Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation
Abstract
:1. Introduction
2. Results
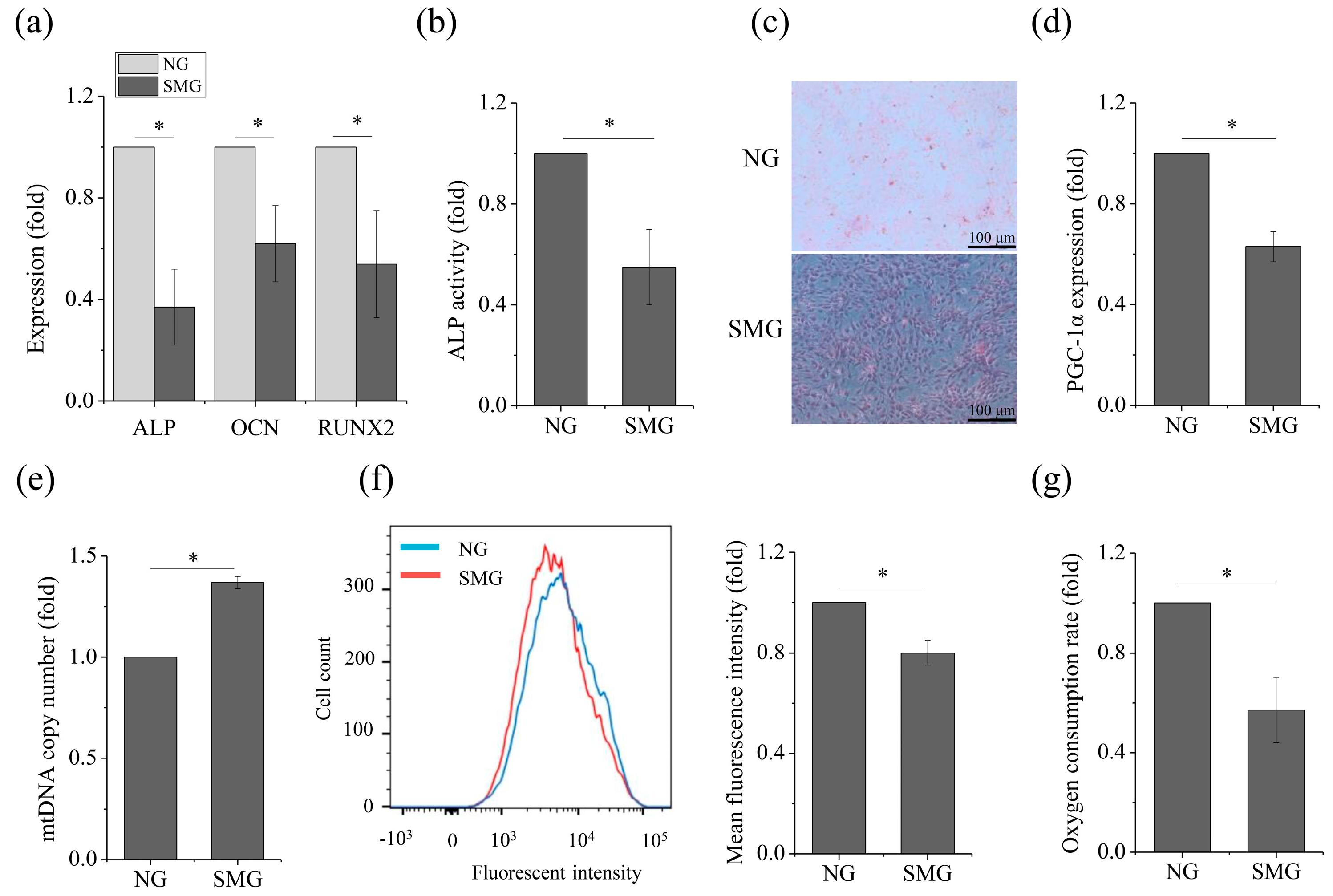
2.1. SMG Hampered Osteogenic Differentiation and OXPHOS in MSCs
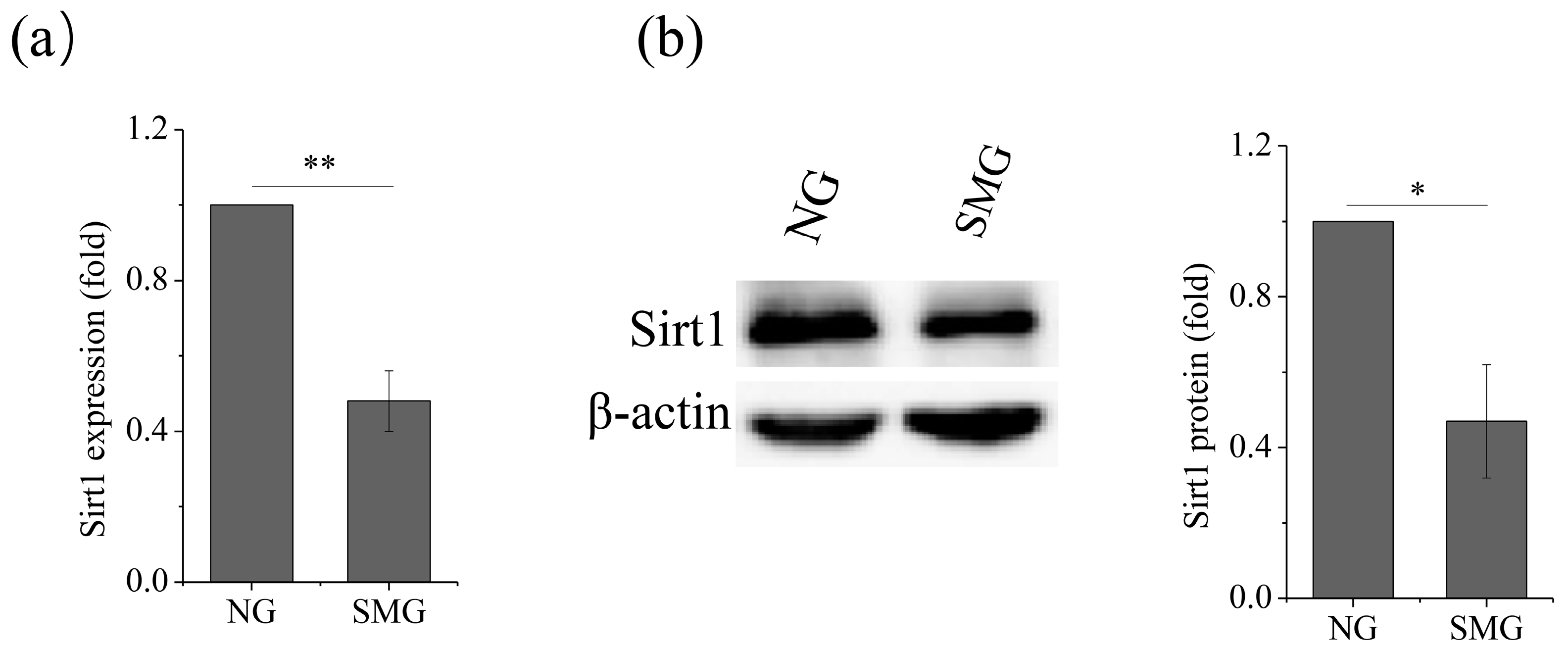
2.2. SMG Inhibited Sirt1 Expression
2.3. Upregulation of Sirt1 Enhanced OXPHOS
2.4. Improving OXPHOS via Sirt1 Rescued Osteogenic Differentiation of MSCs
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Treatments
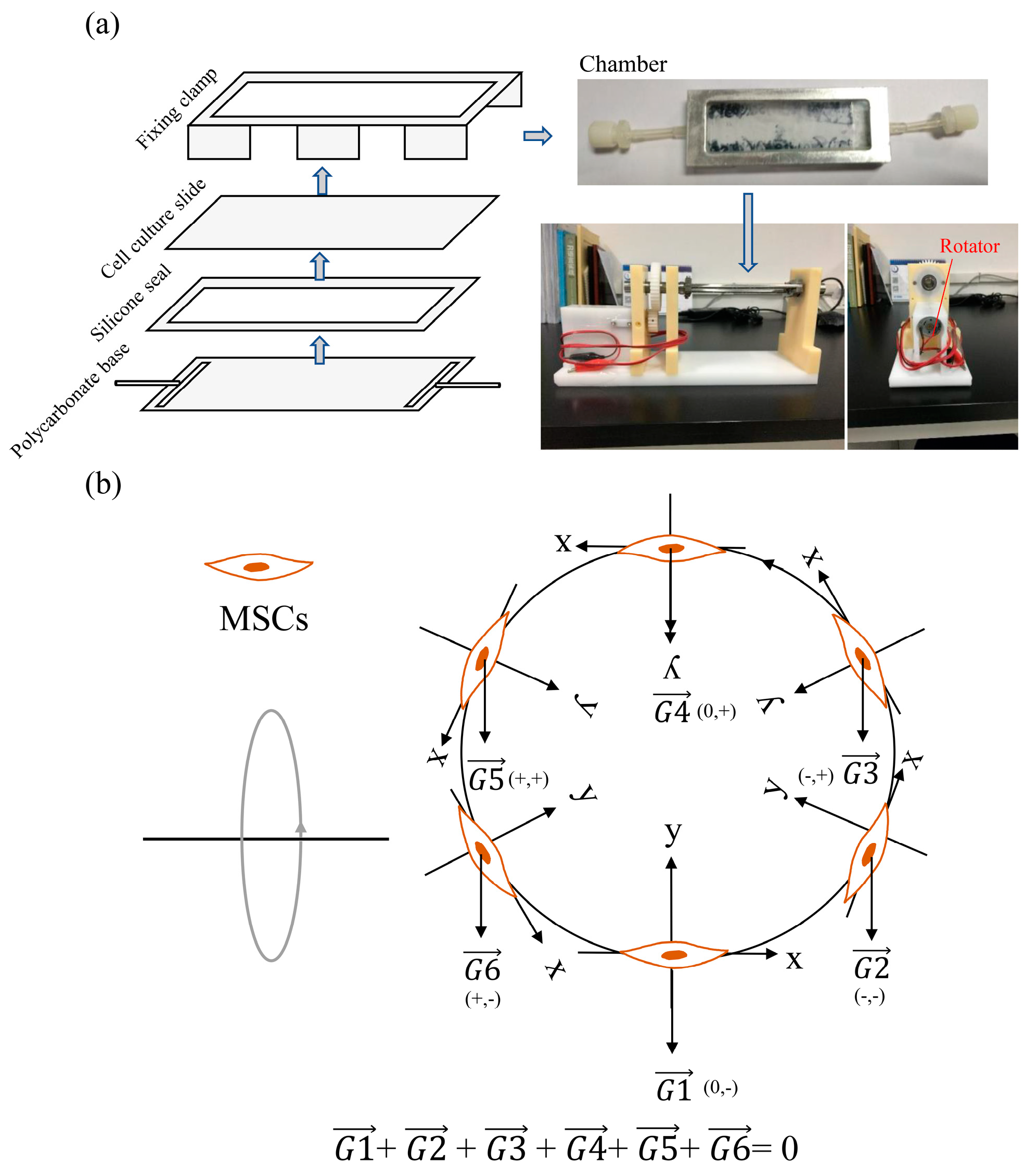
4.2. Production of Simulated Gravity Conditions
4.3. Alkaline Phosphatase Activity Assay
4.4. Alkaline Phosphatase Staining
4.5. Measurement of the Oxygen Consumption Rate
4.6. mtDNA Copy Number Analysis
4.7. Mitochondrial Staining Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Western Blot
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SMG | Simulated Microgravity |
| MSCs | Mesenchymal Stem Cells |
| OXPHOS | Oxidative Phosphorylation |
| PGC-1α | Peroxisome Proliferator Activated Receptor γ Coactivator 1α |
| mtDNA | Mitochondrial DNA |
| OCR | Oxygen Consumption Rate |
| Sirt1 | Sirtuin 1 |
| ALP | Alkaline Phosphatase |
| OCN | Osteocalcin |
| RUNX2 | RUNX Family Transcription Factor 2 |
| Res | Resveratrol |
| NG | Normal Gravity |
References
- Jin, Y.Z.; Lee, J.H. Mesenchymal stem cell therapy for bone regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jiang, Y.; Wang, C.C.; Geng, B.M.; Wang, Y.Q.; Chen, J.L.; Liu, F.; Qiu, P.; Zhai, G.J.; et al. Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 2018, 32, 4444–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibonga, J.D. Spaceflight-induced bone loss: Is there an osteoporosis risk. Curr. Osteoporos. Rep. 2013, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, Q.; Lin, C.C.; Song, G.B. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem. Biophys. Res. Commun. 2015, 468, 21–26. [Google Scholar] [CrossRef]
- Chen, C.T.; Shih, Y.R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Shen, Y.L.; Wu, L.; Wang, J.; Wu, X.; Zhang, X.M. The role of mitochondria in methamphetamine-induced inhibitory effects on osteogenesis of mesenchymal stem cells. Eur. J. Pharmacol. 2018, 826, 56–65. [Google Scholar] [CrossRef]
- Shares, B.H.; Busch, M.; White, N.; Shum, L.; Eliseev, R.A. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J. Biol. Chem. 2018, 293, 16019–16027. [Google Scholar] [CrossRef] [Green Version]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The human sirtuin family: Evolutionary divergences and functions. Hum. Genomics 2011, 5, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Wu, Y.T.; Tsai, C.L.; Wei, Y.H. Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells. Exp. Biol. Med. 2018, 243, 563–575. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Wu, J.; Mei, Y.K.; Zhang, Q.; Zhang, H.W.; Miao, D.S.; Sun, W. Sirt1 promotes osteogenic differentiation and increases alveolar bone mass via Bmi1 activation in mice. J. Bone Miner. Res. 2019, 34, 1169–1181. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, G.D.; Yan, J.K.; Chen, X.; He, F.; Zhu, C.H.; Zhang, J.X.; Lin, J.; Pan, G.Q.; Yu, J.; et al. Upregulation of SIRT1 by kartogenin enhances antioxidant functions and promotes osteogenesis in human mesenchymal stem cells. Oxid. Med. Cell. Longev. 2018, 2018, 1368142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yan, J.K.; Chen, X.; Tam, W.; Zhou, L.; Liu, T.; Pan, G.Q.; Lin, J.; Yang, H.L.; Pei, M.; et al. Spontaneous up-regulation of SIRT1 during osteogenesis contributes to stem cells’ resistance to oxidative stress. J. Cell. Biochem. 2018, 119, 4928–4944. [Google Scholar] [CrossRef]
- Chen, H.Q.; Liu, X.B.; Chen, H.; Cao, J.; Zhang, L.; Hu, X.Y.; Wang, J.A. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res. Rev. 2014, 13, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Jiang, T.L.; Wang, Y.; Guo, L. The role and mechanism of SIRT1 in resveratrol-regulated osteoblast autophagy in osteoporosis rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.H.; Li, Y.S.; Liu, H.F.; Xiao, S.S.; Li, L.J.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F.J. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.W.; Han, D.; Li, X.; Yang, B.; Li, X.J.; Fan, M.M.; Li, C.Y.; et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid. Med. Cell. Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Wu, Y.T.; Yu, T.H.; Wei, Y.H. Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. Semin. Cell. Dev. Biol. 2016, 52, 119–131. [Google Scholar] [CrossRef]
- Lee, A.R.; Moon, D.K.; Siregar, A.; Moon, S.Y.; Jeon, R.H.; Son, Y.B.; Kim, B.G.; Hah, Y.S.; Hwang, S.C.; Byun, J.H.; et al. Involvement of mitochondrial biogenesis during the differentiation of human periosteum-derived mesenchymal stem cells into adipocytes, chondrocytes and osteocytes. Arch. Pharm. Res. 2019, 42, 1052–1062. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Cucina, A.; Bizzarri, M.; Mariggiò, M.A. Antioxidant strategy to prevent simulated microgravity-induced effects on bone osteoblasts. Int. J. Mol. Sci. 2020, 21, 3638. [Google Scholar] [CrossRef]
- Fu, W.Y.; Liu, Y.; Yin, H. Mitochondrial dynamics: Biogenesis, fission, fusion, and mitophagy in the regulation of stem cell behaviors. Stem Cells Int. 2019, 2019, 9757201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.J.; Cruzat, V.F.; Newsholme, P.; Cheng, J.Q.; Chen, Y.N.; Lu, Y.R. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Hock, M.B.; Kralli, A. Transcriptional control of mitochondrial biogenesis and function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yan, J.K.; He, F.; Zhong, D.Y.; Yang, H.L.; Pei, M.; Luo, Z.P. Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic. Biol. Med. 2018, 126, 187–201. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chen, S.D.; Hsu, C.Y.; Chen, S.F.; Chen, N.C.; Jou, S.B. Resveratrol promotes mitochondrial biogenesis and protects against seizure-induced neuronal cell damage in the hippocampus following status epilepticus by activation of the PGC-1α signaling pathway. Int. J. Mol. Sci. 2019, 20, 998. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Chi, Y.Q.; Ren, Y.Z.; Du, C.Y.; Shi, Y.H.; Li, Y. Resveratrol reduces oxidative stress and apoptosis in podocytes via Sir2-related enzymes, sirtuins1 (SIRT1)/peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) axis. Med. Sci. Monit. 2019, 25, 1220–1231. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; Geest, M.D.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Dedolph, R.R.; Dipert, M.H. The physical basis of gravity stimulus nullification by clinostat rotation. Plant Physiol. 1971, 47, 756–764. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Marsboom, G.; Toth, P.T.; Rehman, J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 2013, 8, e77077. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Genes | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| ALP | TCGGACCCTGCCTTACCA | TGTCTCCTCGCCCGTGTT |
| OCN | AGCTCAACCCCAATTGTGAC | AGCTGTGCCGTCCATACTTT |
| RUNX2 | CAGACCAGCAGCACTCCATA | CAGCGTCAACACCATCATTC |
| PGC-1α | TGACCACAAACGATGACCCTC | GACTGCGGTTGTGTATGGGAC |
| Sirt1 | GGAACCTCTGCCTCATCTA | CATACTCGCCACCTAACCT |
| β-actin | ACCGTCAGGTCACTATCG | GGCATAGAGGTCTTTACGGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Cheng, Y.; Wang, J.; Ding, Z.; Halim, A.; Luo, Q.; Song, G. Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation. Int. J. Mol. Sci. 2020, 21, 9747. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249747
Liu L, Cheng Y, Wang J, Ding Z, Halim A, Luo Q, Song G. Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation. International Journal of Molecular Sciences. 2020; 21(24):9747. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249747
Chicago/Turabian StyleLiu, Lin, Yansiwei Cheng, Jie Wang, Zhongjie Ding, Alexander Halim, Qing Luo, and Guanbin Song. 2020. "Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation" International Journal of Molecular Sciences 21, no. 24: 9747. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21249747





