1. Introduction
Natural products may be considered as starting points in the design and development of potential compounds of interest for human medicine. Sesquiterpene lactones are naturally occurring compounds that display a wide array of biological properties and are potential agents against cancer [
1,
2]. Guaianolides belong to a class of sesquiterpene lactones characterized by three fused rings, two five-membered rings and a seven-membered ring structure with a wide range of biological activities [
3]. Some compounds of this class exhibit anti-inflammatory activity due to inhibition of the transcription factor NF-κB [
4,
5,
6].
Halogenated compounds generally contain a chlorine atom and about 5000 of them have been isolated from natural sources. They have a wide range of beneficial bioactivities that have led to applications in the pharmaceutical industry. Currently, half of the molecules in high-performance screening contain halogen atoms [
6]. Many of these halogenated compounds are chlorohydrins isolated together with their corresponding epoxides [
7]. Naturally occurring halogenated sesquiterpene lactones and synthetic derivatives exhibit antitumor and cytotoxic activities and have potential as anticancer agents [
8,
9].
Apoptosis induction is an important response to many chemotherapeutic agents. This is a mode of regulated cell death characterized by the translocation of phosphatidylserine to the outside of the plasma membrane, formation of apoptotic bodies, chromatin condensation and internucleosomal DNA fragmentation. There are at least two major apoptotic pathways, referred to as the intrinsic pathway and the extrinsic pathway, depending on the cell type [
10]. The intrinsic pathway involves the translocation of cytochrome
c from mitochondria to cytoplasm and caspase-9 activation, which cleaves and activates downstream effector caspases-3, -6 and -7, which in turn trigger the cleavage of key structural and regulatory proteins to effect cell death [
11]. The extrinsic pathway involves cell surface death receptors, such as tumor necrosis factor, Fas and TRAIL receptors, and is dependent on the initiator caspase-8, which cleaves and activates the downstream effector caspases [
12]. Both caspase-8 and caspase-9 activate caspase-3, which is responsible for breaking specific cellular proteins during apoptosis [
13]. Caspase-3 is one of the key executioners of apoptosis, being responsible for the proteolytic cleavage of many key proteins, including the nuclear enzyme poly(ADP-ribose)polymerase which is normally involved in DNA repair [
14].
In this study we analyzed the structure–cytotoxicity relationships of eight halogenated guaianolides (seven of them isolated from plants from the genus
Centaurea, family
Asteraceae) against human leukemia and melanoma cells. The compounds were selected from our sesquiterpene library, constructed during previous studies that had shown that guaianolides-type sesquiterpene lactones were the most cytotoxic compounds against different cancer cell lines [
15]. The guaianolides assayed were isolated from natural sources as previously described [
16,
17,
18]. The chlorinated guaianolides chlorohyssopifolins A (
1), B (
2), C (
3), D (
4) and E (
5) were isolated from
Centaurea hyssopifolia Vahl. Linichlorin A (
6) and linichlorin C (
7) were isolated from
Centaurea linifolia Vahl. The compound 11,13-Dihydrochlorohyssopifolin C (
8) was obtained from chlorohyssopifolin A through reduction with Zn-Cu followed by epoxide formation with AgNO
3 [
18] (
Figure 1). The evaluated guaianolides have the following properties in common: (i) the presence of a double bond as a methylene group at position ten (C-10); (ii) the presence of a hydroxy group and/or an acetyl group in position three of the cyclopentane ring; (iii) different substituents on C-8; and (iv) the presence of a chlorine atom either in position C-15, in the ester moiety or both. Some of the most potent compounds against human tumor cells, i.e., chlorohyssopifolins A (
1) and D (
4) and linichlorin A (
6), were evaluated to determine whether the effects on cell viability were due to the activation of the apoptotic pathway. Specifically, we studied the effects on apoptosis induction and caspase activation. The aim of this study was to explore the structure–cytotoxicity relationships of eight selected sesquiterpene lactones belonging to the guaianolide class against human leukemia and melanoma cells, as well as the mechanism of action of the most potent compounds on apoptosis induction using the human leukemia cells U-937. These cells were chosen since they provided a useful model for the study of neoplasia and therapeutics [
19,
20]. So far, the potential use of these halogenated guaianolides in antileukemia therapy has been left largely unexplored. We evaluated whether caspase activation and the release of cytochrome
c were involved in the mechanism of action.
3. Discussion
In recent years there has been a renewed interest in naturally occurring compounds as potential chemopreventive and chemotherapeutic treatments against cancer. It is estimated that about 60% of compounds that are used in the fight against cancer may be considered as derived from natural products. Among these compounds, sesquiterpene lactones have received considerable attention in the last 20 years [
21]. Sesquiterpenes are compounds of fifteen carbon atoms with different skeletons, mainly (but not exclusively) germacrane, guaiano and eudesmane skeletons. Sesquiterpene lactones are characterized by an α-methylene-γ-lactone functional group which acts as an electrophile group able to react with specific nucleophiles functional groups in Michael-type addition reaction [
4]. The germacranolide parthenolide is one of the most studied sesquiterpene lactones. It exhibits anti-inflammatory activity and promising potential anticancer activity [
22,
23]. The guaianolide-type sesquiterpene lactones have also attracted attention because, in general, they exhibit a higher cytotoxic activity compared with the other types of sesquiterpene lactones [
15]. For example, arglabin and micheliolide can selectively inhibit acute myelogenous leukemia stem or progenitor cell growth and dehydroleucodine displayed antitumour activity in a preclinical melanoma model [
24,
25]. Interestingly, phase II clinical trials were recently completed [
3] for mipsagargin, an analogue of the potent sarco-endoplasmic reticulum Ca
2+-ATPase inhibitor thapsigargin in several types of cancer.
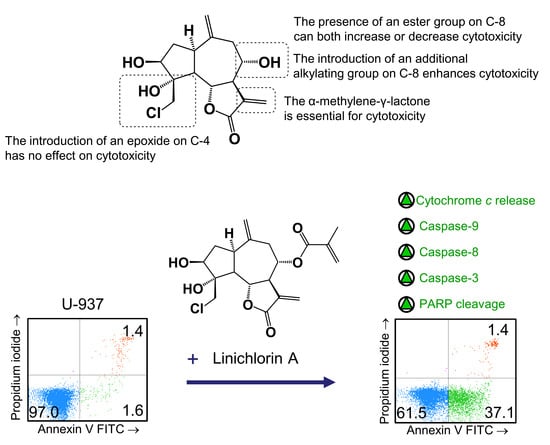
Here we explored the structure–cytotoxicity relationships of eight chlorinated guaianolides, seven obtained from natural sources and one as a semisynthetic derivative, using human leukemia and melanoma cell lines as models. These compounds were characterized by the presence of exocyclic double bonds on carbons C-10 and C-11 and different substituents on carbons C-3, C-4 and C-8. The results of the studies of structure–cytotoxicity relationships revealed that: (i) the reduction of the double bond 11,13 blocked inhibitory activity in tumor cells, independent of the presence of an additional reactive electrophilic group; (ii) acetylation of hydroxy group on C-3 did not enhance cytotoxicity in comparison with the corresponding alcohol; (iii) the introduction of an ester group on C-8 amplified or decreased cytotoxicity; (iv) the increase in polarity of the hydrocarbon chain of the ester group on C-8 reduced cytotoxicity; (v) the introduction of an alkylating group such as an epoxide on C-4 did not enhance cytotoxicity but the introduction of an enone on C-8 did.
The importance of the α-methylene-γ-lactone in determining cytotoxicity in the presence of additional reactive electrophilic groups has been described for repin, a guaianolide isolated from
Centaurea repens [
26]. Previous studies have shown that chlorohyssopifolin A (
1) inhibits cell viability of several cancer cell lines, including 1A9 (ovarian cancer), KB (nasopharyngeal cancer) and KB-V (vincristine-resistant KB subline), and that chlorohyssopifolin C (
3) showed significant cytotoxicity against MCF-7 (estrogen receptor positive breast cancer). In that study the chlorohyssopifolins A (
1) and C (
3) were not assayed against human melanoma cells and the mechanism of cell death was not explored [
8]. Similar to our results in human leukemia and melanoma cells, the study showed that the chlorohydrin on C-4 may be modified to an epoxide on the cyclic skeleton with no change in cytotoxicity, as demonstrated by the comparison of the IC
50 values of chlorohyssopifolin A (
1) and chlorohyssopifolin C (
3). In addition, the presence of a diol in the side chain on C-8 (chlorohyssopifolin E (
5)) rather than a chlorohydrin (chlorohyssopifolin A (
1)) abolished activity. A similar observation has been described for the comparison between babylin A, which contains a diol in the side chain, and chlorohyssopifolin C (
3) which contains a chlorohydrin in the side chain [
8].
Interestingly, the guaianolides were able to inhibit cell viability in U-937/Bcl-2, a subline that overexpresses the survival protein Bcl-2, which has been involved in chemoresistance, especially in hematologic malignancies [
27]. These results suggest that guaianolides seem capable of blocking the growth of human tumor cells by inactivation of the mitochondrial protection by Bcl-2. Moreover, SK-MEL-1 melanoma cells were sensitive to chlorohyssopifolins A (
1), C (
3) and D (
4) and linichlorin A (
6), emphasizing the potential of these compounds, given that melanoma is the most aggressive and lethal skin cancer.
The results of this work revealed that linichlorin A (
6) was one of the most potent guaianolides in reducing the cell viability of the four human tumor cells assayed. Linichlorin A (
6) has been reported as a negative regulator of the degradation of the cyclin-dependent kinases inhibitor p27
Kip1. This guaianolide inhibits the
in vitro ubiquitination of p27
Kip1 by SCF
Skp2—the ubiquitin ligase (SCF) complex with S-phase kinase-associated protein 2- stabilizes p27
Kip1 levels in HeLa cells and inhibits the growth of human and mouse cancer cells [
28]. Linichlorin A (
6) has been shown to display substantial, selective antiproliferative activity against cancer and transformed cells in the micromolar range. The IC
50 values reported for linichlorin A (
6) in HeLa (human cervix carcinoma), tsFT210 (mouse tumor cells) and NIH3T3 (mouse immortalized cells) were 3.2, 1.6 and 12.7 μM, respectively, as determined by WST-8 assay for 48 h. However, little is known about the mechanism of cell death induced by linichlorin A (
6).
Flow cytometry experiments in the present study revealed that inhibition of cell viability by chlorohyssopifolins A (1), C (3) and D (4) and linichlorin A (6) was accompanied by an increase in the sub-G1 fraction. The selected guaianolides chlorohyssopifolins A (1) and D (4) and linichlorin A (6) were able to induce nuclear morphological changes, such as fragmentation and condensation of chromatin, characteristic of apoptotic cell death. Experiments using U-937 cells as a model confirmed that these compounds are potent apoptotic inducers, as demonstrated by phosphatidylserine externalization and poly(ADP-ribose)polymerase cleavage. In addition, selected guaianolides induced a concentration-dependent release of mitochondrial apoptogenic cytochrome c, indicating that the intrinsic apoptotic pathway may play a key role in cell death. Enzymatic analysis revealed activation of caspase-9 and caspase-3, in accordance with the release of cytochrome c. Activation of caspase-8 was also detected in extracts of selected guaianolides-treated U-937 cells. In order to identify the primary targets and early mechanism of action of selected sesquiterpene lactones on U-937 cells, we used concentrations close to or threefold higher than the antiproliferative IC50 values, which were determined at 72 h of treatment, while flow cytometry and assays of caspase activity were analyzed after 24 h of treatment. Concentrations close to the IC50 values of selected sesquiterpene lactones were sufficient to trigger apoptosis. It is interesting to note that a low concentration (3 μM) of chlorohyssopifolins A (1) and D (4) and linichlorin A (6) was sufficient to induce cytochrome c release in U-937 cells. Taken together, these results indicate that both the extrinsic and the intrinsic pathways play an important role in cell death induced by chlorohyssopifolins A (1) and D (4) and linichlorin A (6).
In conclusion, chlorohyssopifolins A (1) and D (4) and linichlorin A (6) were shown to induce apoptosis via caspase activation. Although more research must be carried out to uncover the detailed pathway of cell death, these compounds are potentially interesting and should be considered for further preclinical and in vivo testing.
4. Materials and Methods
4.1. Drugs and Reagents
The guaianolides assayed were isolated from natural sources as previously described. Five sesquiterpene lactones containing chlorine, chlorohyssopifolins A (
1), B (
2), C (
3), D (
4) and E (
5) were isolated from
Centaurea hyssopifolia Vahl, which is an endemic dominant species on Iberian gypsum soils in central Spain [
15,
16]. Linichlorin A (
6) is a sesquiterpene lactone that was first isolated from
Centaurea linifolia Vahl, a native plant from the eastern part of Spain and Italy [
17]. The chemical structures of sesquiterpene lactones were determined spectroscopically (proton nuclear magnetic resonance, infrared spectroscopy and mass spectrometry) as described previously.
1H- NMR,
13C-NMR and mass spectra of guaianolides are shown in
Figures S3–S33 in Supplementary Materials.
4.2. Cell Culture
The human acute myeloid leukemia HL-60 (DSMZ N° ACC 3), the human histiocytic lymphoma U-937 (DSMZ N° ACC 5) and the human melanoma SK-MEL-1 (DSMZ N° ACC 303) were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), cultured in suspension in RPMI 1640 medium containing 10% (v/v) fetal bovine serum and maintained at 0.5–1.0 × 106 cells/mL, except SK-MEL-1 cells, which were maintained at 0.1–0.3 × 106 cells/mL. The U-937 cell line overexpressing human Bcl-2 (designated U-937/Bcl-2) was donated by Dr. Jacqueline Bréard (Faculté de Pharmacie Paris-Sud, Chatenay-Malabry, France) and cultured in RPMI 1640 medium containing 10% (v/v) fetal bovine serum at 37 °C in a humidified atmosphere containing 5% CO2. Cell viability was determined by the trypan blue exclusion test. Cells were resuspended in fresh medium 24 h before treatments to ensure the exponential growth. HL-60 and U-937 cells exhibited characteristic doubling times of about 25 h and 35 h, respectively, while SK-MEL-1 cells exhibited a doubling time of several days (about 48–72 h). Stock solutions of 50 mM guaianolides were made in dimethylsulfoxide (DMSO) and aliquots were frozen at −20 °C. Further dilutions were made in culture medium immediately prior to use. In all experiments, the final concentration of DMSO did not exceed 0.2% (v/v), a concentration that was nontoxic to the cells.
4.3. Assay for Growth Inhibition
The effects of guaianolides on the cell viability of human tumor cells were assessed using the colorimetric 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2
H-tetrazolium bromide (MTT) assay [
29]. Exponentially growing cells (4000 for HL-60 and U-937; 6000 for SK-MEL-1) were seeded in 96-well microculture plates with increasing concentrations of guaianolides for 72 h at 37 °C. Surviving cells were detected based on their ability to metabolize MTT in formazan crystals, measuring the absorbance at 570 nm, and the IC
50 (concentrations inducing a 50% inhibition of cell growth) values were calculated graphically using the curve-fitting algorithm of the computer software Prism 5.0 (GraphPad, La Jolla, CA, USA). Values were calculated as means ± SD from three to five independent experiments, each performed in triplicate.
4.4. Fluorescent Microscopy
Cells were treated with the corresponding compound for the specified time period and then fixed with 3% paraformaldehyde for 10 min at room temperature. Cells were then stained with bisbenzimide (Hoechst 33258) for 15 min and visualized under a fluorescence microscope.
4.5. Analysis by Flow Cytometry
After treatment with guaianolides, cells were fixed in 75% ethanol at −20 °C for at least 1 h, stained with propidium iodide and analyzed by flow cytometry using a BD FACS Verse cytometer. Apoptosis was quantified using an Annexin V-FITC apoptosis detection kit (BD Pharmingen, San Diego, CA, USA), performed according to the manufacturer’s protocol.
4.6. Caspase Activity
Caspase activity was determined in cell lysates using specific colorimetric substrates for caspase-3/7, caspase-8 and caspase-9 [
30]. Briefly, after treatments cells were washed twice in phosphate buffer saline, they were resuspended in 50 mM HEPES pH 7.4, 0.1 mM EDTA, 1 mM dithiothreitol, 0.1% Chaps, and lysed by pushing them several times through a 22-gauge needle. The cell lysates were centrifuged at 17,000×
g at 4 °C for 10 min and the supernatants were analyzed for protein concentration and for caspase activity. Protein concentration was determined by the Bradford assay and caspase activity by the net increase of absorbance at 405 nm. The specific substrates for caspase-3/7, -8 and -9 were DEVD-
pNA, IETD-
pNA and LEHD-
pNA, respectively.
4.7. Western Blot
Immunoblot analyses of caspases and PARP were performed as previously described [
31]. Briefly, cells were treated with the specified compounds, washed twice with PBS and then lysed in a buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM sodium chloride, 1.0% Triton X-100, 0.1 mM phenylmethylsulfonylfluoride and leupeptin, pepstatin A and aprotinin (1 μg/mL each). Equal amounts of proteins were denatured in 2x Laemli buffer (0.125 M Tris-HCl pH 6.8, 4% SDS, 10% mercaptoethanol, 20% glycerol and 0.004% bromophenol blue) at 95 °C for 5 min. The samples were separated on a 7.5% (PARP) or 12.5% (caspases) SDS-polyacrylamide gel and electrotransferred to a polyvinylidene difluoride (PVDF) membrane. After blocking the membrane with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h, it was incubated with the corresponding primary antibodies followed by the corresponding secondary antibodies. The antigen–antibodies complexes were visualized by enhanced chemiluminiscence.
For the cytosolic fractions, cells were washed twice with PBS, resuspended in 20 mM Hepes-KOH pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.1 mM phenylmethylsulfonylfluoride, 1 mM dithiothreitol, 250 mM sucrose and 1 μg/mL leupeptin, pepstatin A and aprotinin, and lysed by pushing them several times through a 22-gauge needle. Lysates were centrifuged at 1000× g for 5 min at 4 °C and the supernatants were spun down again at 105,000× g for 45 min at 4 °C. The resulting supernatants were used as the soluble cytosolic fraction.
The primary antibodies used for Western blots were purchased from the following companies: anti-caspase-3 (ADI-AAP-113), -8 (ADI-AAM-118) and -9 (ADI-AAM-139) from Enzo (Plymouth Meeting, PA, USA); anti-poly(ADP-ribose) polymerase (PARP) (551024) and anti-cytochrome c (556433) from BD Pharmingen (San Diego, CA, USA); anti-β-actin (clone AC-74, A2228) from Sigma-Aldrich (Saint Louis, MO, USA). Secondary antibodies (NA9310 and NA9340) were from GE Healthcare (Little Chalfont, UK). PVDF membranes were from Millipore (Temecula, CA, USA).
4.8. Statistical Analysis
Statistical differences between means of control and treated samples were tested using Student’s t-test. p-values below 0.05 were considered as statistically significant.