Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation
Abstract
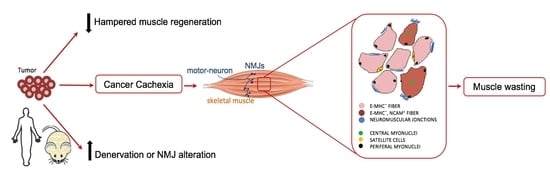
:1. Introduction
2. Results
2.1. Muscle Fibers with Central Nuclei in Adults do not Necessarily Show Markers of Regeneration
2.2. Central Nuclei are Clustered in Association with Neurological Perturbations in Cachexia
2.3. Tumor-Bearing Mice Show Signs of a Myopathy Associated with the Upregulation of Denervation Markers
2.4. Cancer Patients Show Signs of a Myopathy Associated with the Upregulation of Denervation Markers in Overt Cachexia
3. Discussion
4. Methods
4.1. Mice and Tumor Transplant
4.2. Histology and Immunofluorescence Analyses
4.3. Patients’ Characteristics
4.4. Histological Analysis of Human Muscle
4.5. Quantitative PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AchR | acetylcholine receptors |
| CC | cachectic cancer |
| CNM | central nuclear myopathies |
| HDAC | histone deacetylase |
| NCAM | N-cell adhesion molecule |
| RA | rectus Abdominis |
| TA | tibialis anterior |
| WSC | weight stable cancer |
| MUSK | muscle associated receptor tyrosine kinase |
References
- Tseng, B.S.; Kasper, C.E.; Edgerton, V.R. Cytoplasm-to-myonucleus ratios and succinate dehydrogenase activities in adult rat slow and fast muscle fibers. Cell Tissue Res. 1994, 275, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard, J.C.; Liestol, K.; Ekmark, M.; Kollstad, K.; Gundersen, K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J. Physiol. 2003, 551, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.M. Skeletal Muscles Do Not Undergo Apoptosis During Either Atrophy or Programmed Cell Death-Revisiting the Myonuclear Domain Hypothesis. Front. Physiol. 2018, 9, 1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiro, A.J.; Shy, G.M.; Gonatas, N.K. Myotubular myopathy. Persistence of fetal muscle in an adolescent boy. Arch. Neurol. 1966, 14, 1–14. [Google Scholar] [CrossRef]
- Mazzotti, A.L.; Coletti, D. The Need for a Consensus on the Locution "Central Nuclei" in Striated Muscle Myopathies. Front. Physiol. 2016, 7, 577. [Google Scholar] [CrossRef] [PubMed]
- Landisch, R.M.; Kosir, A.M.; Nelson, S.A.; Baltgalvis, K.A.; Lowe, D.A. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve 2008, 38, 1290–1303. [Google Scholar] [CrossRef] [Green Version]
- Capers, C.R. Multinucleation of skeletal muscle in vitro. J. Biophys. Biochem. Cytol. 1960, 7, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Coletti, D.; Teodori, L.; Lin, Z.; Beranudin, J.F.; Adamo, S. Restoration versus reconstruction: Cellular mechanisms of skin, nerve and muscle regeneration compared. Regen. Med. Res. 2013, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Cadot, B.; Gache, V.; Gomes, E.R. Moving and positioning the nucleus in skeletal muscle—One step at a time. Nucleus 2015, 6, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Bruusgaard, J.C.; Liestol, K.; Gundersen, K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 2006, 100, 2024–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, T.; Gache, V.; Xu, M.; Cadot, B.; Folker, E.S.; Richardson, B.E.; Gomes, E.R.; Baylies, M.K. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature 2012, 484, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. (Edinb. Scotl.) 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Coletti, D.; Adamo, S. Highlights on Cachexia, from the 4th Cachexia Conference. Tampa (FL), 6-9 Dec 2007. Eur. J. Transl. Myol. 2008, 18, 109–114. [Google Scholar]
- Blum, D.; Omlin, A.; Fearon, K.; Baracos, V.; Radbruch, L.; Kaasa, S.; Strasser, F.; European Palliative Care Research Centre. Evolving classification systems for cancer cachexia: Ready for clinical practice? Support Care Cancer 2010, 18, 273–279. [Google Scholar] [CrossRef]
- Coletti, D. Chemotherapy-induced muscle wasting: An update. Eur. J. Transl. Myol. 2018, 28, 7587. [Google Scholar] [CrossRef] [Green Version]
- Berardi, E.; Aulino, P.; Murfuni, I.; Toschi, A.; Padula, F.; Scicchitano, B.M.; Coletti, D.; Adamo, S. Skeletal muscle is enriched in hematopoietic stem cells and not inflammatory cells in cachectic mice. Neurol. Res. 2008, 30, 160–169. [Google Scholar] [CrossRef]
- Coletti, D.; Aulino, P.; Pigna, E.; Barteri, F.; Moresi, V.; Annibali, D.; Adamo, S.; Berardi, E. Spontaneous Physical Activity Downregulates Pax7 in Cancer Cachexia. Stem Cells Int. 2016, 2016, 6729268. [Google Scholar] [CrossRef] [Green Version]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. NF-kappaB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef] [Green Version]
- Carotenuto, F.; Coletti, D.; Di Nardo, P.; Teodori, L. Alpha-Linolenic Acid Reduces TNF-Induced Apoptosis in C2C12 Myoblasts by Regulating Expression of Apoptotic Proteins. Eur. J. Transl. Myol. 2016, 26, 6033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carotenuto, F.; Costa, A.; Albertini, M.C.; Rocchi, M.B.; Rudov, A.; Coletti, D.; Minieri, M.; Di Nardo, P.; Teodori, L. Dietary Flaxseed Mitigates Impaired Skeletal Muscle Regeneration: In Vivo, in Vitro and in Silico Studies. Int. J. Med Sci. 2016, 13, 206–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Bennett, A.W.; Zhang, P.; Barrow, K.R.; Kearney, S.R.; Hankey, K.G.; Taylor-Howell, C.; Gibbs, A.M.; Smith, C.P.; MacVittie, T.J. A non-human primate model of radiation-induced cachexia. Sci. Rep. 2016, 6, 23612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampieri, S.; Doria, A.; Adami, N.; Biral, D.; Vecchiato, M.; Savastano, S.; Corbianco, S.; Carraro, U.; Merigliano, S. Subclinical myopathy in patients affected with newly diagnosed colorectal cancer at clinical onset of disease: Evidence from skeletal muscle biopsies. Neurol. Res. 2010, 32, 20–25. [Google Scholar] [CrossRef]
- Moresi, V.; Pristera, A.; Scicchitano, B.M.; Molinaro, M.; Teodori, L.; Sassoon, D.; Adamo, S.; Coletti, D. Tumor necrosis factor-alpha inhibition of skeletal muscle regeneration is mediated by a caspase-dependent stem cell response. Stem Cells (Dayt. Ohio) 2008, 26, 997–1008. [Google Scholar] [CrossRef]
- Moresi, V.; Garcia-Alvarez, G.; Pristera, A.; Rizzuto, E.; Albertini, M.C.; Rocchi, M.; Marazzi, G.; Sassoon, D.; Adamo, S.; Coletti, D. Modulation of caspase activity regulates skeletal muscle regeneration and function in response to vasopressin and tumor necrosis factor. PLoS ONE 2009, 4, e5570. [Google Scholar] [CrossRef]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Drey, M.; Krieger, B.; Sieber, C.C.; Bauer, J.M.; Hettwer, S.; Bertsch, T.; Group, D.S. Motoneuron loss is associated with sarcopenia. J. Am. Med Dir. Assoc. 2014, 15, 435–439. [Google Scholar] [CrossRef]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortes-Lopez, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. eLife 2017, 6, e26464. [Google Scholar] [CrossRef]
- Carraro, U.; Kern, H. Severely Atrophic Human Muscle Fibers with Nuclear Misplacement Survive Many Years of Permanent Denervation. Eur. J. Transl. Myol. 2016, 26, 5894. [Google Scholar] [CrossRef]
- Aulino, P.; Berardi, E.; Cardillo, V.; Rizzuto, E.; Perniconi, B.; Ramina, C. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer 2010, 10, 363. [Google Scholar] [CrossRef]
- Toschi, A.; Severi, A.; Coletti, D.; Catizone, A.; Musaro, A.; Molinaro, M.; Nervi, C.; Adamo, S.; Scicchitano, B.M. Skeletal muscle regeneration in mice is stimulated by local overexpression of V1a-vasopressin receptor. Mol. Endocrinol. 2011, 25, 1661–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veratti, E. Investigations on the fine structure of striated muscle fiber read before the Reale Istituto Lombardo, 13 March 1902. J. Biophys. Biochem. Cytol. 1961, 10, 1–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralston, E.; Lu, Z.; Biscocho, N.; Soumaka, E.; Mavroidis, M.; Prats, C.; Lomo, T.; Capetanaki, Y.; Ploug, T. Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J. Cell. Physiol. 2006, 209, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, S.F.; Jaspers, R.T.; Degens, H. Is the myonuclear domain size fixed? J. Musculoskelet. Neuronal Interact. 2011, 11, 286–297. [Google Scholar]
- Pavlath, G.K.; Rich, K.; Webster, S.G.; Blau, H.M. Localization of muscle gene products in nuclear domains. Nature 1989, 337, 570–573. [Google Scholar] [CrossRef]
- Folker, E.S.; Schulman, V.K.; Baylies, M.K. Muscle length and myonuclear position are independently regulated by distinct Dynein pathways. Development 2012, 139, 3827–3837. [Google Scholar] [CrossRef] [Green Version]
- Folker, E.S.; Baylies, M.K. Nuclear positioning in muscle development and disease. Front. Physiol. 2013, 4, 363. [Google Scholar] [CrossRef] [Green Version]
- Grady, R.M.; Starr, D.A.; Ackerman, G.L.; Sanes, J.R.; Han, M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2005, 102, 4359–4364. [Google Scholar] [CrossRef] [Green Version]
- Brosig, M.; Ferralli, J.; Gelman, L.; Chiquet, M.; Chiquet-Ehrismann, R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int. J. Biochem. Cell Biol. 2010, 42, 1717–1728. [Google Scholar] [CrossRef]
- Coletti, D.; Daou, N.; Hassani, M.; Li, Z.; Parlakian, A. Serum Response Factor in Muscle Tissues: From Development to Ageing. Eur. J. Transl. Myol. 2016, 26, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norwood, F.; de Visser, M.; Eymard, B.; Lochmuller, H.; Bushby, K.; Force, E.G.T. EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. Eur. J. Neurol. 2007, 14, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.B.; Bitoun, M. Centronuclear myopathies. Semin. Pediatric Neurol. 2011, 18, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wanek, L.J.; Snow, M.H. Presence of embryonic myosin in normal postural muscles of the adult rat. Cell Tissue Res. 1995, 280, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. The need to more precisely define aspects of skeletal muscle regeneration. Int. J. Biochem. Cell Biol. 2014, 56, 56–65. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Biral, D.; Kern, H.; Adami, N.; Boncompagni, S.; Protasi, F.; Carraro, U. Atrophy-resistant fibers in permanent peripheral denervation of human skeletal muscle. Neurol. Res. 2008, 30, 137–144. [Google Scholar] [CrossRef]
- Carraro, U.; Boncompagni, S.; Gobbo, V.; Rossini, K.; Zampieri, S.; Mosole, S.; Ravara, B.; Nori, A.; Stramare, R.; Ambrosio, F.; et al. Persistent Muscle Fiber Regeneration in Long Term Denervation. Past, Present, Future. Eur. J. Transl. Myol. 2015, 25, 4832. [Google Scholar] [CrossRef]
- Carraro, U.; Coletti, D.; Kern, H. The Ejtm Specials “The Long-Term Denervated Muscle”. Eur. J. Transl. Myol. 2014, 24, 3292. [Google Scholar] [CrossRef]
- Slettebo, M.; Lindboe, C.F.; Askevold, F. The neuromuscular system in patients with anorexia nervosa: Electrophysiological and histologic studies. Clin. Neuropathol. 1984, 3, 217–224. [Google Scholar]
- Shefer, G.; Van de Mark, D.P.; Richardson, J.B.; Yablonka-Reuveni, Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006, 294, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepple, R.T. Muscle atrophy is not always sarcopenia. J. Appl. Physiol. 2012, 113, 677–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, D.D.; Pimenta, C.A.; Caponero, R. Fatigue in colorectal cancer patients: Prevalence and associated factors. Rev. Lat. Am. De Enferm. 2012, 20, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Models Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [Green Version]
- Fouladiun, M.; Korner, U.; Gunnebo, L.; Sixt-Ammilon, P.; Bosaeus, I.; Lundholm, K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin. Cancer Res. 2007, 13, 6379–6385. [Google Scholar] [CrossRef] [Green Version]
- Coletti, D.; Moresi, V.; Adamo, S.; Molinaro, M.; Sassoon, D. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 2005, 43, 120–128. [Google Scholar] [CrossRef]
- Penna, F.; Costamagna, D.; Fanzani, A.; Bonelli, G.; Baccino, F.M.; Costelli, P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS ONE 2010, 5, e13604. [Google Scholar] [CrossRef]
- Hiroux, C.; Vandoorne, T.; Koppo, K.; De Smet, S.; Hespel, P.; Berardi, E. Physical Activity Counteracts Tumor Cell Growth in Colon Carcinoma C26-Injected Muscles: An Interim Report. Eur. J. Transl. Myol. 2016, 26, 5958. [Google Scholar] [CrossRef] [Green Version]






| Control | WSC | CC | |
|---|---|---|---|
| Number of patients | 3 | 10 | 13 |
| Gender (M / F) | 2 / 1 | 5 / 5 | 11 / 2 |
| Age (years) | 47.3 ± 8.2 | 62.3 ± 3.7 | 63.7 ± 3.7 |
| Height (m) | 1.56 ± 0.03 | 1.64 ± 0.03 | 1.67 ± 0.02 |
| Weight at diagnosis (kg) | 68.2 ± 9.1 | 65.1 ± 4.4 | 62.1 ± 3.5 |
| BMI (kg/m2) | 27.2 ± 3.9 | 24.1 ± 1.4 | 22.1 ± 0.9 |
| Weight loss (%) | 0 ± 0.5 ** | 3.4 ± 2.2 * | 16.7 ± 3.3 |
| Tumor location (stomach / intestine) | NA | 4 / 6 | 4 / 9 |
| Tumor staging | |||
| I | NA | 4 | 2 |
| II | NA | 3 | 3 |
| III | NA | 2 | 2 |
| IV | NA | 1 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daou, N.; Hassani, M.; Matos, E.; De Castro, G.S.; Galvao Figueredo Costa, R.; Seelaender, M.; Moresi, V.; Rocchi, M.; Adamo, S.; Li, Z.; et al. Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation. Int. J. Mol. Sci. 2020, 21, 1092. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031092
Daou N, Hassani M, Matos E, De Castro GS, Galvao Figueredo Costa R, Seelaender M, Moresi V, Rocchi M, Adamo S, Li Z, et al. Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation. International Journal of Molecular Sciences. 2020; 21(3):1092. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031092
Chicago/Turabian StyleDaou, Nissrine, Medhi Hassani, Emidio Matos, Gabriela Salim De Castro, Raquel Galvao Figueredo Costa, Marilia Seelaender, Viviana Moresi, Marco Rocchi, Sergio Adamo, Zhenlin Li, and et al. 2020. "Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation" International Journal of Molecular Sciences 21, no. 3: 1092. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031092