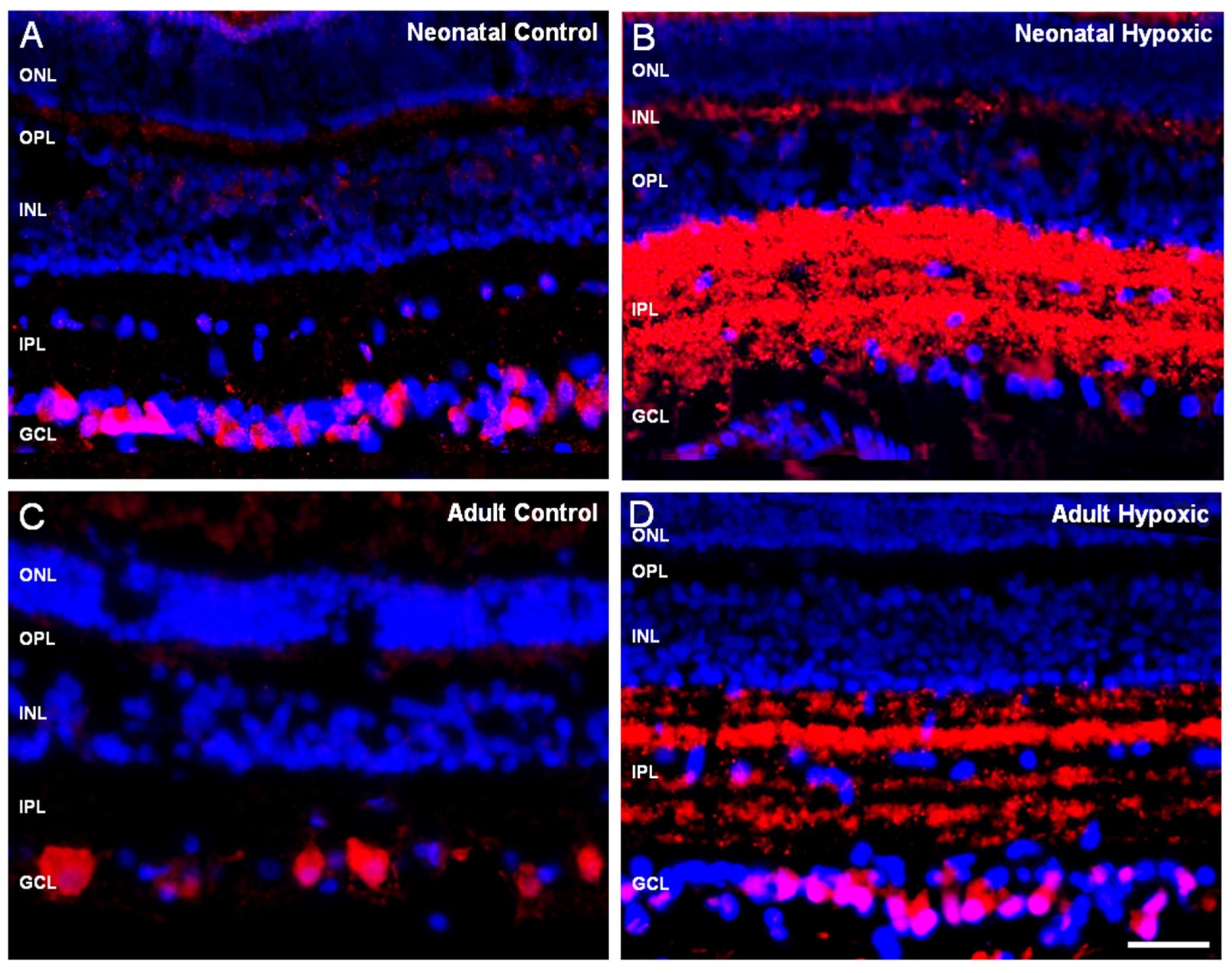
2. Results
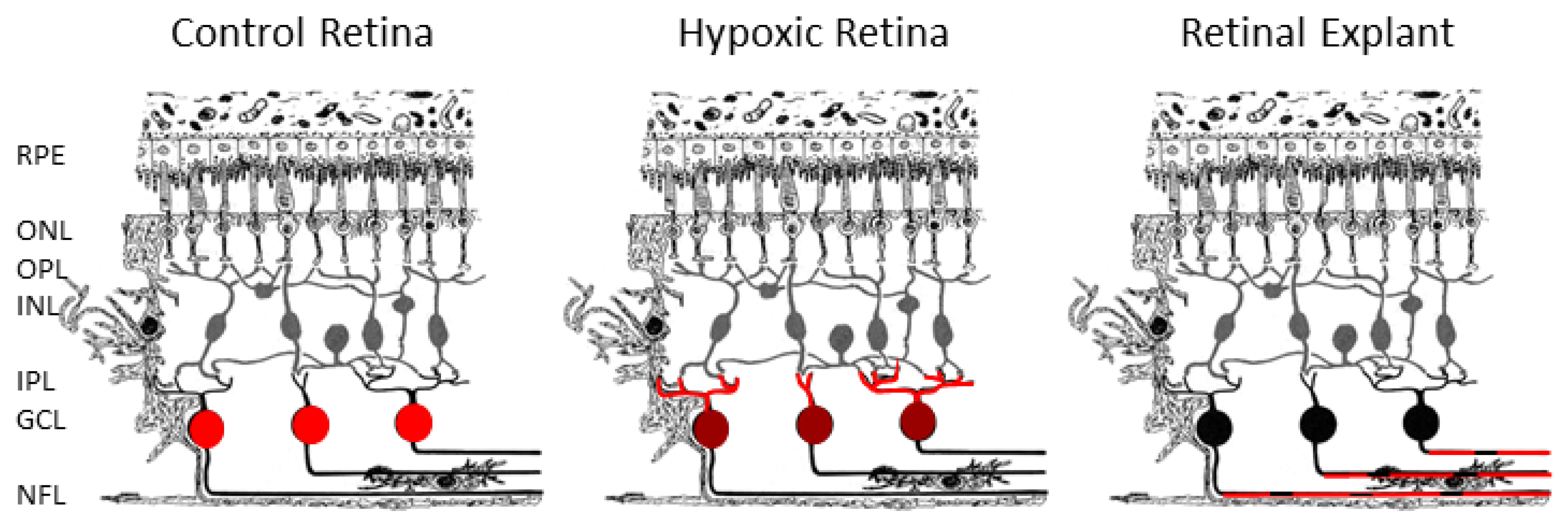
3. Discussion
4. Materials and Methods
4.1. Eye Samples
4.2. Tissue Collection and Cultures
4.3. Immunochemistry
4.4. Image Capture
4.5. Protein Extraction and Western Blotting
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| GCL | Ganglion cell layer |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| NF | Neurofilament |
| NFL | Nerve fiber layer |
| ONL | Outer nuclear layer |
| OPL | Outer plexiform layer |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| RBPMS | RNA binding protein with multiple splicing |
| RGC | Retinal ganglion cell |
| RNP | Ribonucleoprotein |
| RRM | RNA recognition motif |
References
- Gerber, W.V.; Yatskievych, T.A.; Antin, P.B.; Correia, K.M.; Conlon, R.A.; Krieg, P.A. The RNA-binding protein gene, hermes, is expressed at high levels in the developing heart. Mech. Dev. 1999, 80, 77–86. [Google Scholar] [CrossRef]
- Piri, N.; Kwong, J.M.; Song, M.; Caprioli, J. Expression of hermes gene is restricted to the ganglion cells in the retina. Neurosci. Lett. 2006, 405, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Beale, R.; Osborne, N.N. Localization of the Thy-1 antigen to the surfaces of rat retinal ganglion cells. Neurochem. Int. 1982, 4, 587–595. [Google Scholar] [CrossRef]
- Leahy, K.M.; Ornberg, R.L.; Wang, Y.; Zhu, Y.; Gidday, J.M.; Connor, J.R.; Wax, M.B. Quantitative ex vivo detection of rodent retinal ganglion cells by immunolabeling Brn-3b. Exp. Eye Res. 2004, 79, 131–140. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; de Sevilla Muller, L.P.; Brecha, N.C. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, W.; Liu, J.; Huang, X.; Liu, R.; Xia, H.; Brecha, N.C.; Pu, M.; Gao, J. Protective Effect of ALA in Crushed Optic Nerve Cat Retinal Ganglion Cells Using a New Marker RBPMS. PLoS ONE 2016, 11, e0160309. [Google Scholar] [CrossRef] [Green Version]
- McKee, A.E.; Minet, E.; Stern, C.; Riahi, S.; Stiles, C.D.; Silver, P.A. A genome-wide in situ hybridization map of RNA-binding proteins reveals anatomically restricted expression in the developing mouse brain. BMC Dev. Biol. 2005, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Loya, C.M.; Van Vactor, D.; Fulga, T.A. Understanding neuronal connectivity through the post-transcriptional toolkit. Genes Dev. 2010, 24, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Sasaki, Y.; Wen, Z.; Bassell, G.J.; Zheng, J.Q. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat. Neurosci. 2006, 9, 1265–1273. [Google Scholar] [CrossRef]
- Sasaki, Y.; Welshhans, K.; Wen, Z.; Yao, J.; Xu, M.; Goshima, Y.; Zheng, J.Q.; Bassell, G.J. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 9349–9358. [Google Scholar] [CrossRef]
- Welshhans, K.; Bassell, G.J. Netrin-1-induced local beta-actin synthesis and growth cone guidance requires zipcode binding protein 1. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 9800–9813. [Google Scholar] [CrossRef] [PubMed]
- Perycz, M.; Urbanska, A.S.; Krawczyk, P.S.; Parobczak, K.; Jaworski, J. Zipcode binding protein 1 regulates the development of dendritic arbors in hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 5271–5285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasanyan, V.; Arnold, D.B. Actin and myosin-dependent localization of mRNA to dendrites. PLoS ONE 2014, 9, e92349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornberg, H.; Wollerton-van Horck, F.; Maurus, D.; Zwart, M.; Svoboda, H.; Harris, W.A.; Holt, C.E. RNA-binding protein Hermes/RBPMS inversely affects synapse density and axon arbor formation in retinal ganglion cells in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 10384–10395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, M.T.; Sakamoto, H.; Inoue, K. Interaction and colocalization of HERMES/RBPMS with NonO, PSF, and G3BP1 in neuronal cytoplasmic RNP granules in mouse retinal line cells. Genes Cells Devoted Mol. Cell. Mech. 2015, 20, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ederra, J.; Garcia, M.; Hicks, D.; Vecino, E. Comparative study of the three neurofilament subunits within pig and human retinal ganglion cells. Mol. Vis. 2004, 10, 83–92. [Google Scholar]
- Ruiz-Ederra, J.; Hitchcock, P.F.; Vecino, E. Two classes of astrocytes in the adult human and pig retina in terms of their expression of high affinity NGF receptor (TrkA). Neurosci. Lett. 2003, 337, 127–130. [Google Scholar] [CrossRef]
- Garca, M.; Ruiz-Ederra, J.; Hernandez-Barbachano, H.; Vecino, E. Topography of pig retinal ganglion cells. J. Comp. Neurol. 2005, 486, 361–372. [Google Scholar] [CrossRef]
- Prince, J.H.; Ruskell, G.L. The use of domestic animals for experimental ophthalmology. Am. J. Ophthalmol. 1960, 49, 1202–1207. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H. Animal models of optic nerve diseases. Eye 2004, 18, 1066–1074. [Google Scholar] [CrossRef]
- Chekulaeva, M.; Hentze, M.W.; Ephrussi, A. Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell 2006, 124, 521–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cajigas, I.J.; Tushev, G.; Will, T.J.; tom Dieck, S.; Fuerst, N.; Schuman, E.M. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 2012, 74, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tushev, G.; Glock, C.; Heumüller, M.; Biever, A.; Jovanovic, M.; Schuman, E.M. Alternative 3’ UTRs Modify the Localization, Regulatory Potential, Stability, and Plasticity of mRNAs in Neuronal Compartments. Neuron 2018, 98, 495–511.e496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, C.E.; Schuman, E.M. The central dogma decentralized: New perspectives on RNA function and local translation in neurons. Neuron 2013, 80, 648–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glock, C.; Heumüller, M.; Schuman, E.M. mRNA transport & local translation in neurons. Curr. Opin. Neurobiol. 2017, 45, 169–177. [Google Scholar] [CrossRef]
- Fonkeu, Y.; Kraynyukova, N.; Hafner, A.S.; Kochen, L.; Sartori, F.; Schuman, E.M.; Tchumatchenko, T. How mRNA Localization and Protein Synthesis Sites Influence Dendritic Protein Distribution and Dynamics. Neuron 2019, 103, 1109–1122.e1107. [Google Scholar] [CrossRef]
- Martin, K.C.; Zukin, R.S. RNA trafficking and local protein synthesis in dendrites: An overview. J. Neurosci. 2006, 26, 7131–7134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo-Romero, C.; Aviles-Trigueros, M.; Jimenez-Lopez, M.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolas, F.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axotomy-induced retinal ganglion cell death in adult mice: Quantitative and topographic time course analyses. Exp. Eye Res. 2011, 92, 377–387. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Valiente-Soriano, F.J.; Jimenez-Lopez, M.; Garcia-Ayuso, D.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Nicolas, F.M.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Jimenez-Lopez, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Retino-retinal projection in juvenile and young adult rats and mice. Exp. Eye Res. 2015, 134, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Shigeoka, T.; Jung, H.; Jung, J.; Turner-Bridger, B.; Ohk, J.; Lin, J.Q.; Amieux, P.S.; Holt, C.E. Dynamic Axonal Translation in Developing and Mature Visual Circuits. Cell 2016, 166, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briese, M.; Saal, L.; Appenzeller, S.; Moradi, M.; Baluapuri, A.; Sendtner, M. Whole transcriptome profiling reveals the RNA content of motor axons. Nucleic Acids Res. 2016, 44, e33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumy, L.F.; Yeo, G.S.; Tung, Y.C.; Zivraj, K.H.; Willis, D.; Coppola, G.; Lam, B.Y.; Twiss, J.L.; Holt, C.E.; Fawcett, J.W. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 2011, 17, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saal, L.; Briese, M.; Kneitz, S.; Glinka, M.; Sendtner, M. Subcellular transcriptome alterations in a cell culture model of spinal muscular atrophy point to widespread defects in axonal growth and presynaptic differentiation. RNA 2014, 20, 1789–1802. [Google Scholar] [CrossRef] [Green Version]
- Willis, D.E.; van Niekerk, E.A.; Sasaki, Y.; Mesngon, M.; Merianda, T.T.; Williams, G.G.; Kendall, M.; Smith, D.S.; Bassell, G.J.; Twiss, J.L. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 2007, 178, 965–980. [Google Scholar] [CrossRef] [Green Version]
- Zivraj, K.H.; Tung, Y.C.; Piper, M.; Gumy, L.; Fawcett, J.W.; Yeo, G.S.; Holt, C.E. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J. Neurosci. 2010, 30, 15464–15478. [Google Scholar] [CrossRef] [Green Version]
- Sanz, E.; Yang, L.; Su, T.; Morris, D.R.; McKnight, G.S.; Amieux, P.S. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13939–13944. [Google Scholar] [CrossRef] [Green Version]
- Shigeoka, T.; Jung, J.; Holt, C.E.; Jung, H. Axon-TRAP-RiboTag: Affinity Purification of Translated mRNAs from Neuronal Axons in Mouse In Vivo. Methods Mol. Biol. 2018, 1649, 85–94. [Google Scholar] [CrossRef]
- Khalil, B.; Morderer, D.; Price, P.L.; Liu, F.; Rossoll, W. mRNP assembly, axonal transport, and local translation in neurodegenerative diseases. Brain Res. 2018, 1693, 75–91. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereiro, X.; Ruzafa, N.; Urcola, J.H.; Sharma, S.C.; Vecino, E. Differential Distribution of RBPMS in Pig, Rat, and Human Retina after Damage. Int. J. Mol. Sci. 2020, 21, 9330. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239330
Pereiro X, Ruzafa N, Urcola JH, Sharma SC, Vecino E. Differential Distribution of RBPMS in Pig, Rat, and Human Retina after Damage. International Journal of Molecular Sciences. 2020; 21(23):9330. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239330
Chicago/Turabian StylePereiro, Xandra, Noelia Ruzafa, J. Haritz Urcola, Sansar C. Sharma, and Elena Vecino. 2020. "Differential Distribution of RBPMS in Pig, Rat, and Human Retina after Damage" International Journal of Molecular Sciences 21, no. 23: 9330. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21239330





