Sodium Intake and Heart Failure
Abstract
:1. Salt and Sodium
2. Guideline Recommendations for Sodium Intake
3. Low Sodium Intake and Prevention or Management of HF
3.1. Evidence in Favor of Low Sodium Intake in Prevention or Management of HF
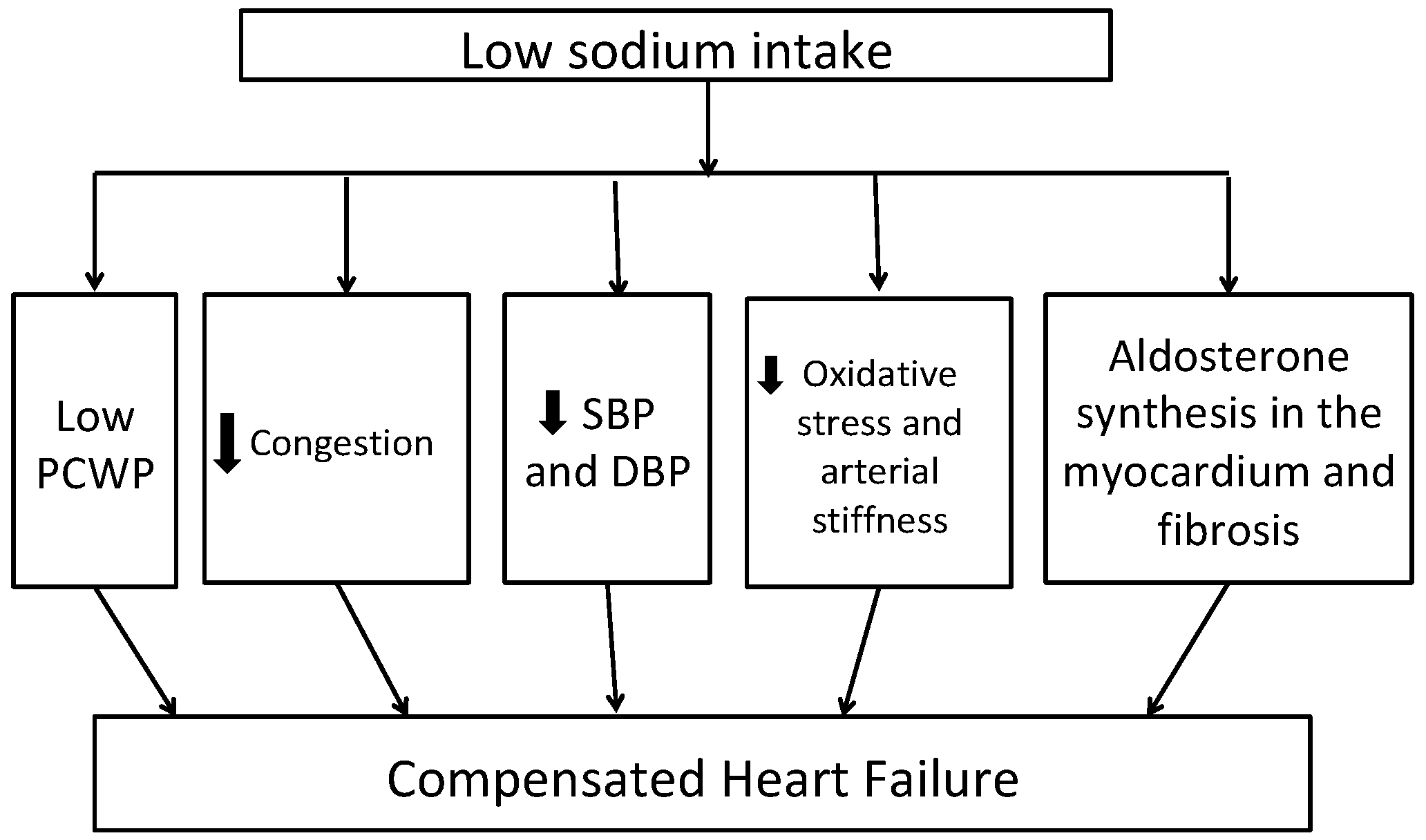
3.2. Pathogenic Mechanisms for Beneficial Effect of Low Sodium Intake in Management of HF
4. Low Sodium Intake and Worsening of HF
4.1. Evidence Against Low Sodium Intake in HF
4.2. Potential Mechanism for Adverse Impact of Low Sodium Intake in HF
5. Potential Molecular Mechanism of Salt Diet and Heart Failure
6. Sodium Intake and Ambulatory Heart Failure
7. Sodium Intake in Selected Patient Populations
8. Serum Sodium Values and HF
9. Future Directions
10. Our Recommendations
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HF | Heart failure |
| HFrEF | Heart failure with reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| DASH | Dietary Approaches to Stop Hypertension |
| NYHA | New York Heart Association |
| TNF | Tumor necrosis factor |
| IL | Interleukin |
| BNP | Brain natriuretic factor |
| RAAS | Renin angiotensin aldosterone system |
| EF-OPTIMIZE-HF | Ejection Fraction Organize Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure registry |
| SODIUM-HF | Study of Dietary Intervention Under 100 MMOL in Heart Failure |
| GOURMET-HF | Geriatric Out of Hospital Randomized Meal Trial in Heart Failure |
References
- Heart Failure Society of America; Lindenfeld, J.; Albert, N.M.; Boehmer, J.P.; Collins, S.P.; Ezekowitz, J.A.; Givertz, M.M.; Katz, S.D.; Klapholz, M.; Moser, D.K.; et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J. Card. Fail. 2010, 16, e1–e194. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef] [PubMed]
- Riegel, B.; Lee, S.; Hill, J.; Daus, M.; Baah, F.O.; Wald, J.W.; Knafl, G.J. Patterns of adherence to diuretics, dietary sodium and fluid intake recommendations in adults with heart failure. Heart Lung 2019, 48, 179–185. [Google Scholar] [CrossRef] [PubMed]
- 2010 Dietary Guidelines for Americans. Available online: https://health.gov/sites/default/files/2020-01/DietaryGuidelines2010.pdf (accessed on 3 December 2020).
- Sodium Intake for Adults and Children. Available online: https://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf (accessed on 3 December 2020).
- Shaking the Salt Habit to Lower High Blood Pressure. Available online: https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/shaking-the-salt-habit-to-lower-high-blood-pressure-:~:text=The%20American%20Heart%20Association%20recommends,blood%20pressure%20and%20heart%20health (accessed on 3 December 2020).
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [Green Version]
- Cut down on Sodium. Available online: https://health.gov/sites/default/files/2019-10/DGA_Cut-Down-On-Sodium.pdf (accessed on 3 December 2020).
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (accessed on 3 December 2020).
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef] [Green Version]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; Dagenais, G.; Lear, S.; McQueen, M.; Diaz, R.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. Lancet 2016, 388, 465–475. [Google Scholar] [CrossRef]
- Schmieder, R.E.; Messerli, F.H.; Garavaglia, G.E.; Nunez, B.D. Dietary salt intake. A determinant of cardiac involvement in essential hypertension. Circulation 1988, 78, 951–956. [Google Scholar] [CrossRef] [Green Version]
- Safar, M.E.; Temmar, M.; Kakou, A.; Lacolley, P.; Thornton, S.N. Sodium intake and vascular stiffness in hypertension. Hypertension 2009, 54, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Langenfeld, M.R.; Schobel, H.; Veelken, R.; Weihprecht, H.; Schmieder, R.E. Impact of dietary sodium intake on left ventricular diastolic filling in early essential hypertension. Eur. Heart J. 1998, 19, 951–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jula, A.M.; Karanko, H.M. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation 1994, 89, 1023–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonski, K.L.; Racine, M.L.; Geolfos, C.J.; Gates, P.E.; Chonchol, M.; McQueen, M.B.; Seals, D.R. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J. Am. Coll. Cardiol. 2013, 61, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, P.; Giannattasio, C.; Parati, G. High sodium intake and arterial stiffness. J. Hypertens. 2018, 36, 754–758. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst. Rev. 2013, CD004937. [Google Scholar] [CrossRef]
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch. Intern. Med. 1997, 157, 657–667. [Google Scholar] [CrossRef]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007, 334, 885–888. [Google Scholar] [CrossRef] [Green Version]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch. Intern. Med. 2002, 162, 1619–1624. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Georgiopoulou, V.V.; Kalogeropoulos, A.P.; Dunbar, S.B.; Reilly, C.M.; Sands, J.M.; Fonarow, G.C.; Jessup, M.; Gheorghiade, M.; Yancy, C.; et al. Dietary sodium intake in heart failure. Circulation 2012, 126, 479–485. [Google Scholar] [CrossRef]
- Levitan, E.B.; Wolk, A.; Mittleman, M.A. Consistency with the DASH diet and incidence of heart failure. Arch. Intern. Med. 2009, 169, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.J.; Covit, A.B.; Schaer, G.L.; Laragh, J.H.; Sealey, J.E.; Feldschuh, J. Sodium and water balance in chronic congestive heart failure. J. Clin. Investig. 1986, 77, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, G.; Di Pasquale, P.; Licata, G.; Torres, D.; Giammanco, M.; Fasullo, S.; Mezzero, M.; Paterna, S. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J. Card. Fail. 2009, 15, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Huang, L.H.; Ku, C.H. Use of dietary sodium intervention effect on neurohormonal and fluid overload in heart failure patients: Review of select research based literature. Appl. Nurs. Res. 2018, 42, 17–21. [Google Scholar] [CrossRef]
- Hummel, S.L.; Seymour, E.M.; Brook, R.D.; Kolias, T.J.; Sheth, S.S.; Rosenblum, H.R.; Wells, J.M.; Weder, A.B. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension 2012, 60, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Vidal, E.R.; Cabrini, R.; Figueroa, M.A.; Bazzi, L.R.; Parisier, H.; Sanchez Zinny, J. Nodular goiter in young patients. Frequency of thyroid cancer. Rev. Argent. Cir. 1965, 8, 99–104. [Google Scholar]
- Aliti, G.B.; Rabelo, E.R.; Clausell, N.; Rohde, L.E.; Biolo, A.; Beck-da-Silva, L. Aggressive fluid and sodium restriction in acute decompensated heart failure: A randomized clinical trial. JAMA Intern. Med. 2013, 173, 1058–1064. [Google Scholar] [CrossRef]
- Velloso, L.G.; Alonso, R.R.; Ciscato, C.M.; Barretto, A.C.; Bellotti, G.; Pileggi, F. Diet with usual quantity of salt in hospital treatment of congestive heart insufficiency. Arq. Bras. Cardiol. 1991, 57, 465–468. [Google Scholar]
- Paterna, S.; Fasullo, S.; Parrinello, G.; Cannizzaro, S.; Basile, I.; Vitrano, G.; Terrazzino, G.; Maringhini, G.; Ganci, F.; Scalzo, S.; et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am. J. Med. Sci. 2011, 342, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Kalogeropoulos, A.; Papadimitriou, L.; Georgiopoulou, V.V.; Dunbar, S.B.; Skopicki, H.; Butler, J. Low-Versus Moderate-Sodium Diet in Patients With Recent Hospitalization for Heart Failure: The PROHIBIT (Prevent Adverse Outcomes in Heart Failure by Limiting Sodium) Pilot Study. Circ. Heart Fail. 2020, 13, e006389. [Google Scholar] [CrossRef]
- Miller, W.L.; Borgeson, D.D.; Grantham, J.A.; Luchner, A.; Redfield, M.M.; Burnett, J.C., Jr. Dietary sodium modulation of aldosterone activation and renal function during the progression of experimental heart failure. Eur. J. Heart Fail. 2015, 17, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Machado d’Almeida, K.S.; Rabelo-Silva, E.R.; Souza, G.C.; Trojahn, M.M.; Santin Barilli, S.L.; Aliti, G.; Rohde, L.E.; Biolo, A.; Beck-da-Silva, L. Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: Results from a randomized clinical trial. Nutrition 2018, 54, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mahtani, K.R.; Heneghan, C.; Onakpoya, I.; Tierney, S.; Aronson, J.K.; Roberts, N.; Hobbs, F.D.R.; Nunan, D. Reduced Salt Intake for Heart Failure: A Systematic Review. JAMA Intern. Med. 2018, 178, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Fabricio, C.G.; Tanaka, D.M.; Souza Gentil, J.R.; Ferreira Amato, C.A.; Marques, F.; Schwartzmann, P.V.; Schmidt, A.; Simoes, M.V. A normal sodium diet preserves serum sodium levels during treatment of acute decompensated heart failure: A prospective, blind and randomized trial. Clin. Nutr. ESPEN 2019, 32, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Selektor, Y.; Weber, K.T. The salt-avid state of congestive heart failure revisited. Am. J. Med. Sci. 2008, 335, 209–218. [Google Scholar] [CrossRef]
- Schrier, R.W. Body fluid volume regulation in health and disease: A unifying hypothesis. Ann. Intern. Med. 1990, 113, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Alvelos, M.; Ferreira, A.; Bettencourt, P.; Serrao, P.; Pestana, M.; Cerqueira-Gomes, M.; Soares-Da-Silva, P. The effect of dietary sodium restriction on neurohumoral activity and renal dopaminergic response in patients with heart failure. Eur. J. Heart Fail. 2004, 6, 593–599. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2011, CD004022. [Google Scholar] [CrossRef]
- Colombo, P.C.; Banchs, J.E.; Celaj, S.; Talreja, A.; Lachmann, J.; Malla, S.; DuBois, N.B.; Ashton, A.W.; Latif, F.; Jorde, U.P.; et al. Endothelial cell activation in patients with decompensated heart failure. Circulation 2005, 111, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Reduced Dietary Sodium Intake Increases Heart Rate. A Meta-Analysis of 63 Randomized Controlled Trials Including 72 Study Populations. Front. Physiol. 2016, 7, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, R.C.M.; Benetti, A.; Girardi, A.C.C.; Forechi, L.; de Oliveira, R.M.; Vassallo, P.F.; Mill, J.G. Influence of Long-Term Salt Diets on Cardiac Ca2+ Handling and Contractility Proteins in Hypertensive Rats. Am. J. Hypertens. 2018, 31, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Venetsanou, K.; Chedrese, P.J.; Pinto, V.; Takemori, H.; Franco-Cereceda, A.; Eriksson, P.; Mochizuki, N.; Soares-da-Silva, P.; Bertorello, A.M. Increases in intracellular sodium activate transcription and gene expression via the salt-inducible kinase 1 network in an atrial myocyte cell line. Am. J. Physiol.-Heart Circ. Physiol. 2012, 303, H57–H65. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Gupta, M.P. Cardiac hypertrophy: Old concepts, new perspectives. Mol. Cell. Biochem. 1997, 176, 273–279. [Google Scholar] [CrossRef]
- Lowes, B.D.; Minobe, W.; Abraham, W.T.; Rizeq, M.N.; Bohlmeyer, T.J.; Quaife, R.A.; Roden, R.L.; Dutcher, D.L.; Robertson, A.D.; Voelkel, N.F.; et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J. Clin. Investig. 1997, 100, 2315–2324. [Google Scholar] [CrossRef]
- Ling, Q.; Chen, T.H.; Guo, Z.G. Inhibition of beta-myosin heavy chain gene expression in pressure overload rat heart by losartan and captopril. Zhongguo Yao Li Xue Bao 1997, 18, 63–66. [Google Scholar]
- Cantilina, T.; Sagara, Y.; Inesi, G.; Jones, L.R. Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. Effect of a phospholamban antibody on enzyme activation by Ca2+. J. Biol. Chem. 1993, 268, 17018–17025. [Google Scholar]
- Kadambi, V.J.; Ponniah, S.; Harrer, J.M.; Hoit, B.D.; Dorn, G.W., 2nd; Walsh, R.A.; Kranias, E.G. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J. Clin. Investig. 1996, 97, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Masaki, H.; Sako, H.; Kadambi, V.J.; Sato, Y.; Kranias, E.G.; Yatani, A. Overexpression of phospholamban alters inactivation kinetics of L-type Ca2+ channel currents in mouse atrial myocytes. J. Mol. Cell. Cardiol. 1998, 30, 317–325. [Google Scholar] [CrossRef]
- Colin Ramirez, E.; Castillo Martinez, L.; Orea Tejeda, A.; Rebollar Gonzalez, V.; Narvaez David, R.; Asensio Lafuente, E. Effects of a nutritional intervention on body composition, clinical status, and quality of life in patients with heart failure. Nutrition 2004, 20, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Colin-Ramirez, E.; McAlister, F.A.; Zheng, Y.; Sharma, S.; Armstrong, P.W.; Ezekowitz, J.A. The long-term effects of dietary sodium restriction on clinical outcomes in patients with heart failure. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure): A pilot study. Am. Heart J. 2015, 169, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Philipson, H.; Ekman, I.; Forslund, H.B.; Swedberg, K.; Schaufelberger, M. Salt and fluid restriction is effective in patients with chronic heart failure. Eur. J. Heart Fail. 2013, 15, 1304–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessler, J.D.; Maurer, M.S.; Hummel, S.L. Evaluating the safety and efficacy of sodium-restricted/Dietary Approaches to Stop Hypertension diet after acute decompensated heart failure hospitalization: Design and rationale for the Geriatric OUt of hospital Randomized MEal Trial in Heart Failure (GOURMET-HF). Am. Heart J. 2015, 169, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Arcand, J.; Ivanov, J.; Sasson, A.; Floras, V.; Al-Hesayen, A.; Azevedo, E.R.; Mak, S.; Allard, J.P.; Newton, G.E. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: A prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Doukky, R.; Avery, E.; Mangla, A.; Collado, F.M.; Ibrahim, Z.; Poulin, M.F.; Richardson, D.; Powell, L.H. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC Heart Fail. 2016, 4, 24–35. [Google Scholar] [CrossRef]
- Graudal, N.; Hubeck-Graudal, T.; Jurgens, G.; Taylor, R.S. Dose-response relation between dietary sodium and blood pressure: A meta-regression analysis of 133 randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1273–1278. [Google Scholar] [CrossRef]
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef] [Green Version]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Ah, S.T.L.; Wei, L.; Diaz, R.; et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: A community-level prospective epidemiological cohort study. Lancet 2018, 392, 496–506. [Google Scholar] [CrossRef]
- Lennie, T.A.; Song, E.K.; Wu, J.R.; Chung, M.L.; Dunbar, S.B.; Pressler, S.J.; Moser, D.K. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J. Card. Fail. 2011, 17, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Dolansky, M.A.; Schaefer, J.T.; Hawkins, M.A.; Gunstad, J.; Basuray, A.; Redle, J.D.; Fang, J.C.; Josephson, R.A.; Moore, S.M.; Hughes, J.W. The association between cognitive function and objective adherence to dietary sodium guidelines in patients with heart failure. Patient Prefer. Adherence 2016, 10, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masterson Creber, R.; Topaz, M.; Lennie, T.A.; Lee, C.S.; Puzantian, H.; Riegel, B. Identifying predictors of high sodium excretion in patients with heart failure: A mixed effect analysis of longitudinal data. Eur. J. Cardiovasc. Nurs. 2014, 13, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basuray, A.; Dolansky, M.; Josephson, R.; Sattar, A.; Grady, E.M.; Vehovec, A.; Gunstad, J.; Redle, J.; Fang, J.; Hughes, J.W. Dietary sodium adherence is poor in chronic heart failure patients. J. Card. Fail. 2015, 21, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghiade, M.; Abraham, W.T.; Albert, N.M.; Gattis Stough, W.; Greenberg, B.H.; O’Connor, C.M.; She, L.; Yancy, C.W.; Young, J.; Fonarow, G.C.; et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur. Heart J. 2007, 28, 980–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokko, J.P. The role of the renal concentrating mechanisms in the regulation of serum sodium concentration. Am. J. Med. 1977, 62, 165–169. [Google Scholar] [CrossRef]
- Vicent, L.; Alvarez-Garcia, J.; Gonzalez-Juanatey, J.R.; Rivera, M.; Segovia, J.; Worner, F.; Bover, R.; Pascual-Figal, D.; Vazquez, R.; Cinca, J.; et al. Prognostic impact of hyponatremia and hypernatremia at admission and discharge in heart failure patients with preserved, mid-range, and reduced ejection fraction. Intern. Med. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rusinaru, D.; Tribouilloy, C.; Berry, C.; Richards, A.M.; Whalley, G.A.; Earle, N.; Poppe, K.K.; Guazzi, M.; Macin, S.M.; Komajda, M.; et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: An individual patient data meta-analysis(dagger): Meta-Analysis Global Group in Chronic heart failure (MAGGIC). Eur. J. Heart Fail. 2012, 14, 1139–1146. [Google Scholar] [CrossRef]
- Patel, Y.R.; Kurgansky, K.E.; Imran, T.F.; Orkaby, A.R.; McLean, R.R.; Ho, Y.L.; Cho, K.; Gaziano, J.M.; Djousse, L.; Gagnon, D.R.; et al. Prognostic Significance of Baseline Serum Sodium in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e007529. [Google Scholar] [CrossRef] [Green Version]
- Imran, T.F.; Kurgansky, K.E.; Patel, Y.R.; Orkaby, A.R.; McLean, R.R.; Ho, Y.L.; Cho, K.; Gaziano, J.M.; Djousse, L.; Gagnon, D.R.; et al. Serial sodium values and adverse outcomes in heart failure with preserved ejection fraction. Int. J. Cardiol. 2019, 290, 119–124. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Yin, X. Improvement of hyponatremia is associated with lower mortality risk in patients with acute decompensated heart failure: A meta-analysis of cohort studies. Heart Fail. Rev. 2019, 24, 209–217. [Google Scholar] [CrossRef]
- Colin-Ramirez, E.; Arcand, J.; Woo, E.; Brum, M.; Morgan, K.; Christopher, W.; Velazquez, L.; Sharifzad, A.; Feeney, S.; Ezekowitz, J.A. Design and Region-Specific Adaptation of the Dietary Intervention Used in the SODIUM-HF Trial: A Multicentre Study. CJC Open 2020, 2, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Year, Name of Guideline | Sodium Restriction |
|---|---|
| 2010, Dietary Guidelines for Americans [4] | <2.3 g/d in all adults <1.5 g/d in adults aged more than 50 years who are African American or with hypertension, diabetes, or chronic kidney disease |
| 2013, World Health Organization [5] | <2 g/d in all adults |
| 2020, American Heart Association [6] | <1.5 g/d in all adults |
| 2010, Heart Failure Society of America [1] | 2–3 g/d in all heart failure patients<2 g/d in patients with moderate to severe heart failure |
| 2019, American Diabetic Association [7] | <2.3 g/d in patients with diabetes<1.5 g/d in patients with diabetes and hypertension |
| 2016, European Society of Cardiology [8] | <5 g/d in all adults |
| 2017, Canadian Cardiovascular Society [9] | <2 g/d in all adults |
| 2015–2020 Dietary Guidelines for Americans [10] | 2.3 g/d in all adults |
| 2012, The Kidney disease: Improving Global Outcomes (KDIGO) [11] | <2 g/d in all patients with chronic disease not on dialysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, Y.; Joseph, J. Sodium Intake and Heart Failure. Int. J. Mol. Sci. 2020, 21, 9474. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249474
Patel Y, Joseph J. Sodium Intake and Heart Failure. International Journal of Molecular Sciences. 2020; 21(24):9474. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249474
Chicago/Turabian StylePatel, Yash, and Jacob Joseph. 2020. "Sodium Intake and Heart Failure" International Journal of Molecular Sciences 21, no. 24: 9474. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249474




