Dynamic Transcriptome Analysis of Anther Response to Heat Stress during Anthesis in Thermotolerant Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Thermotolerance Assay of SDWG005 and 9311 in Growth Chamber
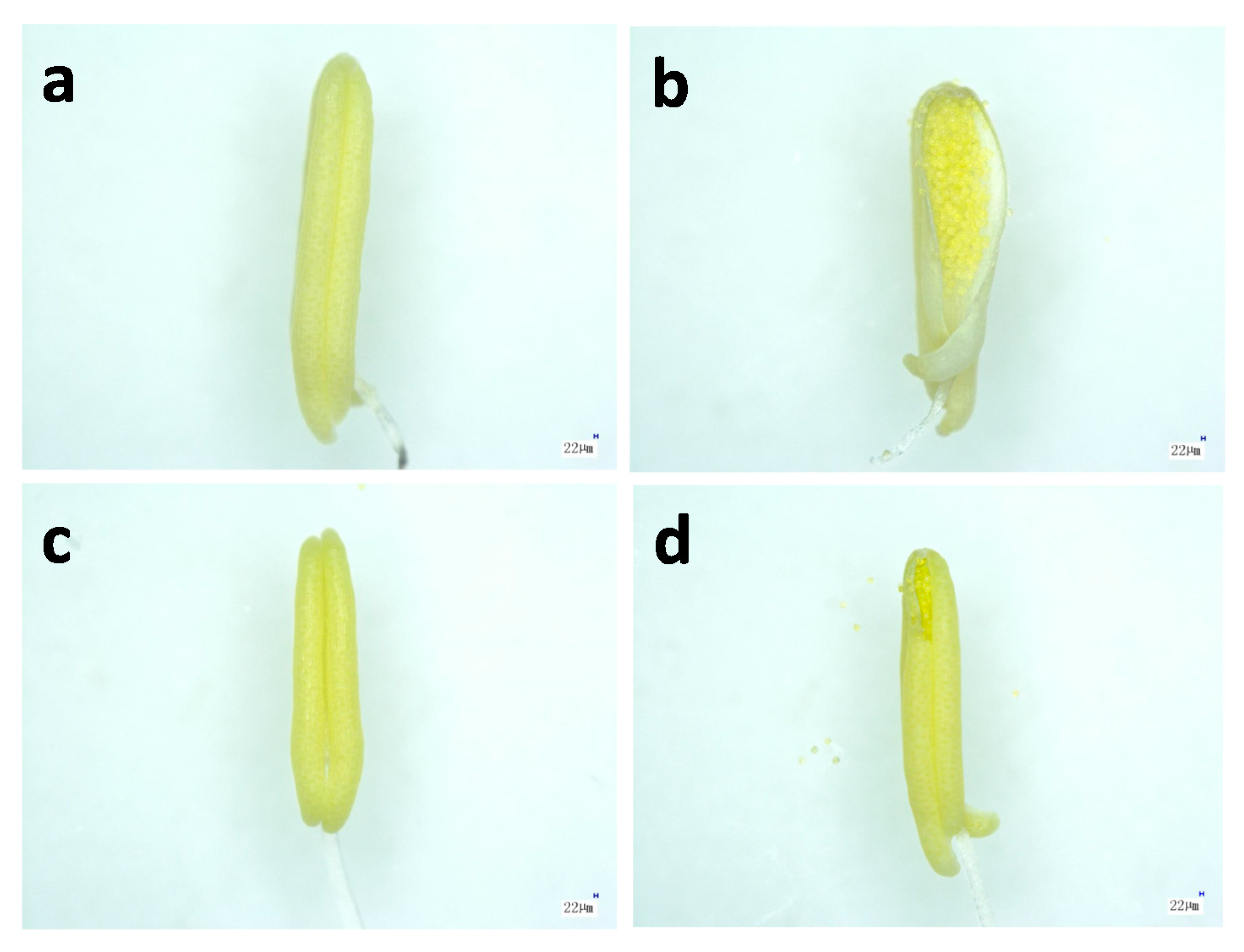
2.2. Microstructure of Anthers in Thermotolerant SDWG005 under Heat Stress
2.3. Quality of RNA-Seq Data
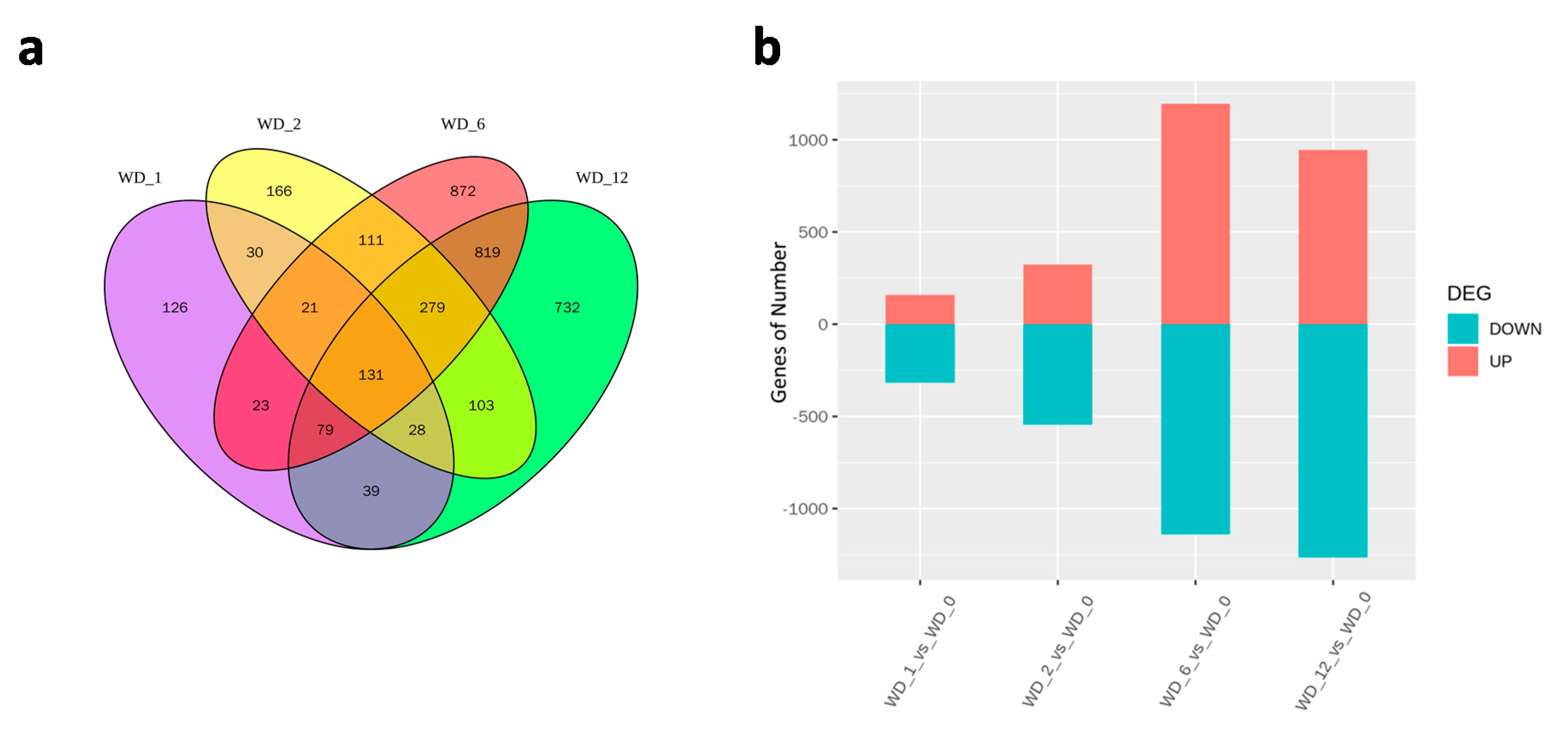
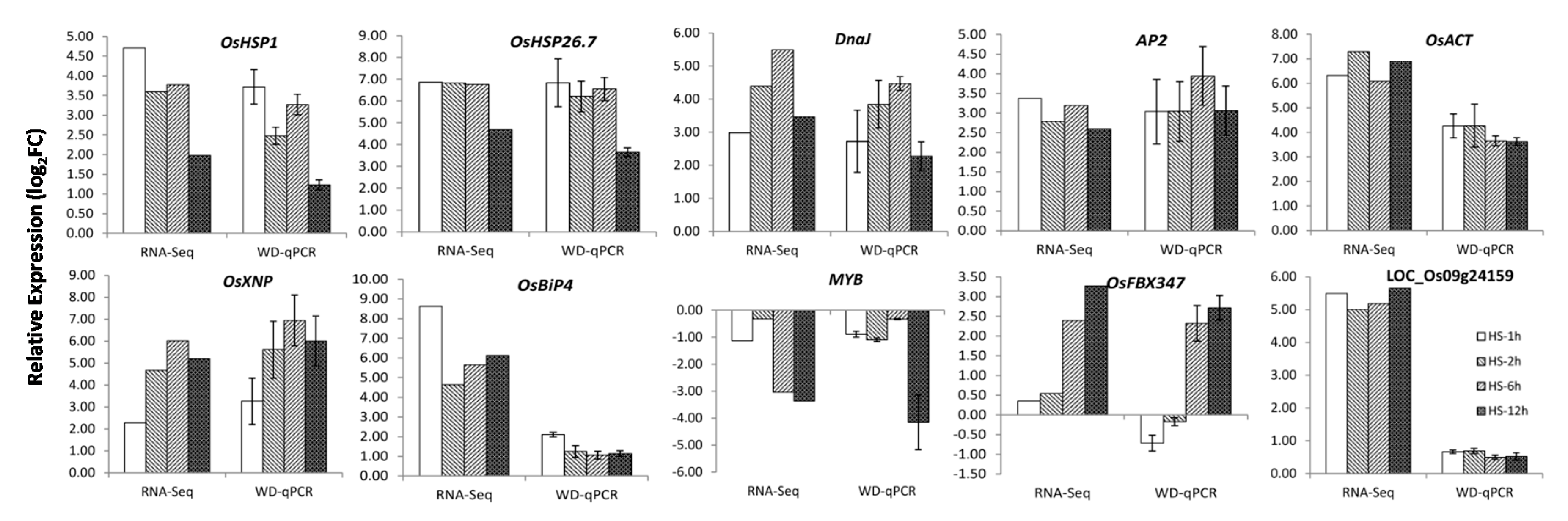
2.4. Identification and Validation of DEGs
2.5. DEGs in Response to Different Heat Treatment Exposure
2.6. Genes Involved in Multiple Biological Processes Are Responsive to Heat Stress
2.6.1. Transcription Factors
2.6.2. Nucleic Acid Synthesis and Modification
2.6.3. Protein Synthesis and Posttranslational Modification
2.6.4. Physiological Processes Involved in the Heat Stress Response in Rice Anthers
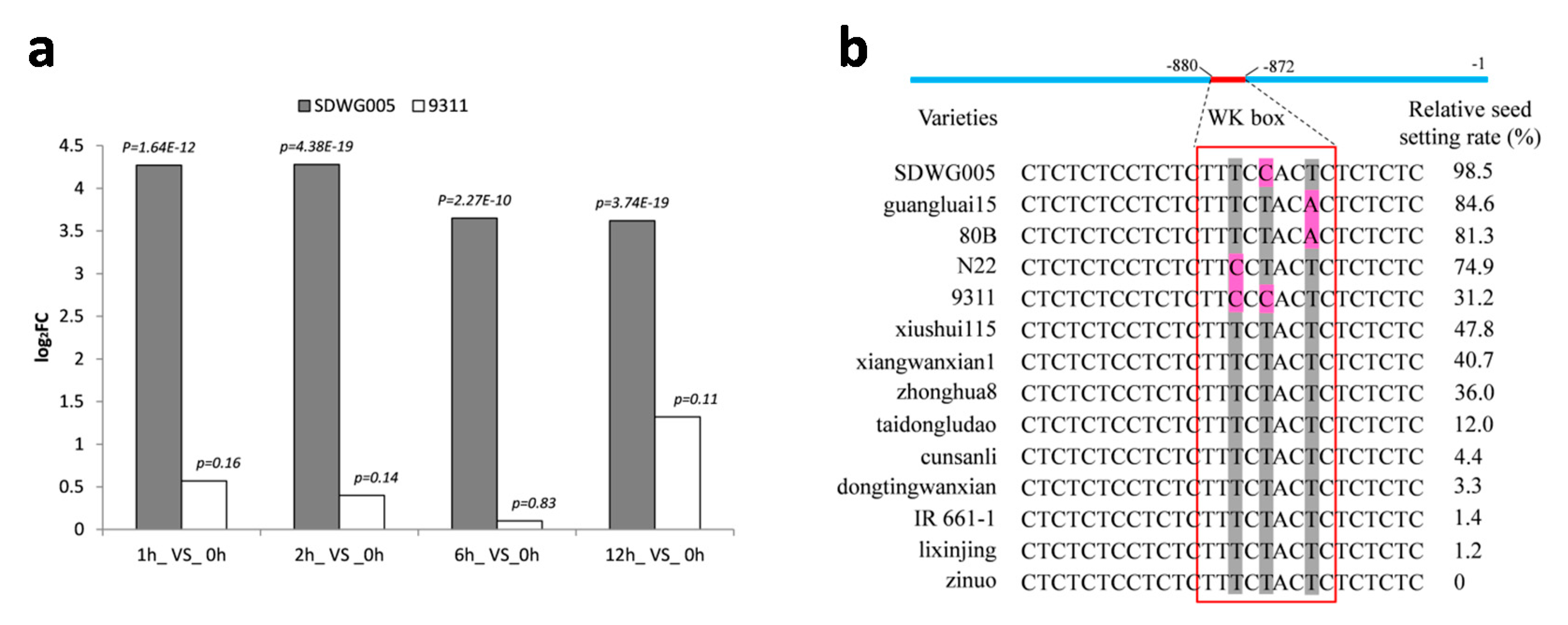
2.7. Characterization of Anther Specific Gene OsACT
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Treatments
4.2. Microscopic Observations of Rice Anthers
4.3. Seed Setting Rate
4.4. Sample Collection for RNA-Seq and Real-Time PCR
4.5. Total RNA Extraction and RNA Sequencing
4.6. Quality Control and Comparative Analysis of RNA-seq Data
4.7. Differential Expression Analysis
4.8. Quantitative Real-Time PCR
4.9. Gene Functional Annotation and Enrichment Analysis
4.10. Cloning and Sequence Analysis of OsACT Gene in Rice
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittal, D.; Madhyastha, D.A.; Grover, A. Genome-wide transcriptional profiles during temperature and oxidative stress reveal coordinated expression patterns and overlapping regulons in rice. PLoS ONE 2012, 7, e40899. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Lawas, L.M.F.; Raju, B.R.; Jagadish, S.V.K. Acquired thermo-tolerance and trans-generational heat stress response at flowering in rice. J. Agron. Crop Sci. 2016, 202, 309–319. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; BBroome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci. 2008, 48, 1140–1146. [Google Scholar] [CrossRef]
- Coast, O.; Murdoch, A.J.; Ellis, R.H.; Hay, F.R.; Jagadish, K.S.V. Resilience of rice (Oryza spp.) pollen germination and tube growth to temperature stress. Plant Cell Environ. 2016, 39, 26–37. [Google Scholar] [CrossRef]
- Satake, T.; Yoshida, S. High temperature-induced sterility in indica rices at flowering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Omasa, K. Rice (Oryza sativa L.) Cultivars tolerant to high temperature at flowering: Anther characteristics. Ann. Bot. 2002, 89, 683–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, T.; Kagata, H. Characteristics of floral organs related to reliable self-pollination in rice (Oryza sativa L.). Ann. Bot. 2003, 91, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Sheehy, J.E.; Thomas, J.M.G. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Qin, D.; Wu, H.; Peng, H.; Yao, Y.; Ni, Z.; Li, Z.; Zhou, C.; Sun, Q. Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using Wheat Genome Array. BMC Genom. 2008, 9, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bita, C.E.; Zenoni, S.; Vriezen, W.H.; Mariani, C.; Pezzotti, M.; Gerats, T. Temperature stress differentially modulates transcription in meiotic anthers of heat-tolerant and heat-sensitive tomato plants. BMC Genom. 2011, 12, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginzberg, I.; Barel, G.; Ophir, R.; Tzin, E.; Tanami, Z.; Muddarangappa, T.; De Jong, W.; Fogelman, E. Transcriptomic profiling of heat-stress response in potato periderm. J. Exp. Bot. 2009, 60, 4411. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.L.; Zhou, Q.; Wang, Y.Y.; Wang, W.E.; Bao, M.Z.; Zhang, J.W. Identification of heat-responsive genes in carnation (Dianthus caryophyllus L.) by RNA-seq. Front. Plant Sci. 2015, 6, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.; Tsuchiya, T.; Hamada, K.; Kawamura, S.; Yano, K.; Ohshima, M.; Higashitani, A.; Watanabe, M.; Kawagishi-Kobayashi, M. High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 2009, 50, 1911–1922. [Google Scholar] [CrossRef]
- González-Schain, N.; Dreni, L.; Lawas, L.M.F.; Galbiati, M.; Colombo, L.; Heuer, S.; Jagadish, K.S.V.; Kater, M.M. Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 2016, 57, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lawas, L.M.F.; Malo, R.; Glaubitz, U.; Erban, A.; Mauleon, R.; Heuer, S.; Zuther, E.; Kopka, J.; Hincha, D.K.; et al. Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ. 2015, 38, 2171–2192. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Liu, A.; Zou, J.; Zhou, X.; Xiang, J.; Rerksiri, W.; Peng, Y.; Xiong, X.; Chen, X. Expression profile in rice panicle: Insights into heat response mechanism at reproductive stage. PLoS ONE 2012, 7, e49652. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Rerksiri, W.; Liu, A.; Zhou, X.; Xiong, H.; Xiang, J.; Chen, X.; Xiong, X. Transcriptome profile reveals heat response mechanism at molecular and metabolic levels in rice flag leaf. Gene 2013, 530, 185–192. [Google Scholar] [CrossRef]
- Zha, Z.; Yin, D.; Wan, B.; Jiao, C. An analysis on the heat resistance of rice germplasm resources during flowering period. Asian Agric. Res. 2016, 55, 17–23. (In Chinese) [Google Scholar]
- Van Verk, M.C.; Neeleman, L.; Bol, J.F.; Linthorst, H.J.M. Tobacco transcription factor NtWRKY12 interacts with TGA2.2 in vitro and in vivo. Front. Plant Sci. 2011, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Vikram, P.; Swamy, B.P.M.; Dixit, S.; Ahmed, H.U.; Teresa Sta Cruz, M.; Singh, A.K.; Kumar, A. qDTY 1.1, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet. 2011, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Tenorio, F.A.; Redoña, E.D.; Morales-Cortezano, P.S.; Cabrega, G.A.; Jagadish, K.S.V.; Gregorio, G.B. Fine-mapping and validating qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice. Theor. Appl. Genet. 2015, 128, 1507–1517. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Raveendran, M.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Dong, X.; Yi, H.; Lee, J.; Nou, I.S.; Han, C.T.; Hur, Y. Global gene-expression analysis to identify differentially expressed genes critical for the heat stress response in brassica rapa. PLoS ONE 2015, 10, e0130451. [Google Scholar] [CrossRef]
- Frey, F.P.; Urbany, C.; Hüttel, B.; Reinhardt, R.; Stich, B. Genome-wide expression profiling and phenotypic evaluation of European maize inbreds at seedling stage in response to heat stress. BMC Genom. 2015, 16, 123. [Google Scholar] [CrossRef] [Green Version]
- Mangelsen, E.; Kilian, J.; Harter, K.; Jansson, C.; Wanke, D.; Sundberg, E. Transcriptome analysis of high-temperature stress in developing barley caryopses: Early stress responses and effects on storage compound biosynthesis. Mol. Plant 2011, 4, 97–115. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Kim, Y.K.; Grover, A. Coexpression network analysis associated with call of rice seedlings for encountering heat stress. Plant Mol. Biol. 2014, 84, 125–143. [Google Scholar] [CrossRef]
- Boston, R.S.; Viitanen, P.V.; Vierling, E. Molecular chaperones and protein folding in plants. Plant Mol. Biol. 1996, 32, 191–222. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [Green Version]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Grover, A.; Mittal, D.; Negi, M.; Lavania, D. Generating high temperature tolerant transgenic plants: Achievements and challenges. Plant Sci. 2013, 205, 38–47. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, J.C. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bassard, J.E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Grienenberger, E.; Besseau, S.; Geoffroy, P.; Debayle, D.; Heintz, D.; Lapierre, C.; Pollet, B.; Heitz, T.; Legrand, M. A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J. 2009, 58, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Onkokesung, N.; Gaquerel, E.; Kotkar, H.; Kaur, H.; Baldwin, I.T.; Galis, I. MYB8 controls inducible phenolamide levels by activating three novel hydroxycinnamoyl-coenzyme A: Polyamine transferases in Nicotiana attenuata. Plant Physiol. 2012, 158, 389–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, Q.; Dong, Q.; Zhang, Z.; Lin, C.; Hu, W.; Pan, R.; Guan, Y.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Routine procedure for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Los Banos, Philippines, 1976; pp. 53–57. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Schulze, S.K.; Kanwar, R.; Gölzenleuchter, M.; Therneau, T.M.; Beutler, A.S. SERE: Single-parameter quality control and sample comparison for RNA-Seq. BMC Genom. 2012, 13, 524. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, L.; Li, Y.; Hu, Q.; Zhu, L.; Gao, W.; Wu, Y.; Ding, Y.; Liu, S.; Yang, X.; Zhang, X. Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol. 2014, 164, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]




| Gene ID | WD_1 | WD_2 | WD_6 | WD_12 | Functional Annotation | ||||
|---|---|---|---|---|---|---|---|---|---|
| log2FC | p Value | log2FC | p Value | log2FC | p Value | log2FC | p Value | ||
| LOC_Os11g13980 | 7.07 | 8.01 × 10−5 | 7.39 | 4.48 × 10−18 | 8.09 | 2.01 × 10−31 | 8.05 | 1.87 × 10−29 | hsp20 |
| LOC_Os03g14180 | 6.86 | 2.02 × 10−17 | 6.83 | 1.65 × 10−134 | 6.77 | 2.44 × 10−99 | 4.69 | 9.23 × 10−32 | hsp20 |
| LOC_Os04g01740 | 4.71 | 2.27 × 10−28 | 3.60 | 1.00 × 10−38 | 3.77 | 4.50 × 10−43 | 1.97 | 9.91 × 10−9 | hsp90 |
| LOC_Os04g36750 | 3.57 | 3.35 × 10−22 | 2.60 | 3.67 × 10−18 | 1.64 | 2.63 × 10−5 | 2.13 | 6.51 × 10−5 | hsp20 |
| LOC_Os02g52150 | 3.40 | 5.69 × 10−32 | 3.00 | 4.65 × 10−40 | 2.56 | 3.06 × 10−24 | 3.08 | 2.04 × 10−5 | hsp20 |
| LOC_Os04g45480 | 3.08 | 9.62 × 10−21 | 2.58 | 5.52 × 10−54 | 2.10 | 5.23 × 10−20 | 1.42 | 5.63 × 10−6 | uncharacterized protein |
| LOC_Os03g16920 | 2.05 | 5.14 × 10−7 | 1.93 | 1.36 × 10−18 | 2.35 | 2.28 × 10−20 | 2.63 | 1.54 × 10−12 | hsp70 |
| LOC_Os04g28420 | 2.41 | 1.36 × 10−41 | 1.63 | 5.46 × 10−21 | 1.22 | 4.19 × 10−8 | – | – | 70 kDa peptidyl-prolyl isomerase |
| LOC_Os02g15930 | 1.94 | 1.90 × 10−15 | 1.07 | 2.10 × 10−8 | 1.42 | 1.23 × 10−16 | – | – | uncharacterized protein |
| LOC_Os01g08860 | 3.05 | 1.20 × 10−28 | 1.88 | 5.74 × 10−24 | – | – | −1.71 | 2.36 × 10−9 | hsp20 |
| LOC_Os11g05170 | 2.76 | 1.00 × 10−11 | – | – | −2.15 | 5.63 × 10−5 | −2.14 | 1.20 × 10−3 | uncharacterized protein |
| LOC_Os11g32890 | 2.56 | 6.08 × 10−20 | – | – | −1.68 | 7.10 × 10−7 | −1.87 | 1.65 × 10−7 | uncharacterized protein |
| LOC_Os02g54140 | 1.95 | 2.28 × 10−7 | – | – | −1.80 | 8.55 × 10−6 | −3.68 | 1.23 × 10−17 | hsp20 |
| LOC_Os01g55270 | 1.37 | 3.70 × 10−12 | – | – | −1.31 | 1.22 × 10−14 | −1.26 | 1.24 × 10−13 | calcyclin-binding protein |
| LOC_Os01g04370 | 2.85 | 2.73 × 10−17 | 1.98 | 7.64 × 10−19 | – | – | – | – | hsp20 |
| LOC_Os03g16020 | 2.09 | 2.88 × 10−7 | 1.02 | 3.61 × 10−5 | – | – | – | – | hsp20 |
| LOC_Os05g46480 | – | – | – | – | −2.58 | 1.42 × 10−16 | −3.56 | 1.35 × 10−27 | late embryogenesis abundant protein |
| LOC_Os03g16030 | 3.98 | 1.97 × 10−3 | – | – | – | – | – | – | hsp20 |
| LOC_Os03g16040 | 2.20 | 5.80 × 10−12 | – | – | – | – | – | – | hsp20 |
| LOC_Os01g18080 | – | – | −1.11 | 1.33×10−5 | – | – | – | – | uncharacterized protein |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zha, Z.; Cai, H.; Qin, D.; Jia, H.; Liu, C.; Qiu, D.; Zhang, Z.; Wan, Z.; Yang, Y.; et al. Dynamic Transcriptome Analysis of Anther Response to Heat Stress during Anthesis in Thermotolerant Rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1155. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031155
Liu G, Zha Z, Cai H, Qin D, Jia H, Liu C, Qiu D, Zhang Z, Wan Z, Yang Y, et al. Dynamic Transcriptome Analysis of Anther Response to Heat Stress during Anthesis in Thermotolerant Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2020; 21(3):1155. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031155
Chicago/Turabian StyleLiu, Gang, Zhongping Zha, Haiya Cai, Dandan Qin, Haitao Jia, Changyan Liu, Dongfeng Qiu, Zaijun Zhang, Zhenghuang Wan, Yuanyuan Yang, and et al. 2020. "Dynamic Transcriptome Analysis of Anther Response to Heat Stress during Anthesis in Thermotolerant Rice (Oryza sativa L.)" International Journal of Molecular Sciences 21, no. 3: 1155. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21031155




