High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy
Abstract
:1. Introduction
2. Results
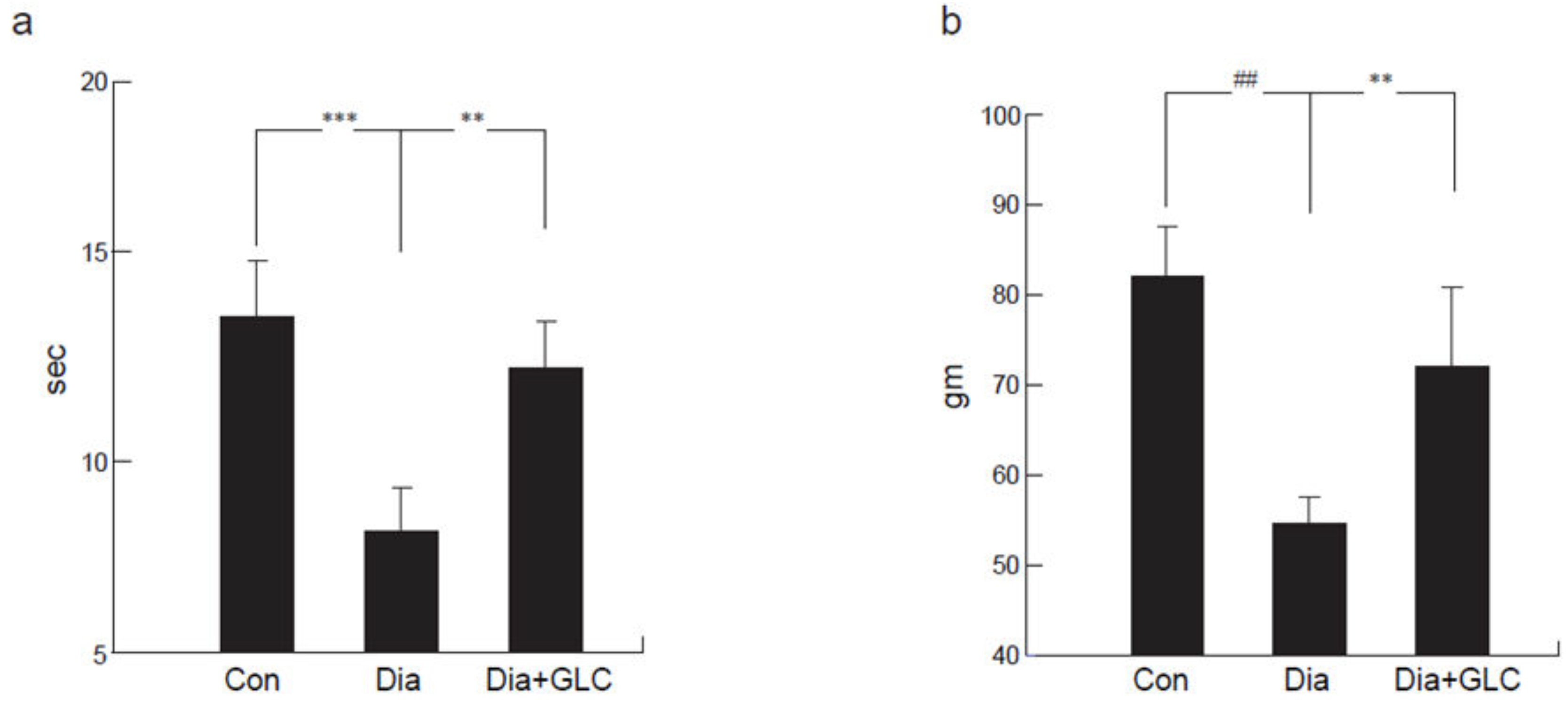
2.1. Type 2 Diabetic ZDF Animals Exhibited a Significant Change in Mechanical and Thermal Hyperalgesia after Treatment with HMGB1 Inhibitor
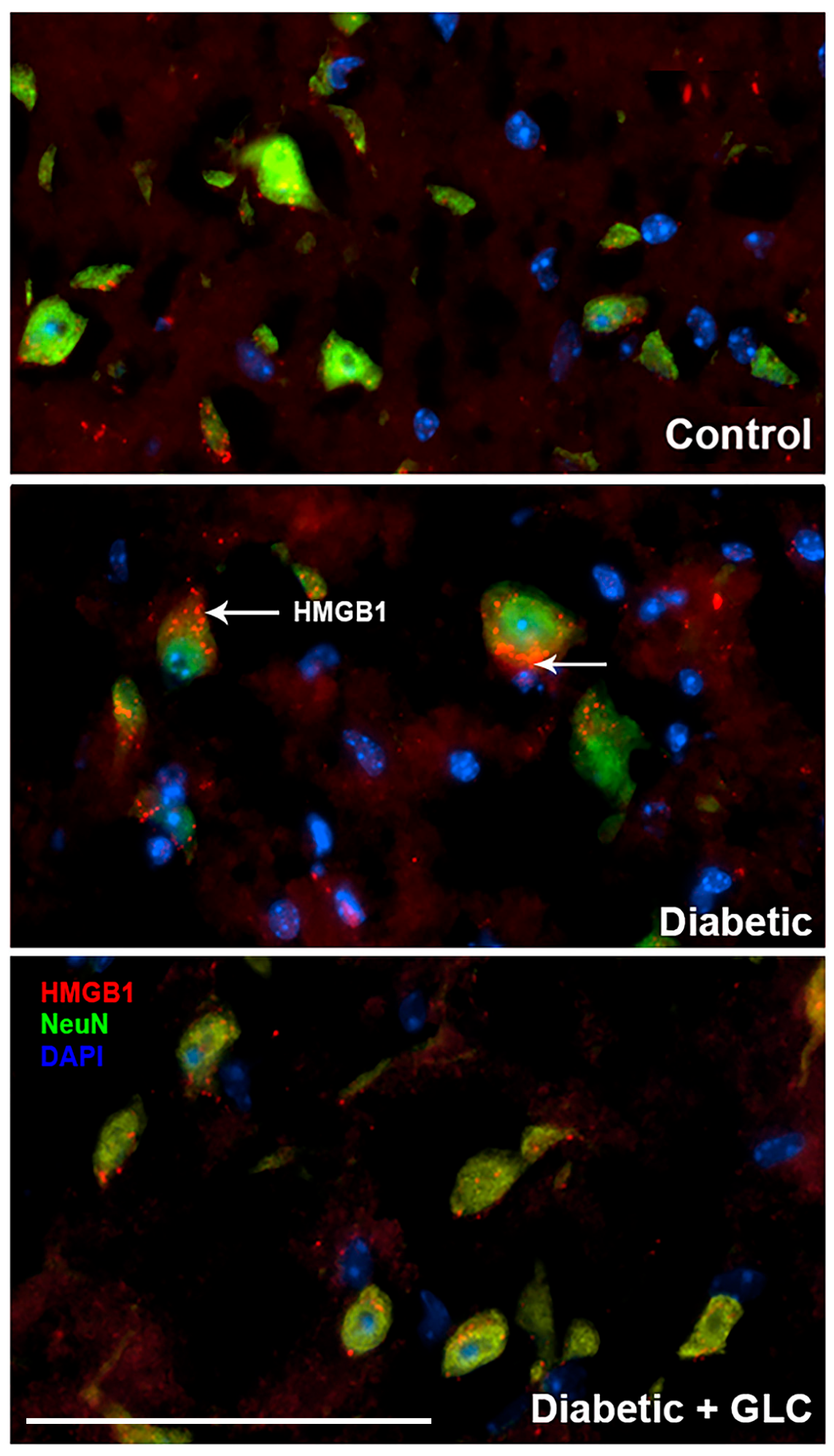
2.2. Increased Neuroinflammation in DRG of ZDF Rats with Painful Neuropathy was Ameliorated by Glycyrrhizin Treatment
2.3. Glycyrrhizin Prevents Hyperglycemia-Induced HMGB1 Cytoplasmic Relocation in DRG and Extracellular Release in Spinal Cord Neurons as well as Alleviates TRPC6 Expression
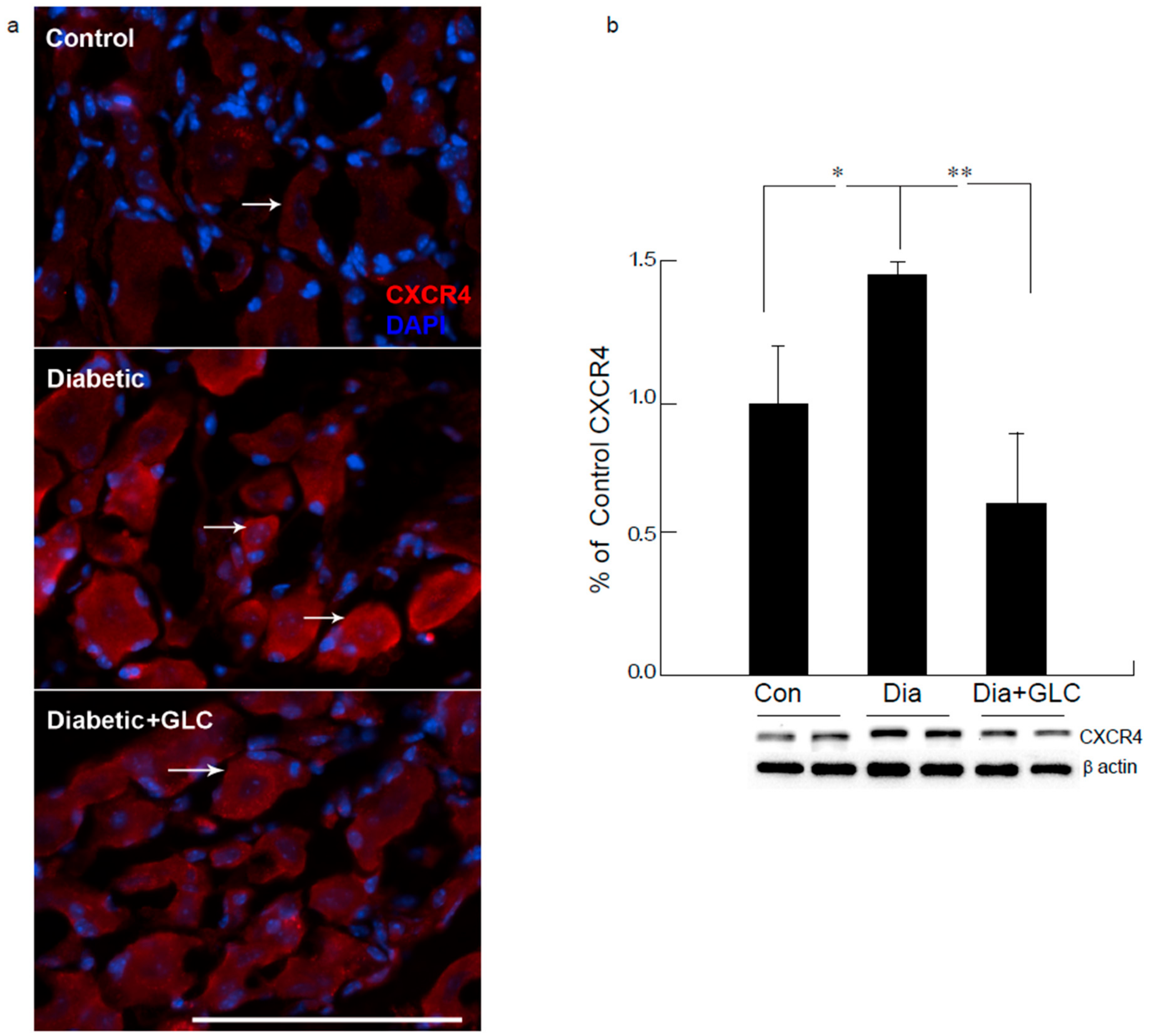
2.4. GLC Inhibited Hyperglycemia Induced Activation of JNK in Spinal Cord and CXCR4 Expression in DRG
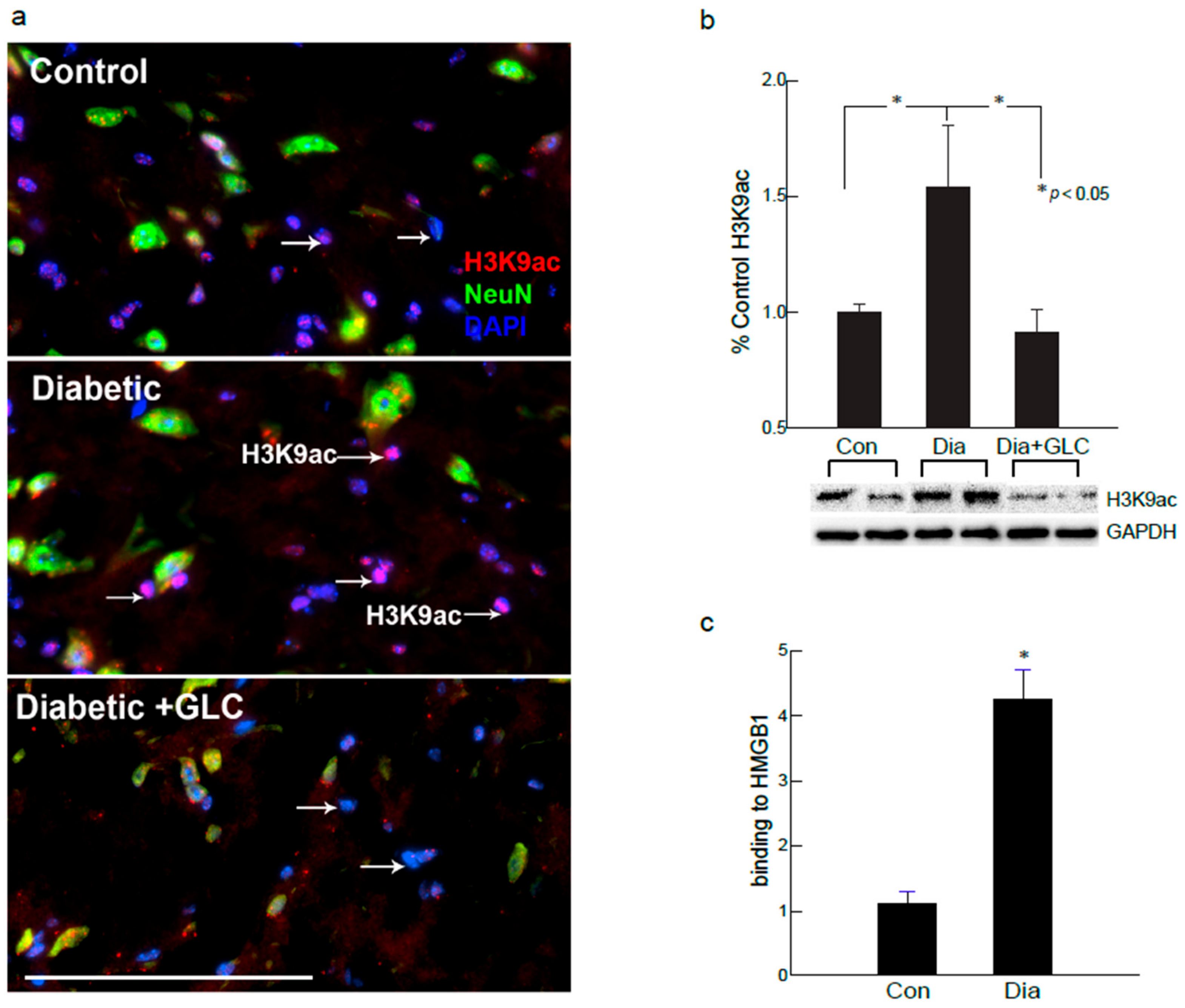
2.5. Global Acetylation of H3K9 was Increased in the Spinal Cord Dorsal Horn of Diabetic Animals as well as in the Hyperglycemic DRG Neuronal Cell Line
2.6. H3K9ac-Dependent Spinal HMGB1 Expression in Diabetic Animals with Painful Neuropathy
2.7. Hyperglycemic DRG Neuronal Cell Line Exhibited Increased HMGB1 Expression with Increased Global Acetylation of H3K9
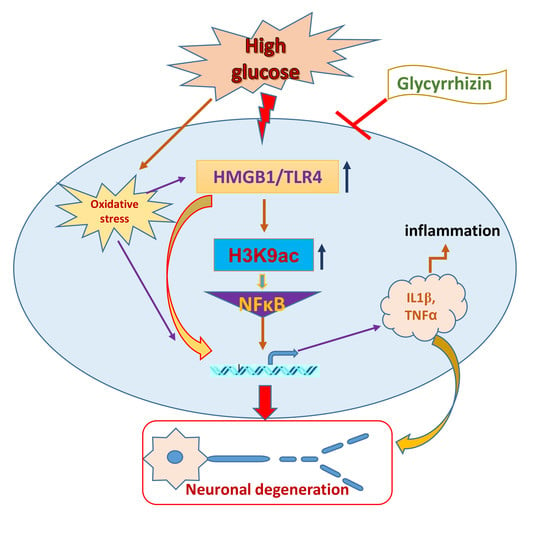
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Behavioral Studies
4.2.1. Thermal Hyperalgesia
4.2.2. Mechanical Hyperalgesia
4.3. Western Blot
4.4. Chromatin Immunoprecipitation (ChIP)
4.5. Immunohistochemistry
4.6. Comparative Analysis
4.7. F11 DRG Cell Culture
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DRG | dorsal root ganglia |
| HMGB1 | high mobility group box 1 |
| CXCR4 | chemokine C-X-C motif receptor 4 |
| RAGE | receptor for advanced glycation end products |
| TLR4 | Toll-like receptor 4 |
| NLRP3 | Nod-like receptor protein 3 |
| JNK | c-Jun N-terminal kinases |
| ZDF | Zucker diabetic fatty |
| GLC | Glycyrrhizin |
| H3K9ac | Histone 3 Lysine (K) 9 acetylation |
References
- Tavakoli, M.; Malik, R.A. Management of painful diabetic neuropathy. Expert Opin. Pharmacother. 2008, 9, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A. Effects of aldose reductase inhibitors on the progression of nerve fiber damage in diabetic neuropathy. J. Diabetes Complications 1992, 6, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Gordois, A.; Scuffham, P.; Shearer, A.; Oglesby, A.; Tobian, J.A. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003, 26, 1790–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahroni, J.H.; Boyko, E.J. Responsiveness of the SF-36 among veterans with diabetes mellitus. J. Diabetes Complications 2000, 14, 31–39. [Google Scholar] [CrossRef]
- Benbow, S.J.; Wallymahmed, M.E.; MacFarlane, I.A. Diabetic peripheral neuropathy and quality of life. QJM 1998, 91, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.T.; Brandle, M.; Zhou, H.; Marriott, D.; Burke, R.; Tabaei, B.P.; Engelgau, M.M.; Kaplan, R.M.; Herman, W.H. Valuing health-related quality of life in diabetes. Diabetes Care 2002, 25, 2238–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, M.; Brandenburg, N.A.; Dukes, E.; Hoffman, D.L.; Tai, K.S.; Stacey, B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manag. 2005, 30, 374–385. [Google Scholar] [CrossRef]
- Wang, F.C.; Pei, J.X.; Zhu, J.; Zhou, N.J.; Liu, D.S.; Xiong, H.F.; Liu, X.Q.; Lin, D.J.; Xie, Y. Overexpression of HMGB1 A-box reduced lipopolysaccharide-induced intestinal inflammation via HMGB1/TLR4 signaling in vitro. World J. Gastroenterol. 2015, 21, 7764–7776. [Google Scholar] [CrossRef]
- Zabini, D.; Crnkovic, S.; Xu, H.; Tscherner, M.; Ghanim, B.; Klepetko, W.; Olschewski, A.; Kwapiszewska, G.; Marsh, L.M. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J. Cell Mol. Med. 2015, 19, 1151–1161. [Google Scholar] [CrossRef]
- Ong, S.P.; Lee, L.M.; Leong, Y.F.; Ng, M.L.; Chu, J.J. Dengue virus infection mediates HMGB1 release from monocytes involving PCAF acetylase complex and induces vascular leakage in endothelial cells. PLoS ONE 2012, 7, e41932. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Wang, Z.; Liu, X.; Wu, B.; Wang, J.; Ward, T.; Zhang, L.; Ding, X.; Gibbons, G.; Shi, Y.; et al. EB1 acetylation by P300/CBP-associated factor (PCAF) ensures accurate kinetochore-microtubule interactions in mitosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16564–16569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evankovich, J.; Cho, S.W.; Zhang, R.; Cardinal, J.; Dhupar, R.; Zhang, L.; Klune, J.R.; Zlotnicki, J.; Billiar, T.; Tsung, A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J. Biol. Chem. 2010, 285, 39888–39897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Guo, L.; Collage, R.D.; Stripay, J.L.; Tsung, A.; Lee, J.S.; Rosengart, M.R. Calcium/calmodulin-dependent protein kinase (CaMK) Ialpha mediates the macrophage inflammatory response to sepsis. J. Leukoc. Biol. 2011, 90, 249–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agalave, N.M.; Larsson, M.; Abdelmoaty, S.; Su, J.; Baharpoor, A.; Lundback, P.; Palmblad, K.; Andersson, U.; Harris, H.; Svensson, C.I. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 2014, 155, 1802–1813. [Google Scholar] [CrossRef]
- Tanaka, J.; Yamaguchi, K.; Ishikura, H.; Tsubota, M.; Sekiguchi, F.; Seki, Y.; Tsujiuchi, T.; Murai, A.; Umemura, T.; Kawabata, A. Bladder pain relief by HMGB1 neutralization and soluble thrombomodulin in mice with cyclophosphamide-induced cystitis. Neuropharmacology 2014, 79, 112–118. [Google Scholar] [CrossRef]
- Tanaka, J.; Seki, Y.; Ishikura, H.; Tsubota, M.; Sekiguchi, F.; Yamaguchi, K.; Murai, A.; Umemura, T.; Kawabata, A. Recombinant human soluble thrombomodulin prevents peripheral HMGB1-dependent hyperalgesia in rats. Br. J. Pharmacol. 2013, 170, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Allette, Y.M.; Due, M.R.; Wilson, S.M.; Feldman, P.; Ripsch, M.S.; Khanna, R.; White, F.A. Identification of a functional interaction of HMGB1 with Receptor for Advanced Glycation End-products in a model of neuropathic pain. Brain Behav. Immun. 2014, 42, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Feldman, P.; Due, M.R.; Ripsch, M.S.; Khanna, R.; White, F.A. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J. Neuroinflamm. 2012, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Morioka, N.; Abe, H.; Zhang, F.F.; Hisaoka-Nakashima, K.; Liu, K.; Nishibori, M.; Nakata, Y. Neuropathic pain in rats with a partial sciatic nerve ligation is alleviated by intravenous injection of monoclonal antibody to high mobility group box-1. PLoS ONE 2013, 8, e73640. [Google Scholar] [CrossRef] [Green Version]
- Bhangoo, S.K.; Ren, D.; Miller, R.J.; Chan, D.M.; Ripsch, M.S.; Weiss, C.; McGinnis, C.; White, F.A. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav. Immun. 2007, 21, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Hu, X.M.; Liu, Y.N.; Han, Y.; Chen, L.P.; Wang, C.C.; Song, C. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J. Neuroinflamm. 2014, 11, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Tai, W.L.; Sun, L.; Qiu, Q.; Xia, Z.; Chung, S.K.; Cheung, C.W. Central administration of C-X-C chemokine receptor type 4 antagonist alleviates the development and maintenance of peripheral neuropathic pain in mice. PLoS ONE 2014, 9, e104860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.J.; Wang, L.; Zhang, B.; Gao, F.; Yang, C.M. Glycyrrhizin, an HMGB1 inhibitor, exhibits neuroprotective effects in rats after lithium-pilocarpine-induced status epilepticus. J. Pharm. Pharmacol. 2019, 71, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Soufy, H.; Yassein, S.; Ahmed, A.R.; Khodier, M.H.; Kutkat, M.A.; Nasr, S.M.; Okda, F.A. Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.Y.; Chu, Y.L.; Jiang, Z.B.; Chen, X.M.; Zhang, X.; Zeng, X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell Physiol. Biochem. 2014, 33, 375–388. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.T.; Wang, F.; Shao, M.; Wang, Y.; Zhu, H.Q. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vascul. Pharmacol. 2017, 88, 48–55. [Google Scholar] [CrossRef]
- Liu, R.; Luo, Q.; You, W.; Jin, M. MicroRNA-106 attenuates hyperglycemia-induced vascular endothelial cell dysfunction by targeting HMGB1. Gene 2018, 677, 142–148. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Aspects Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [Green Version]
- Shibasaki, M.; Sasaki, M.; Miura, M.; Mizukoshi, K.; Ueno, H.; Hashimoto, S.; Tanaka, Y.; Amaya, F. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain 2010, 149, 514–521. [Google Scholar] [CrossRef]
- Topalova, D.; Ugrinova, I.; Pashev, I.G.; Pasheva, E.A. HMGB1 protein inhibits DNA replication in vitro: A role of the acetylation and the acidic tail. Int. J. Biochem. Cell Biol. 2008, 40, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Yang, H.; Tracey, K.J.; Bustin, M.; Oppenheim, J.J. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 2007, 81, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002, 3, 995–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, M.E.; Manfredi, A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007, 220, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Palumbo, R.; Bianchi, M.E. HMGB1: A signal of necrosis. Autoimmunity 2007, 40, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, L.; Kanneganti, T.D.; Lamkanfi, M. HMGB1 release by inflammasomes. Virulence 2011, 2, 162–165. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wu, W.; Hu, Y.C.; Li, H.; Zhang, D.; Li, S.; Li, W.; Li, W.D.; Ma, B.; Zhu, J.H.; et al. Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J. Neuroinflamm. 2014, 11, 106. [Google Scholar] [CrossRef] [Green Version]
- Fang, P.; Schachner, M.; Shen, Y.Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012, 45, 499–506. [Google Scholar] [CrossRef]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Lin, J.J.; Du, Y.; Cai, W.K.; Kuang, R.; Chang, T.; Zhang, Z.; Yang, Y.X.; Sun, C.; Li, Z.Y.; Kuang, F. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci. Rep. 2015, 5, 12549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorina, R.; Font-Nieves, M.; Marquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006, 83, 711–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Li, J.; Qiang, X.; Czura, C.J.; Ochani, M.; Ochani, K.; Ulloa, L.; Yang, H.; Tracey, K.J.; Wang, P.; et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: An additional mechanism for its therapeutic effects in experimental sepsis. J. Lipid Res. 2005, 46, 623–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, R.; Zhang, Q.; Hou, W.; Yan, Z.; Chen, R.; Bonaroti, J.; Bansal, P.; Billiar, T.R.; Tsung, A.; Wang, Q.; et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 2014, 146, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Wang, H.; Yuan, R.; Li, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Czura, C.J.; Huston, J.M.; Miller, E.; et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006, 203, 1637–1642. [Google Scholar] [CrossRef] [Green Version]
- Bonaldi, T.; Talamo, F.; Scaffidi, P.; Ferrera, D.; Porto, A.; Bachi, A.; Rubartelli, A.; Agresti, A.; Bianchi, M.E. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003, 22, 5551–5560. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Sig Choi, J.; Yu, Y.M.; Nam, K.; Piao, C.S.; Kim, S.W.; Lee, M.H.; Han, P.L.; Park, J.S.; Lee, J.K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006, 26, 6413–6421. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Mi, Y.; Yang, H.; Hu, A.; Zhang, Q.; Shang, C. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Mol. Cell. Biochem. 2013, 380, 249–257. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamakuchi, M.; Biswas, K.K.; Aryal, B.; Yamada, S.; Hashiguchi, T.; Maruyama, I. HMGB1 is secreted by 3T3-L1 adipocytes through JNK signaling and the secretion is partially inhibited by adiponectin. Obesity 2016, 24, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Kratz, A.; Arner, E.; Saito, R.; Kubosaki, A.; Kawai, J.; Suzuki, H.; Carninci, P.; Arakawa, T.; Tomita, M.; Hayashizaki, Y.; et al. Core promoter structure and genomic context reflect histone 3 lysine 9 acetylation patterns. BMC Genomics 2010, 11, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.; Lin, J.C.; Wei, V.; Yoo, C.; Cheng, J.C.; Nguyen, C.T.; Weisenberger, D.J.; Egger, G.; Takai, D.; Gonzales, F.A.; et al. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc. Natl. Acad. Sci. USA 2004, 101, 7357–7362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, H.; Suzuki, T.; Kondo, S.; Miura, H.; Fujimura, Y.; Hayashizaki, Y. Histone H3 acetylated at lysine 9 in promoter is associated with low nucleosome density in the vicinity of transcription start site in human cell. Chromosome Res. 2006, 14, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sukumaran, P.; Bandyopadhyay, B.C.; Singh, B.B. Physiological Function and Characterization of TRPCs in Neurons. Cells 2014, 3, 455–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandri-Haber, N.; Dina, O.A.; Chen, X.; Levine, J.D. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 2009, 29, 6217–6228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.M.; Liu, Y.N.; Zhang, H.L.; Cao, S.B.; Zhang, T.; Chen, L.P.; Shen, W. CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J. Neurochem. 2015, 132, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar]
- Chattopadhyay, M.; Mata, M.; Fink, D.J. Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J. Neurosci. 2008, 28, 6652–6658. [Google Scholar] [CrossRef]
- Verdu, E.; Vilches, J.J.; Rodriguez, F.J.; Ceballos, D.; Valero, A.; Navarro, X. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve 1999, 22, 329–340. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, V.; Sadanandan, J.; Chattopadhyay, M. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. Int. J. Mol. Sci. 2020, 21, 881. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21030881
Thakur V, Sadanandan J, Chattopadhyay M. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. International Journal of Molecular Sciences. 2020; 21(3):881. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21030881
Chicago/Turabian StyleThakur, Vikram, Jayanarayanan Sadanandan, and Munmun Chattopadhyay. 2020. "High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy" International Journal of Molecular Sciences 21, no. 3: 881. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21030881