Neurosteroids and Focal Epileptic Disorders
Abstract
:1. Introduction
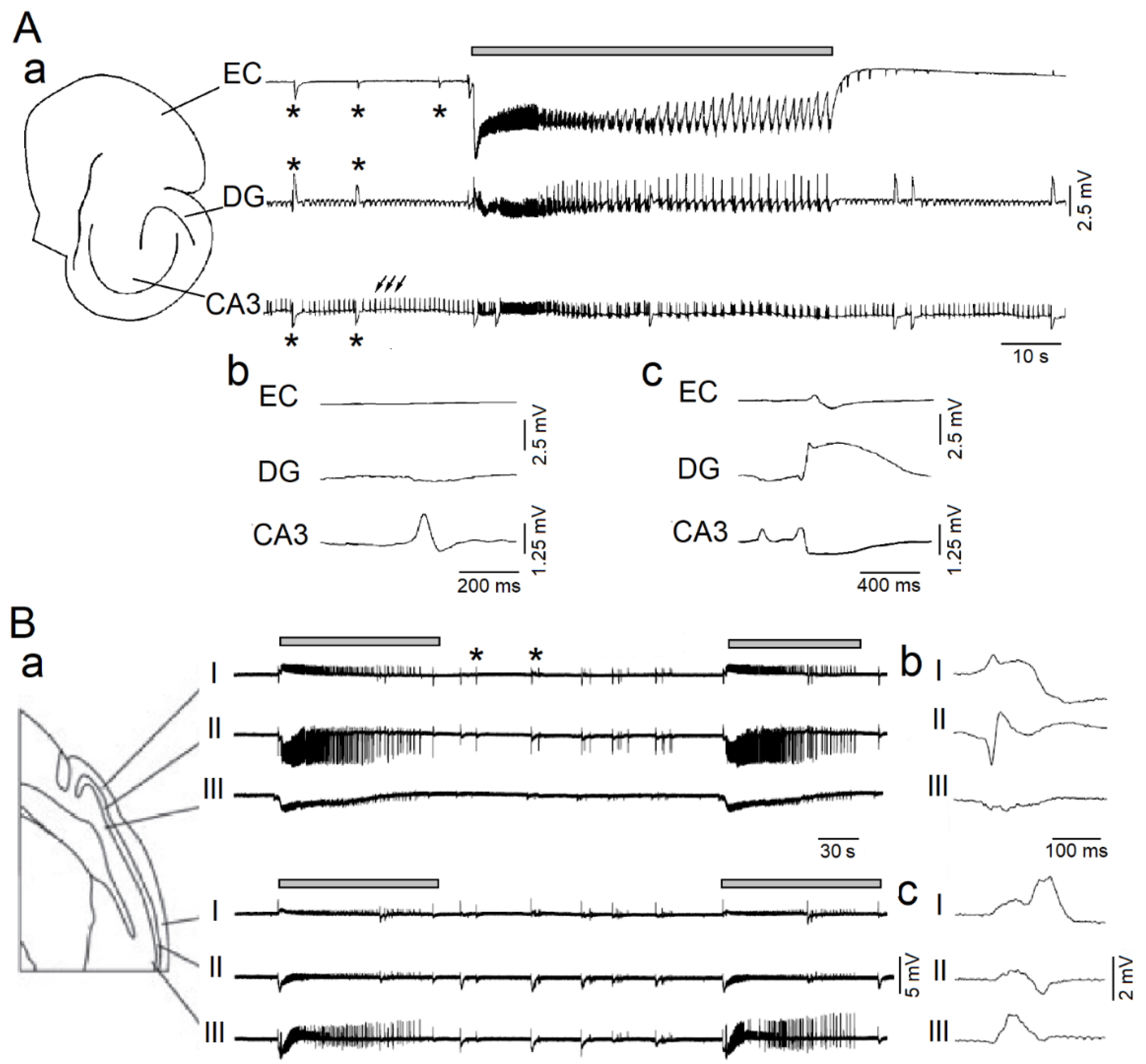
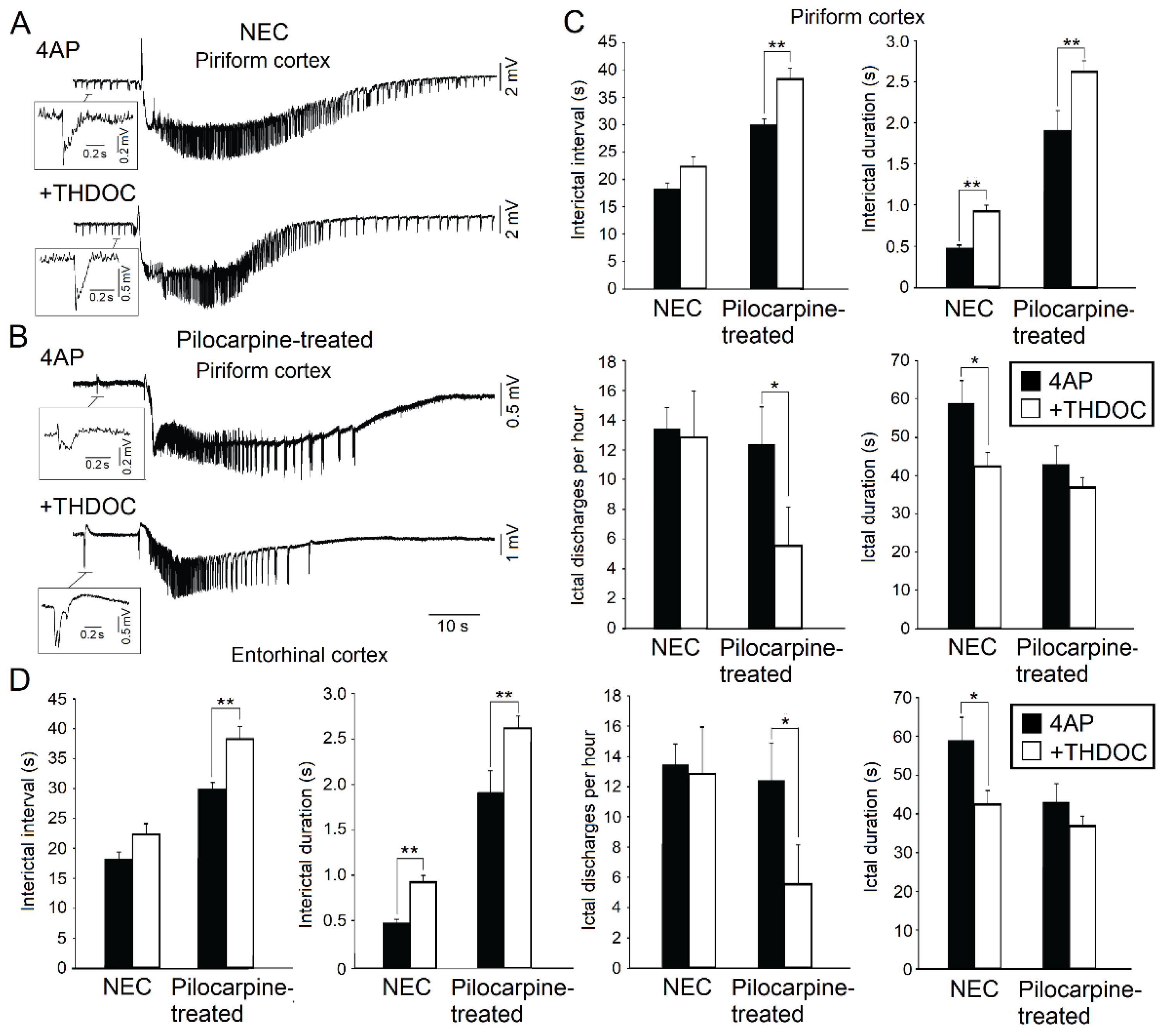
2. Effects on 4-Aminopyridine-Induced Epileptiform Synchronization In Vitro
3. Effects in Animal Models of Mesial Temporal Lobe Epilepsy In Vivo
4. Human Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4AP | 4-aminopyridine |
| P450scc | P450 cholesterol side chain cleavage |
| HFOs | high-frequency oscillations |
| MTLE | mesial temporal lobe epilepsy |
| NEC | non-epileptic control |
| PCDH19 | Protocadherin 19 |
| PTX | picrotoxin |
| SE | status epilepticus |
| THDOC | Allotetrahydrodeoxycorticosterone |
References
- Akk, G.; Covey, D.F.; Evers, A.S.; Steinbach, J.H.; Zorumski, C.F.; Mennerick, S. The influence of the membrane on neurosteroid actions at GABAA receptors. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S59–S66. [Google Scholar] [CrossRef] [Green Version]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef]
- MacKenzie, G.; Maguire, J. Neurosteroids and GABAergic signaling in health and disease. Biomol. Concepts 2013, 4, 29–42. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids: Endogenous Role in the Human Brian and Therapeutic Potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–395. [Google Scholar] [CrossRef]
- Park-Chung, M.; Malayev, A.; Purdy, R.H.; Gibbs, T.T.; Farb, D.H. Sulfated and unsulfated steroids modulate γ-aminobutyric acid(A) receptor function through distinct sites. Brain Res. 1999, 830, 72–87. [Google Scholar] [CrossRef]
- Sousa, A.; Ticku, M.K. Interactions of the neurosteroid dehydroepiandrosterone sulfate with the GABA(A) receptor complex reveals that it may act via the picrotoxin site. J. Pharmacol. Exp. Ther. 1997, 282, 827–833. [Google Scholar] [PubMed]
- Wu, F.S.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate: A positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 1991, 40, 333–336. [Google Scholar]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Reddy, D.S. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front. Endocrin. 2011, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.S.; Rogawski, M.A. Neurosteroids—Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Biagini, G.; Panuccio, G.; Avoli, M. Neurosteroids and epilepsy. Curr. Opin. Neurol. 2010, 23, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, D.S.; Rogawski, M.A. Neurosteroids as endogenous regulators of seizure susceptibility and use in the treatment of epilepsy. Epilepsia 2010, 51, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévesque, M.; Herrington, R.; Leclerc, L.; Rogawski, M.A.; Avoli, M. Allopregnanolone decreases interictal spiking and fast ripples in an animal model of mesial temporal lobe epilepsy. Neuropharmacology 2017. [Google Scholar] [CrossRef] [PubMed]
- Herrington, R.; Lévesque, M.; Avoli, M. Neurosteroids differentially modulate fast and slow interictal discharges in the hippocampal CA3 area. Eur. J. Neurosci. 2015, 41, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Herrington, R.; Lévesque, M.; Avoli, M. Neurosteroids modulate epileptiform activity and associated high frequency oscillations in the piriform cortex. Neuroscience 2013. [Google Scholar] [CrossRef] [Green Version]
- Shiri, Z.; Herrington, R.; Lévesque, M.; Avoli, M. Neurosteroidal modulation of in vitro epileptiform activity is enhanced in pilocarpine-treated epileptic rats. Neurobiol. Dis. 2015, 78, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.S. Pharmacology of Endogenous Neuroactive Steroids. Crit. Rev. Neurobiol. 2004, 15, 197–234. [Google Scholar] [CrossRef]
- Rupprecht, R.; Hauser, C.A.E.; Trapp, T.; Holsboer, F. Neurosteroids: Molecular mechanisms of action and psychopharmacological significance. J. Steroid Biochem. Mol. Biol. 1996, 56, 163–168. [Google Scholar] [CrossRef]
- Belelli, D.; Casula, A.; Ling, A.; Lambert, J.J. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 2002, 43, 651–661. [Google Scholar] [CrossRef]
- Gangisetty, O.; Reddy, D.S. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 2010, 170, 865–880. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, R.M.; Marini, H.; Kim, W.-J.; Rogawski, M.A. Anticonvulsant Activity of Androsterone and Etiocholanolone. Epilepsia 2005, 46, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agís-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strömstedt, M.; Waterman, M.R. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res. Mol. Brain Res. 1995, 34, 75–88. [Google Scholar] [CrossRef]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.J.; Vicini, S. Neurosteroid Prolongs GABAA Channel Deactivation by Altering Kinetics of Desensitized States. J. Neurosci. 1997, 17, 4022–4031. [Google Scholar] [CrossRef]
- Wohlfarth, K.M.; Bianchi, M.T.; Macdonald, R.L. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 2002, 22, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Mihalek, R.M.; Banerjee, P.K.; Korpi, E.R.; Quinlan, J.J.; Firestone, L.L.; Mi, Z.-P.; Lagenaur, C.; Tretter, V.; Sieghart, W.; Anagnostaras, S.G.; et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12905–12910. [Google Scholar] [CrossRef] [Green Version]
- Glykys, J.; Mody, I. Activation of GABAA Receptors: Views from Outside the Synaptic Cleft. Neuron 2007, 56, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Hosie, A.M.; Wilkins, M.E.; da Silva, H.M.A.; Smart, T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 2006, 444, 486–489. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABAA receptors: Their function in the CNS and implications for disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Salazar, P.; Tapia, R.; Rogawski, M.A. Effects of neurosteroids on epileptiform activity induced by picrotoxin and 4-aminopyridine in the rat hippocampal slice. Epilepsy Res. 2003, 55, 71–82. [Google Scholar] [CrossRef]
- Avoli, M.; D’Antuono, M.; Louvel, J.; Köhling, R.; Biagini, G.; Pumain, R.; D’Arcangelo, G.; Tancredi, V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog. Neurobiol. 2002, 68, 167–207. [Google Scholar] [CrossRef]
- Avoli, M.; de Curtis, M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog. Neurobiol. 2011, 95, 104–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, P.; Avoli, M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J. Neurosci. 1992, 12, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Avoli, M.; Barbarosie, M.; Lücke, A.; Nagao, T.; Lopantsev, V.; Köhling, R. Synchronous GABA-Mediated Potentials and Epileptiform Discharges in the Rat Limbic System In Vitro. J. Neurosci. 1996, 16, 3912–3924. [Google Scholar] [CrossRef] [Green Version]
- Panuccio, G.; Sanchez, G.; Lévesque, M.; Salami, P.; de Curtis, M.; Avoli, M. On the ictogenic properties of the piriform cortex in vitro. Epilepsia 2012, 53, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Sudbury, J.R.; Avoli, M. Epileptiform synchronization in the rat insular and perirhinal cortices in vitro. Eur. J. Neurosci. 2007, 26, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Panuccio, G.; Curia, G.; Colosimo, A.; Cruccu, G.; Avoli, M. Epileptiform synchronization in the cingulate cortex. Epilepsia 2009, 50, 521–536. [Google Scholar] [CrossRef] [Green Version]
- Bragin, A.; Engel, J., Jr.; Wilson, C.L.; Fried, I.; Mathern, G.W. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid—Treated rats with chronic seizures. Epilepsia 1999, 40, 127–137. [Google Scholar] [CrossRef]
- Staba, R.J.; Wilson, C.L.; Bragin, A.; Fried, I.; Engel, J., Jr. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J. Neurophysiol. 2002, 88, 1743–1752. [Google Scholar] [CrossRef]
- Jirsch, J.D.; Urrestarazu, E.; LeVan, P.; Olivier, A.; Dubeau, F.; Gotman, J. High-frequency oscillations during human focal seizures. Brain 2006, 129, 1593–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévesque, M.; Bortel, A.; Gotman, J.; Avoli, M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol. Dis. 2011, 42, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferys, J.G.R.; Menendez de la Prida, L.; Wendling, F.; Bragin, A.; Avoli, M.; Timofeev, I.; Lopes da Silva, F.H. Mechanisms of physiological and epileptic HFO generation. Prog. Neurobiol. 2012, 98, 250–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzsáki, G.; Chrobak, J.J. Temporal structure in spatially organized neuronal ensembles: A role for interneuronal networks. Curr. Opin. Neurobiol. 1995, 5, 504–510. [Google Scholar] [CrossRef]
- Ylinen, A.; Bragin, A.; Nadasdy, Z.; Jando, G.; Szabo, I.; Sik, A.; Buzsaki, G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. J. Neurosci. 1995, 15, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Bragin, A.; Benassi, S.K.; Kheiri, F.; Engel, J., Jr. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia 2011, 52, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Dzhala, V.I.; Staley, K.J. Mechanisms of Fast Ripples in the Hippocampus. J. Neurosci. 2004, 24, 8896–8906. [Google Scholar] [CrossRef] [Green Version]
- Engel, J., Jr.; Bragin, A.; Staba, R.; Mody, I. High-frequency oscillations: What is normal and what is not? Epilepsia 2009, 50, 598–604. [Google Scholar] [CrossRef]
- Foffani, G.; Uzcategui, Y.G.; Gal, B.; Menendez de la Prida, L. Reduced Spike-Timing Reliability Correlates with the Emergence of Fast Ripples in the Rat Epileptic Hippocampus. Neuron 2007, 55, 930–941. [Google Scholar] [CrossRef] [Green Version]
- Ibarz, J.M.; Foffani, G.; Cid, E.; Inostroza, M.; de la Prida, L.M. Emergent Dynamics of Fast Ripples in the Epileptic Hippocampus. J. Neurosci. 2010, 30, 16249–16261. [Google Scholar] [CrossRef] [Green Version]
- Teschemacher, A.; Zeise, M.L.; Holsboer, F.; Zieglgänsberger, W. The Neuroactive Steroid 5α-Tetrahydrodeoxycorticosterone Increases GABAergic Postsynaptic Inhibition in Rat Neocortical Neurons in vitro. J. Neuroendocrinol. 1995, 7, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Stell, B.M.; Brickley, S.G.; Tang, C.Y.; Farrant, M.; Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by? Subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 14439–14444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, D.S. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004, 62, 99–118. [Google Scholar] [CrossRef]
- Buzsaki, G.; Horvath, Z.; Urioste, R.; Hetke, J.; Wise, K. High-frequency network oscillation in the hippocampus. Science 1992, 256, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Demont-Guignard, S.; Benquet, P.; Gerber, U.; Biraben, A.; Martin, B.; Wendling, F. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann. Neurol. 2012, 71, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Wendling, F.; Bartolomei, F.; Mina, F.; Huneau, C.; Benquet, P. Interictal spikes, fast ripples and seizures in partial epilepsies—Combining multi-level computational models with experimental data. Eur. J. Neurosci. 2012, 36, 2164–2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritschy, J.-M.; Kiener, T.; Bouilleret, V.; Loup, F. GABAergic neurons and GABAA-receptors in temporal lobe epilepsy. Neurochem. Int. 1999, 34, 435–445. [Google Scholar] [CrossRef]
- Loup, F.; Wieser, H.G.; Yonekawa, Y.; Aguzzi, A.; Fritschy, J.M. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J. Neurosci. 2000, 20, 5401–5419. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, K.; Joshi, S.; Sun, C.; Mtchedlishvilli, Z.; Kapur, J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol. Dis. 2010, 40, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Huang, C.S.; Stell, B.M.; Mody, I.; Houser, C.R. Altered Expression of the δ Subunit of the GABAA Receptor in a Mouse Model of Temporal Lobe Epilepsy. J. Neurosci. 2004, 24, 8629–8639. [Google Scholar] [CrossRef]
- Leroy, C.; Poisbeau, P.; Keller, A.F.; Nehlig, A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J. Physiol. 2004, 557, 473–487. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, P.; Inaba, Y.; Biagini, G.; Baldelli, E.; Mollinari, C.; Merlo, D.; Avoli, M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus 2006, 16, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Knopp, A.; Frahm, C.; Fidzinski, P.; Witte, O.W.; Behr, J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain 2008, 131, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovici, C.; D’Alfonso, S.; Dann, M.; Poulter, M.O. Kindling-induced alterations in GABAA receptor-mediated inhibition and neurosteroid activity in the rat piriform cortex. Eur. J. Neurosci. 2006, 24, 1373–1384. [Google Scholar] [CrossRef]
- Høgskilde, S.; Wagner, J.; Carl, P.; Anker, N.; Angelo, H.R.; Sørensen, M.B. Anticonvulsive properties of pregnanolone emulsion compared with althesin and thiopentone in mice. Br. J. Anaesth. 1988, 61, 462–467. [Google Scholar] [CrossRef]
- Belelli, D.; Bolger, M.B.; Gee, K.W. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur. J. Pharmacol. 1989, 166, 325–329. [Google Scholar] [CrossRef]
- Kokate, T.G.; Svensson, B.E.; Rogawski, M.A. Anticonvulsant activity of neurosteroids: Correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J. Pharmacol. Exp. Ther. 1994, 270, 1223–1229. [Google Scholar]
- Kaminski, R.M.; Livingood, M.R.; Rogawski, M.A. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 2004, 45, 864–867. [Google Scholar] [CrossRef]
- Kokate, T.G.; Cohen, A.L.; Karp, E.; Rogawski, M.A. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 1996, 35, 1049–1056. [Google Scholar] [CrossRef]
- Turski, L.; Ikonomidou, C.; Turski, W.A.; Bortolotto, Z.A.; Cavalheiro, E.A. Review: Cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: A novel experimental model of intractable epilepsy. Synapse 1989, 3, 154–171. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Lagowska, J.; Tremblay, E.; Le Gal La Salle, G. A new model of focal status epilepticus: Intra-amygdaloid application of kainic acid elicits repetitive secondarily generalized convulsive seizures. Brain Res. 1979, 163, 176–179. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Lagowska, J. Epileptogenic action of intra-amygdaloid injection of kainic acid. Comptes Rendus Hebd. Séances Acad. Sci. Sér. Sci. Nat. 1978, 287, 813–816. [Google Scholar]
- Cavalheiro, E.A.; Riche, D.A.; Le Gal La Salle, G. Long-term effects of intrahippocampal kainic acid injection in rats: A method for inducing spontaneous recurrent seizures. Electroencephalogr. Clin. Neurophysiol. 1982, 53, 581–589. [Google Scholar] [CrossRef]
- Nadler, J.V. Kainic acid: Neurophysiological and neurotoxic actions. Life Sci. 1979, 24, 289–299. [Google Scholar] [CrossRef]
- Saporito, M.S.; Gruner, J.A.; DiCamillo, A.; Hinchliffe, R.; Barker-Haliski, M.; White, H.S. Intravenously Administered Ganaxolone Blocks Diazepam-Resistant Lithium-Pilocarpine-Induced Status Epilepticus in Rats: Comparison with Allopregnanolone. J. Pharmacol. Exp. Ther. 2019, 368, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Biagini, G.; Baldelli, E.; Longo, D.; Pradelli, L.; Zini, I.; Rogawski, M.A.; Avoli, M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp. Neurol. 2006, 201, 519–524. [Google Scholar] [CrossRef]
- Burnham, W.M. The GABA hypothesis of kindling: Recent assay studies. Neurosci. Biobehav. Rev. 1989, 13, 281–288. [Google Scholar] [CrossRef]
- Biagini, G.; Longo, D.; Baldelli, E.; Zoli, M.; Rogawski, M.A.; Bertazzoni, G.; Avoli, M. Neurosteroids and epileptogenesis in the pilocarpine model: Evidence for a relationship between P450scc induction and length of the latent period. Epilepsia 2009, 50, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Rajasekaran, K.; Williamson, J.; Kapur, J. Neurosteroid-sensitive δ-GABA-A receptors: A role in epileptogenesis? Epilepsia 2017, 58, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Behr, C.; Lévesque, M.; Ragsdale, D.; Avoli, M. Lacosamide modulates interictal spiking and high-frequency oscillations in a model of mesial temporal lobe epilepsy. Epilepsy Res. 2015, 115, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévesque, M.; Behr, C.; Avoli, M. The anti-ictogenic effects of levetiracetam are mirrored by interictal spiking and high-frequency oscillation changes in a model of temporal lobe epilepsy. Seizure 2015, 25, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchi, C.; Costa, A.M.; Rustichelli, C.; Biagini, G. Allopregnanolone and pregnanolone are reduced in the hippocampus of epileptic rats, but only allopregnanolone correlates with the seizure frequency. Neuroendocrinology 2020. [Google Scholar] [CrossRef] [PubMed]
- Pieribone, V.A.; Tsai, J.; Soufflet, C.; Rey, E.; Shaw, K.; Giller, E.; Dulac, O. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia 2007, 48, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.R.; Klein, P.; Tsai, J. Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 2017, 58, 558–564. [Google Scholar] [CrossRef] [Green Version]
- Trivisano, M.; Lucchi, C.; Rustichelli, C.; Terracciano, A.; Cusmai, R.; Ubertini, G.M.; Giannone, G.; Bertini, E.S.; Vigevano, F.; Gecz, J.; et al. Reduced steroidogenesis in patients with PCDH19-female limited epilepsy. Epilepsia 2017, 58, e91–e95. [Google Scholar] [CrossRef]
- Samanta, D. PCDH19-Related Epilepsy Syndrome: A Comprehensive Clinical Review. Pediatr. Neurol. 2020, 105, 3–9. [Google Scholar] [CrossRef]
- Joshi, S.; Roden, W.H.; Kapur, J.; Jansen, L.A. Reduced neurosteroid potentiation of GABAA receptors in epilepsy and depolarized hippocampal neurons. Ann. Clin. Transl. Neurol. 2020, 7, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, A.O. Place of neurosteroids in the treatment of status epilepticus. Epilepsia 2018, 59 (Suppl. S2), 216–219. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, E.S.; Claassen, J.; Wainwright, M.S.; Husain, A.M.; Vaitkevicius, H.; Raines, S.; Hoffmann, E.; Colquhoun, H.; Doherty, J.J.; Kanes, S.J. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann. Neurol. 2017, 82, 342–352. [Google Scholar] [CrossRef]
- Meletti, S.; Lucchi, C.; Monti, G.; Giovannini, G.; Bedin, R.; Trenti, T.; Rustichelli, C.; Biagini, G. Low levels of progesterone and derivatives in cerebrospinal fluid of patients affected by status epilepticus. J. Neurochem. 2018, 147, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meletti, S.; Lucchi, C.; Monti, G.; Giovannini, G.; Bedin, R.; Trenti, T.; Rustichelli, C.; Biagini, G. Decreased allopregnanolone levels in cerebrospinal fluid obtained during status epilepticus. Epilepsia 2017, 58, e16–e20. [Google Scholar] [CrossRef] [PubMed]
- Espallergues, J.; Mamiya, T.; Vallée, M.; Koseki, T.; Nabeshima, T.; Temsamani, J.; Laruelle, C.; Maurice, T. The antidepressant-like effects of the 3β-hydroxysteroid dehydrogenase inhibitor trilostane in mice is related to changes in neuroactive steroid and monoamine levels. Neuropharmacology 2012, 62, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Corpéchot, C.; Perché, F.; Eychenne, B.; Haug, M.; Baulieu, E.E.; Robel, P. Neurosteroids in the mouse brain: Behavioral and pharmacological effects of a 3 beta-hydroxysteroid dehydrogenase inhibitor. Steroids 1996, 61, 144–149. [Google Scholar] [CrossRef]
- Lucchi, C.; Costa, A.M.; Senn, L.; Messina, S.; Rustichelli, C.; Biagini, G. Augmentation of endogenous neurosteroid synthesis alters experimental status epilepticus dynamics. Epilepsia 2020. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G.; Fowler, K.M.; Smithson, S.D.; Kalayjian, L.A.; Heck, C.N.; Sperling, M.R.; Liporace, J.D.; Harden, C.L.; Dworetzky, B.A.; Pennell, P.B.; et al. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology 2012, 78, 1959–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, A.G.; Frye, C.A. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann. Neurol. 2003, 53, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Pugnaghi, M.; Monti, G.; Biagini, G.; Meletti, S. Temporal lobe epilepsy exacerbation during pharmacological inhibition of endogenous neurosteroid synthesis. Case Rep. 2013, 2013, bcr2012008204. [Google Scholar] [CrossRef] [Green Version]
- Avoli, M.; de Curtis, M.; Gnatkovsky, V.; Gotman, J.; Köhling, R.; Lévesque, M.; Manseau, F.; Shiri, Z.; Williams, S. Specific imbalance of excitatory/inhibitory signaling establishes seizure onset pattern in temporal lobe epilepsy. J. Neurophysiol. 2016, 115, 3229–3237. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lévesque, M.; Biagini, G.; Avoli, M. Neurosteroids and Focal Epileptic Disorders. Int. J. Mol. Sci. 2020, 21, 9391. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249391
Lévesque M, Biagini G, Avoli M. Neurosteroids and Focal Epileptic Disorders. International Journal of Molecular Sciences. 2020; 21(24):9391. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249391
Chicago/Turabian StyleLévesque, Maxime, Giuseppe Biagini, and Massimo Avoli. 2020. "Neurosteroids and Focal Epileptic Disorders" International Journal of Molecular Sciences 21, no. 24: 9391. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249391




