Effect of Cholesterol on the Organic Cation Transporter OCTN1 (SLC22A4)
Abstract
:1. Introduction
2. Results
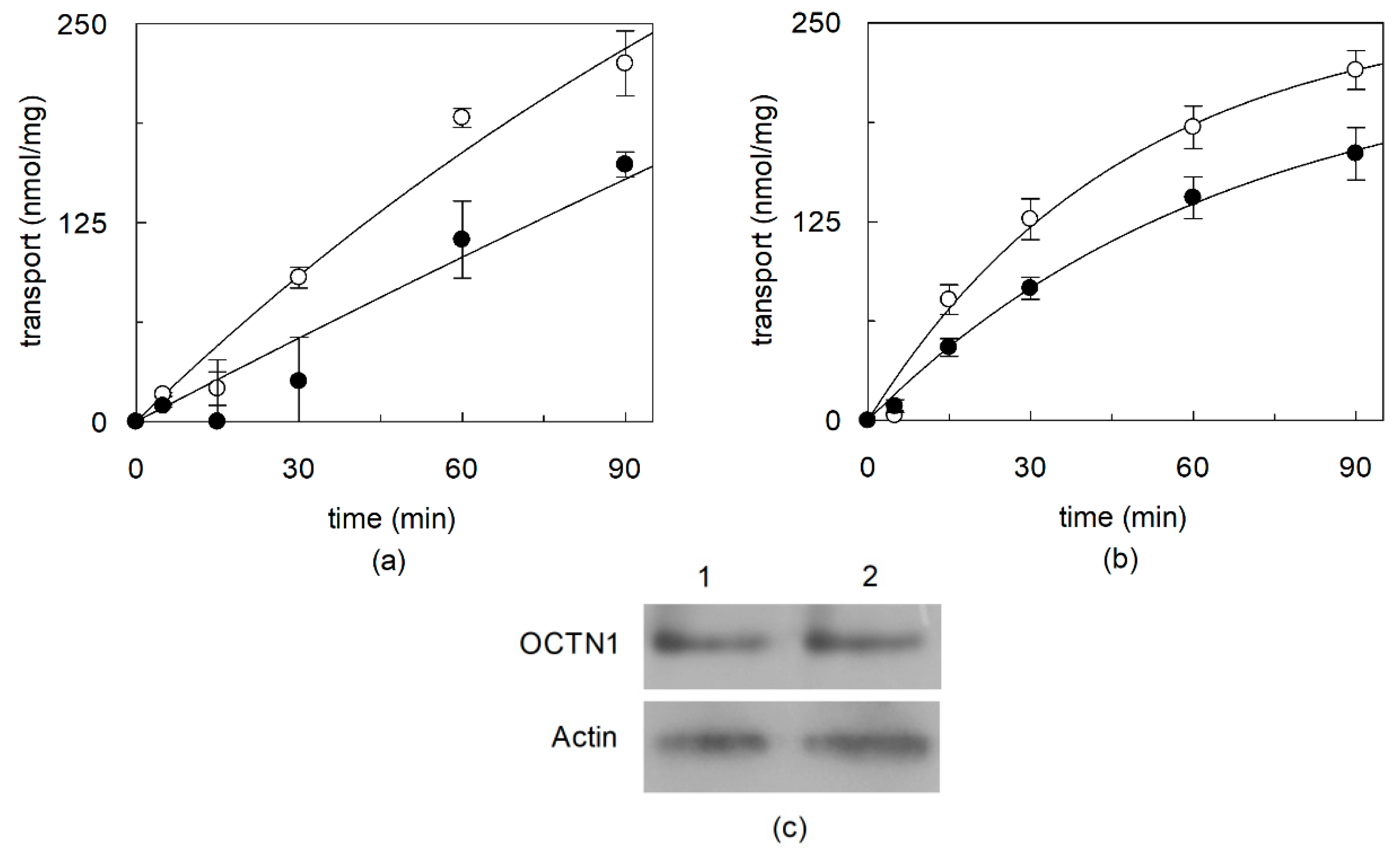
2.1. Effect of Cholesterol Removal on the Native or Recombinant OCTN1
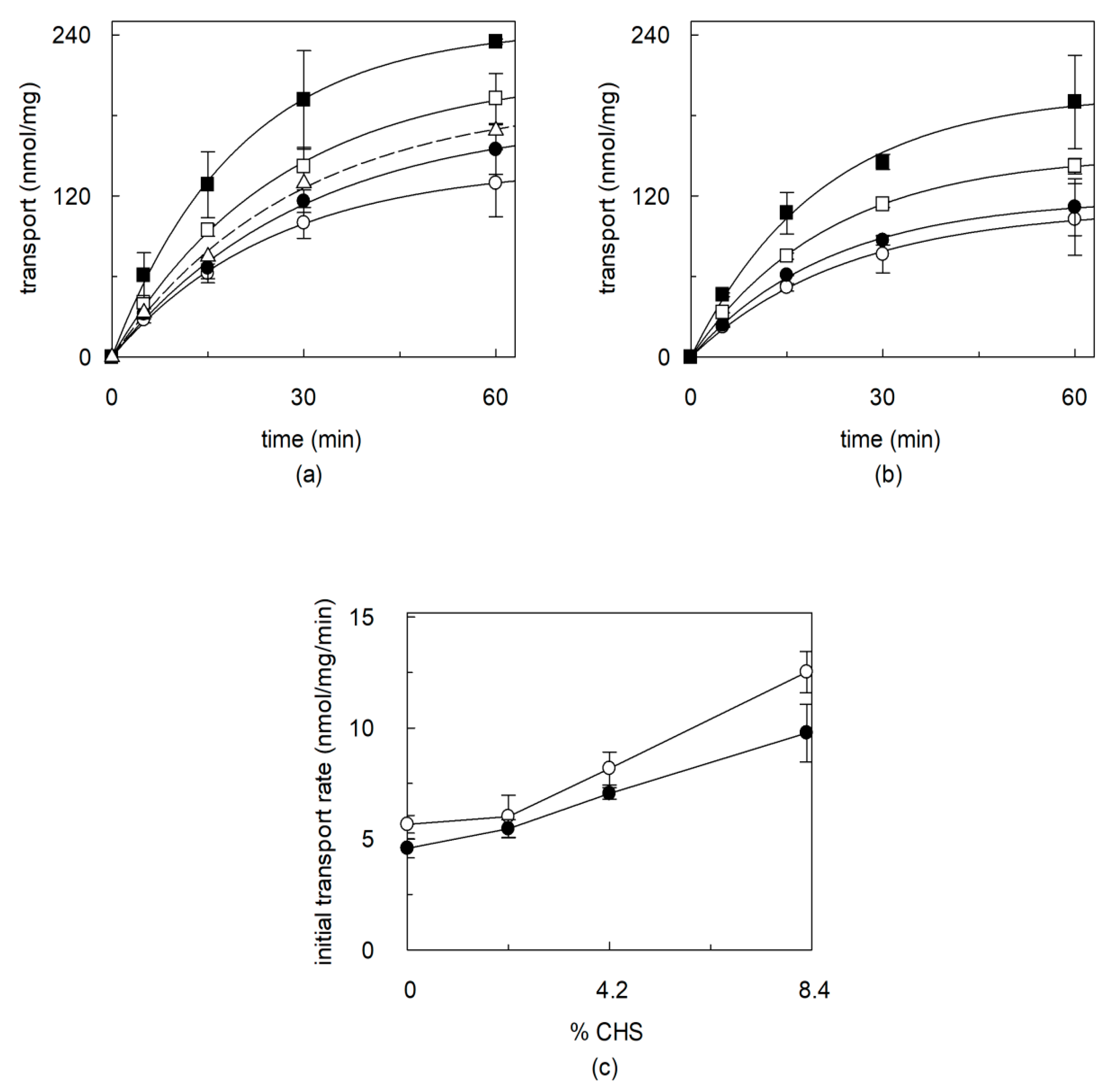
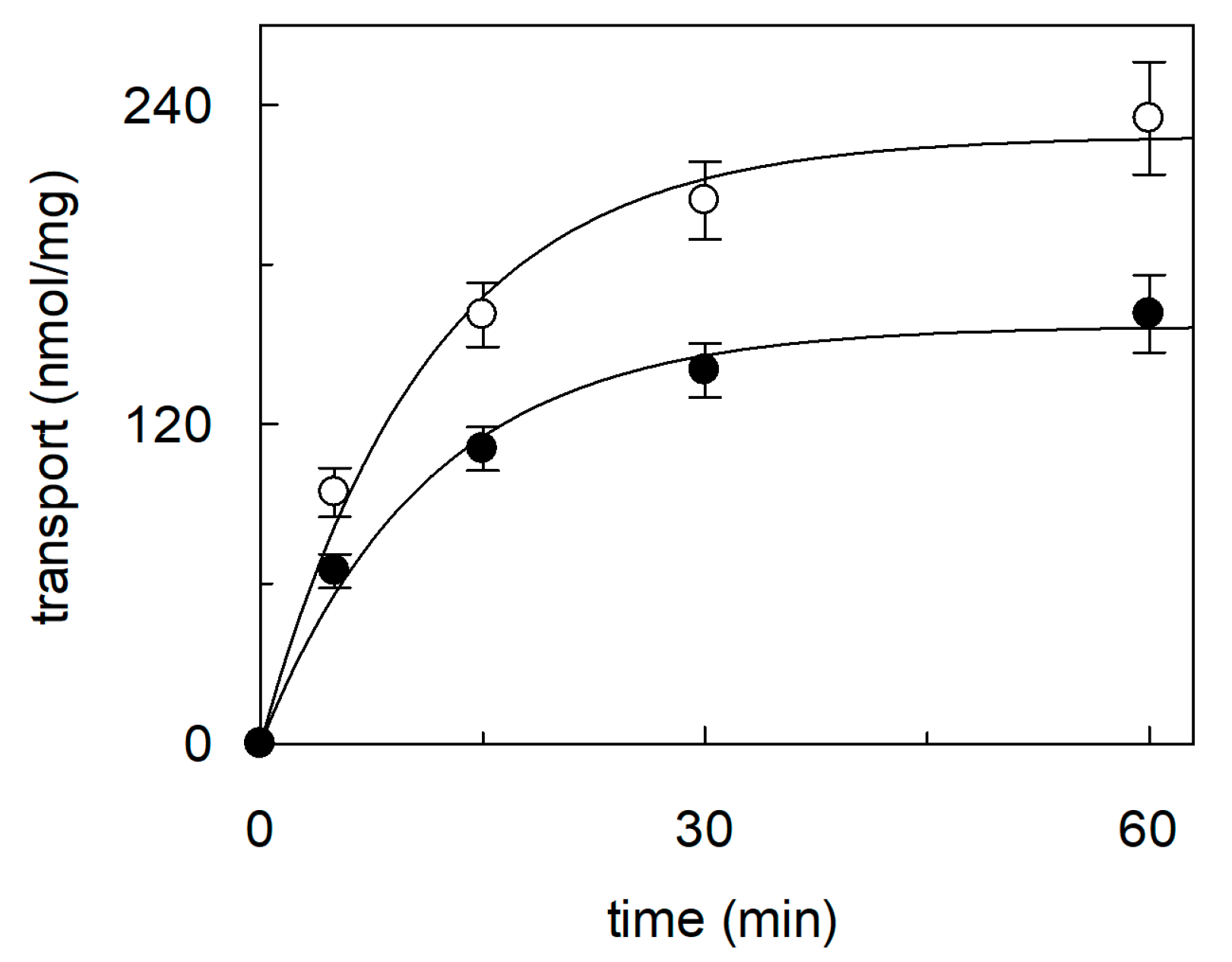
2.2. Dependence of CHS Addition on the Recombinant OCTN1
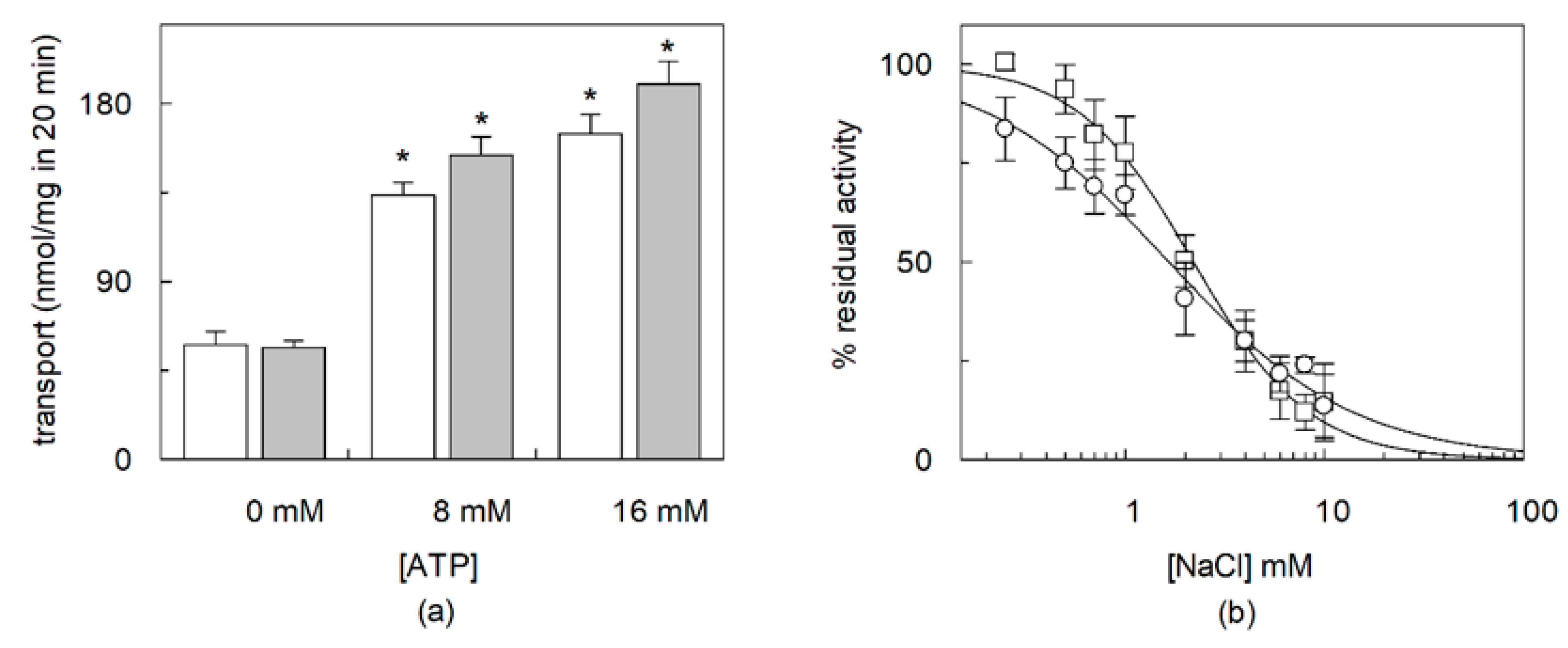
2.3. Effects of CHS on Regulation of OCTN1 by Physiological or Non-Physiological Effectors
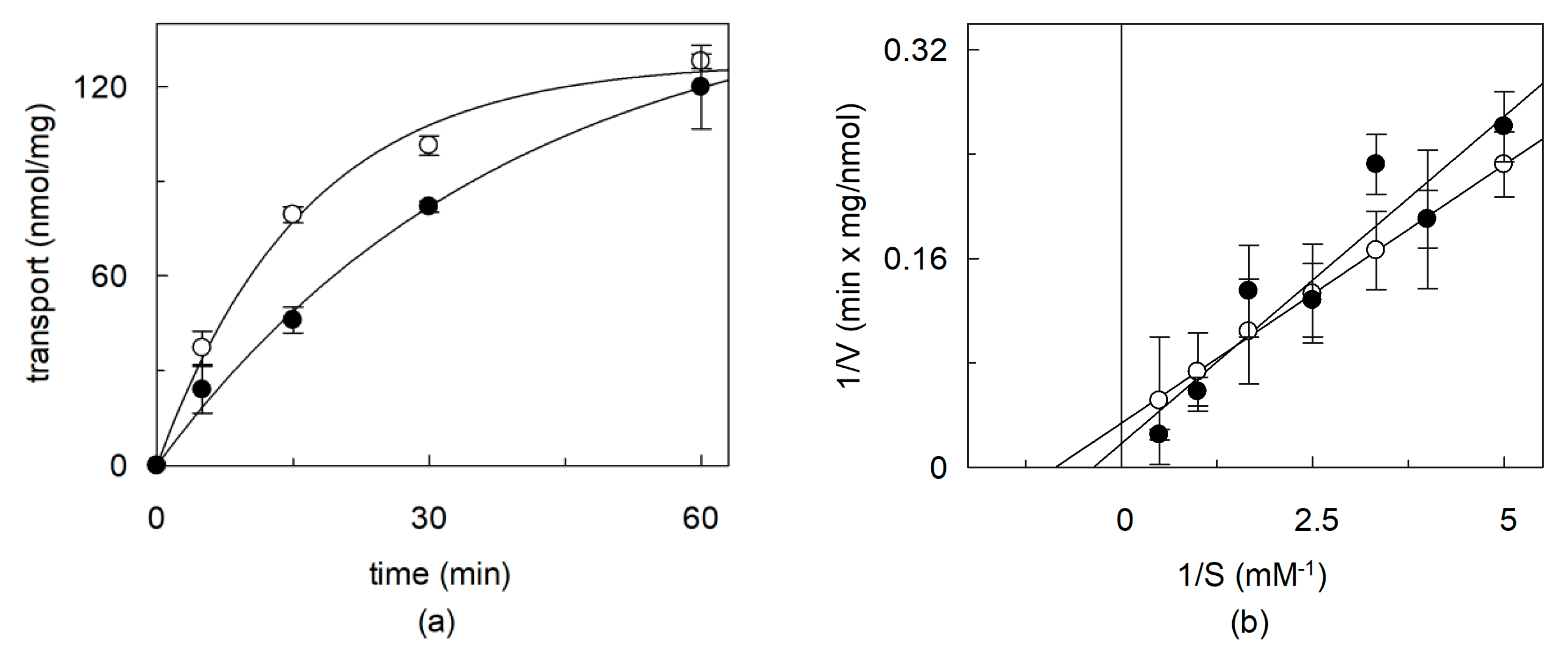
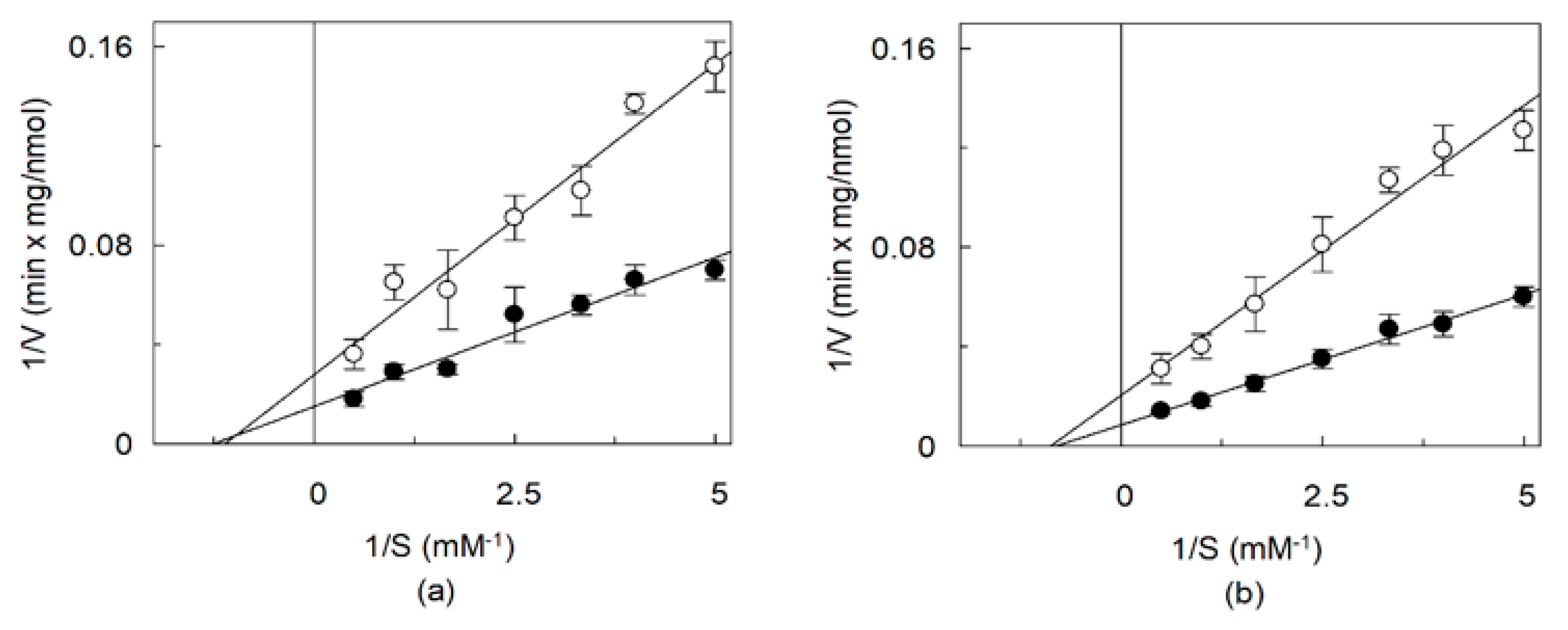
2.4. Effects of CHS on the Kinetics of OCTN1 Mediated Transport
2.5. In Silico Analysis of the Interaction of OCTN1 with Cholesterol
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. CHS Solubilization
4.3. Cell culture
4.4. Reconstitution of the OCTN1 Transporter from HeLa Cell Extract into Proteoliposomes
4.5. Reconstitution of the Recombinant OCTN1 Transporter into Liposomes
4.6. MβCD Treatment
4.7. Transport Measurements
4.8. Quantitation of Cholesterol
4.9. Electrophoretic and Western Blotting Analysis
4.10. Docking
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OCTN | Novel Organic Cation Transporters |
| SLC | SoLute Carrier |
| CHS | Cholesteryl HemiSuccinate |
| MβCD | Methyl-beta-CycloDextrin |
| DDM | n-Dodecyl-β-D-maltoside |
| TEA | Tetraethylammonium |
| TEBA | Benzyltriethylammonium |
References
- Tamai, I.; Ohashi, R.; Nezu, J.I.; Sai, Y.; Kobayashi, D.; Oku, A.; Shimane, M.; Tsuji, A. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J. Biol. Chem. 2000, 275, 40064–40072. [Google Scholar] [CrossRef] [Green Version]
- Eraly, S.A.; Monte, J.C.; Nigam, S.K. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol. Genom. 2004, 18, 12–24. [Google Scholar] [CrossRef]
- Mihaljevic, I.; Popovic, M.; Zaja, R.; Smital, T. Phylogenetic, syntenic, and tissue expression analysis of slc22 genes in zebrafish (Danio rerio). BMC Genom. 2016, 17, 626. [Google Scholar] [CrossRef] [Green Version]
- Scalise, M.; Galluccio, M.; Pochini, L.; Indiveri, C. Over-expression in Escherichia coli, purification and reconstitution in liposomes of the third member of the OCTN sub-family: The mouse carnitine transporter OCTN3. Biochem. Biophys. Res. Commun. 2012, 422, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Galluccio, M.; Scalise, M.; Console, L.; Indiveri, C. OCTN: A Small Transporter Subfamily with Great Relevance to Human Pathophysiology, Drug Discovery, and Diagnostics. SLAS Discov. 2019, 24, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. OCTN cation transporters in health and disease: Role as drug targets and assay development. J. Biomol. Screen. 2013, 18, 851–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pochini, L.; Scalise, M.; Di Silvestre, S.; Belviso, S.; Pandolfi, A.; Arduini, A.; Bonomini, M.; Indiveri, C. Acetylcholine and acetylcarnitine transport in peritoneum: Role of the SLC22A4 (OCTN1) transporter. Biochim. Biophys. 2016, 1858, 653–660. [Google Scholar] [CrossRef]
- Tamai, I.; Yabuuchi, H.; Nezu, J.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997, 419, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Yabuuchi, H.; Tamai, I.; Nezu, J.; Sakamoto, K.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J. Pharmacol. Exp. Ther. 1999, 289, 768–773. [Google Scholar]
- Peltekova, V.D.; Wintle, R.F.; Rubin, L.A.; Amos, C.I.; Huang, Q.; Gu, X.; Newman, B.; Van Oene, M.; Cescon, D.; Greenberg, G.; et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat. Genet. 2004, 36, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Tamai, I.; Nakanishi, T.; Kobayashi, D.; China, K.; Kosugi, Y.; Nezu, J.; Sai, Y.; Tsuji, A. Involvement of OCTN1 (SLC22A4) in pH-dependent transport of organic cations. Mol. Pharm. 2004, 1, 57–66. [Google Scholar] [CrossRef]
- Grundemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schomig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Kubo, Y.; Iwata, D.; Kato, S.; Sudo, T.; Sugiura, T.; Kagaya, T.; Wakayama, T.; Hirayama, A.; Sugimoto, M.; et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm. Res. 2010, 27, 832–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamichi, N.; Taguchi, T.; Hosotani, H.; Wakayama, T.; Shimizu, T.; Sugiura, T.; Iseki, S.; Kato, Y. Functional expression of carnitine/organic cation transporter OCTN1 in mouse brain neurons: Possible involvement in neuronal differentiation. Neurochem. Int. 2012, 61, 1121–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamichi, N.; Kato, Y. Physiological Roles of Carnitine/Organic Cation Transporter OCTN1/SLC22A4 in Neural Cells. Biol. Pharm. Bull. 2017, 40, 1146–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pochini, L.; Scalise, M.; Galluccio, M.; Pani, G.; Siminovitch, K.A.; Indiveri, C. The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn’s disease. Biochim. Biophys. 2012, 1818, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Amelio, L.; Indiveri, C. Reconstitution in liposomes of the functionally active human OCTN1 (SLC22A4) transporter overexpressed in Escherichia coli. Biochem. J. 2011, 439, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Regulation by physiological cations of acetylcholine transport mediated by human OCTN1 (SLC22A4). Implications in the non-neuronal cholinergic system. Life Sci. 2012, 91, 1013–1016. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef] [Green Version]
- Kummer, W.; Krasteva-Christ, G. Non-neuronal cholinergic airway epithelium biology. Curr. Opin. Pharmacol. 2014, 16, 43–49. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Indiveri, C. Immuno-detection of OCTN1 (SLC22A4) in HeLa cells and characterization of transport function. Int. Immunopharmacol. 2015, 29, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Furuichi, K.; Toyama, T.; Kitajima, S.; Hara, A.; Iwata, Y.; Sakai, N.; Shimizu, M.; Kaneko, S.; Isozumi, N.; et al. Impairment of the carnitine/organic cation transporter 1-ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nakamichi, N.; Hosotani, H.; Masuo, Y.; Sugiura, T.; Kato, Y. Organic cation transporter-mediated ergothioneine uptake in mouse neural progenitor cells suppresses proliferation and promotes differentiation into neurons. PLoS ONE 2014, 9, e89434. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nakamichi, N.; Nishijima, H.; Masuo, Y.; Kato, Y. Carnitine/Organic Cation Transporter OCTN1 Negatively Regulates Activation in Murine Cultured Microglial Cells. Neurochem. Res. 2018, 43, 107–119. [Google Scholar] [CrossRef]
- Masuo, Y.; Ohba, Y.; Yamada, K.; Al-Shammari, A.H.; Seba, N.; Nakamichi, N.; Ogihara, T.; Kunishima, M.; Kato, Y. Combination Metabolomics Approach for Identifying Endogenous Substrates of Carnitine/Organic Cation Transporter OCTN1. Pharm. Res. 2018, 35, 224. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Bai, M.; Li, C.; Lu, S.; Ma, Z.; Zhao, Y.; Zhou, H.; Jiang, H.; Sun, D.; Zheng, C. Multiple drug transporters contribute to the placental transfer of emtricitabine. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm. Drug Dispos. 2013, 34, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Weng, Y.; Wang, W.; Bai, M.; Lei, H.; Zhou, H.; Jiang, H. Multiple organic cation transporters contribute to the renal transport of sulpiride. Biopharm. Drug Dispos. 2017, 38, 526–534. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Z.; Zhou, S.; Weng, Y.; Lei, H.; Zeng, S.; Li, L.; Jiang, H. Multiple Drug Transporters Are Involved in Renal Secretion of Entecavir. Antimicrob. Agents Chemother. 2016, 60, 6260–6270. [Google Scholar] [CrossRef] [Green Version]
- Futatsugi, A.; Masuo, Y.; Kawabata, S.; Nakamichi, N.; Kato, Y. L503F variant of carnitine/organic cation transporter 1 efficiently transports metformin and other biguanides. J. Pharm. Pharmacol. 2016, 68, 1160–1169. [Google Scholar] [CrossRef]
- Nakamichi, N.; Shima, H.; Asano, S.; Ishimoto, T.; Sugiura, T.; Matsubara, K.; Kusuhara, H.; Sugiyama, Y.; Sai, Y.; Miyamoto, K.; et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J. Pharm. Sci. 2013, 102, 3407–3417. [Google Scholar] [CrossRef]
- Drenberg, C.D.; Gibson, A.A.; Pounds, S.B.; Shi, L.; Rhinehart, D.P.; Li, L.; Hu, S.; Du, G.; Nies, A.T.; Schwab, M.; et al. OCTN1 Is a High-Affinity Carrier of Nucleoside Analogues. Cancer Res. 2017, 77, 2102–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Kijima, A.; Masuo, Y.; Ishimoto, T.; Sugiura, T.; Takahashi, S.; Nakamichi, N.; Kato, Y. Gene ablation of carnitine/organic cation transporter 1 reduces gastrointestinal absorption of 5-aminosalicylate in mice. Biol. Pharm. Bull. 2015, 38, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Chan, T.; Zhu, L.; Yan, X.; Cao, Z.; Wang, Y.; Zhou, F. The inhibitory effects of camptothecin (CPT) and its derivatives on the substrate uptakes mediated by human solute carrier transporters (SLCs). Xenobiotica 2016, 46, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Dickens, D.; Chiduza, G.N.; Wright, G.S.; Pirmohamed, M.; Antonyuk, S.V.; Hasnain, S.S. Modulation of LAT1 (SLC7A5) transporter activity and stability by membrane cholesterol. Sci. Rep. 2017, 7, 43580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeppelin, T.; Ladefoged, L.K.; Sinning, S.; Periole, X.; Schiott, B. A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput. Biol. 2018, 14, e1005907. [Google Scholar] [CrossRef] [Green Version]
- Scalise, M.; Pochini, L.; Cosco, J.; Aloe, E.; Mazza, T.; Console, L.; Esposito, A.; Indiveri, C. Interaction of Cholesterol with the Human SLC1A5 (ASCT2): Insights Into Structure/Function Relationships. Front. Mol. Biosci. 2019, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Scalise, M.; Galluccio, M.; Pochini, L.; Cosco, J.; Trotta, M.; Rebsamen, M.; Superti-Furga, G.; Indiveri, C. Insights into the transport side of the human SLC38A9 transceptor. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1558–1567. [Google Scholar] [CrossRef]
- Hoermann, S.; Gai, Z.; Kullak-Ublick, G.A.; Visentin, M. Plasma membrane cholesterol regulates the allosteric binding of 1-methyl-4-phenylpyridinium (MPP+) to organic cation transporter 2 (OCT2, SLC22A2). J. Pharmacol. Exp. Ther. 2019, 372, 46–53. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, M.; Pochini, L.; Peta, V.; Ianni, M.; Scalise, M.; Indiveri, C. Functional and molecular effects of mercury compounds on the human OCTN1 cation transporter: C50 and C136 are the targets for potent inhibition. Toxicol. Sci. 2015, 144, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Listowski, M.A.; Leluk, J.; Kraszewski, S.; Sikorski, A.F. Cholesterol Interaction with the MAGUK Protein Family Member, MPP1, via CRAC and CRAC-Like Motifs: An In Silico Docking Analysis. PLoS ONE 2015, 10, e0133141. [Google Scholar] [CrossRef] [Green Version]
- Kulig, W.; Tynkkynen, J.; Javanainen, M.; Manna, M.; Rog, T.; Vattulainen, I.; Jungwirth, P. How well does cholesteryl hemisuccinate mimic cholesterol in saturated phospholipid bilayers? J. Mol. Model. 2014, 20, 2121. [Google Scholar] [CrossRef]
- Rog, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. 2009, 1788, 97–121. [Google Scholar] [CrossRef] [Green Version]
- Kulig, W.; Jurkiewicz, P.; Olzynska, A.; Tynkkynen, J.; Javanainen, M.; Manna, M.; Rog, T.; Hof, M.; Vattulainen, I.; Jungwirth, P. Experimental determination and computational interpretation of biophysical properties of lipid bilayers enriched by cholesteryl hemisuccinate. Biochim. Biophys. 2015, 1848, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, S.M.; Williams, D.C.; Schloss, P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry 2001, 40, 10507–10513. [Google Scholar] [CrossRef]
- Jones, K.T.; Zhen, J.; Reith, M.E. Importance of cholesterol in dopamine transporter function. J. Neurochem. 2012, 123, 700–715. [Google Scholar] [CrossRef] [Green Version]
- Indiveri, C.; Prezioso, G.; Dierks, T.; Kramer, R.; Palmieri, F. Kinetic characterization of the reconstituted dicarboxylate carrier from mitochondria: A four-binding-site sequential transport system. Biochim. Biophys. 1993, 1143, 310–318. [Google Scholar] [CrossRef]
- Indiveri, C.; Palmieri, L.; Palmieri, F. Kinetic characterization of the reconstituted ornithine carrier from rat liver mitochondria. Biochim. Biophys. 1994, 1188, 293–301. [Google Scholar] [CrossRef]
- Palmieri, F.; Klingenberg, M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol. 1979, 56, 279–301. [Google Scholar] [PubMed]
- Brizio, C.; Brandsch, R.; Bufano, D.; Pochini, L.; Indiveri, C.; Barile, M. Over-expression in Escherichia coli, functional characterization and refolding of rat dimethylglycine dehydrogenase. Protein Expr. Purif. 2004, 37, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochini, L.; Pappacoda, G.; Galluccio, M.; Pastore, F.; Scalise, M.; Indiveri, C. Effect of Cholesterol on the Organic Cation Transporter OCTN1 (SLC22A4). Int. J. Mol. Sci. 2020, 21, 1091. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031091
Pochini L, Pappacoda G, Galluccio M, Pastore F, Scalise M, Indiveri C. Effect of Cholesterol on the Organic Cation Transporter OCTN1 (SLC22A4). International Journal of Molecular Sciences. 2020; 21(3):1091. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031091
Chicago/Turabian StylePochini, Lorena, Gilda Pappacoda, Michele Galluccio, Francesco Pastore, Mariafrancesca Scalise, and Cesare Indiveri. 2020. "Effect of Cholesterol on the Organic Cation Transporter OCTN1 (SLC22A4)" International Journal of Molecular Sciences 21, no. 3: 1091. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031091







