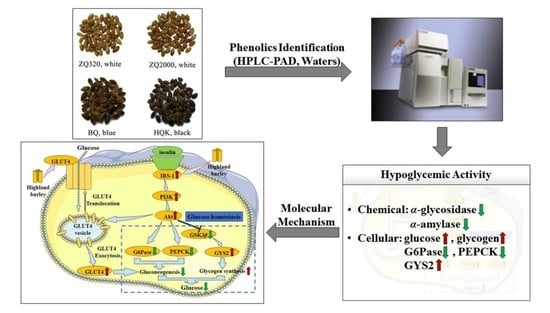
Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Moisture Content of Highland Barley Varieties
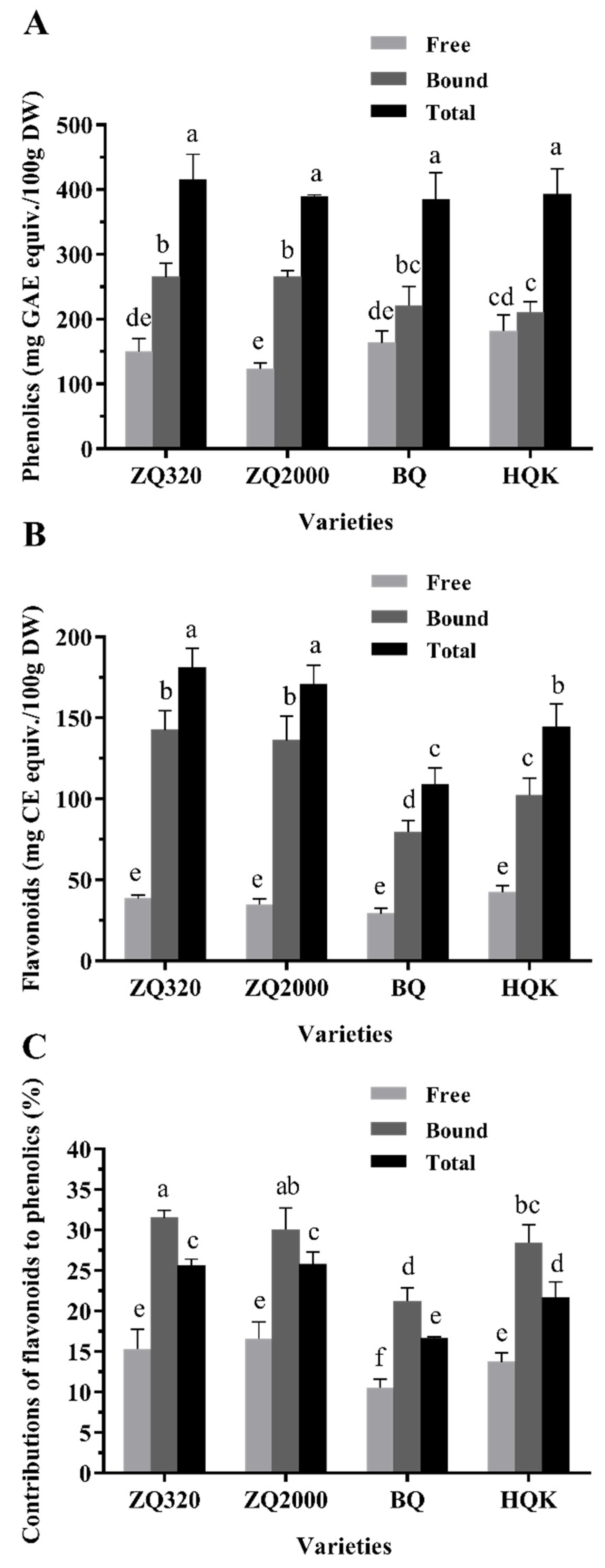
2.2. Phenolic, Flavonoid, and Anthocyanin Contents of Highland Barley Varieties
2.3. Phenolic Profiles of Highland Barley Varieties
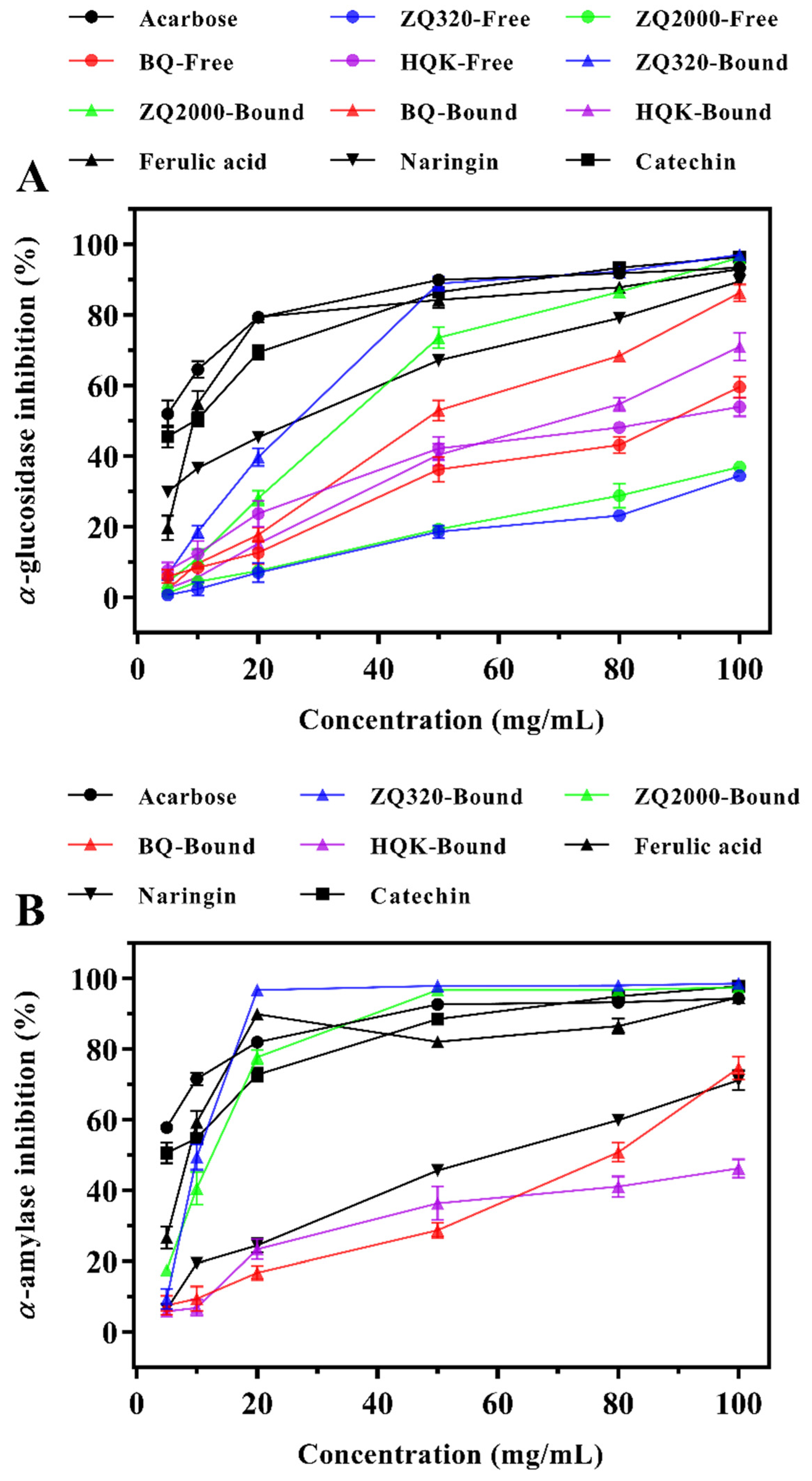
2.4. Chemical-based Hypoglycemic Activity of Highland Barley Varieties
2.4.1. α-Glucosidase Inhibitory Effects
2.4.2. α-Amylase Inhibitory Effects
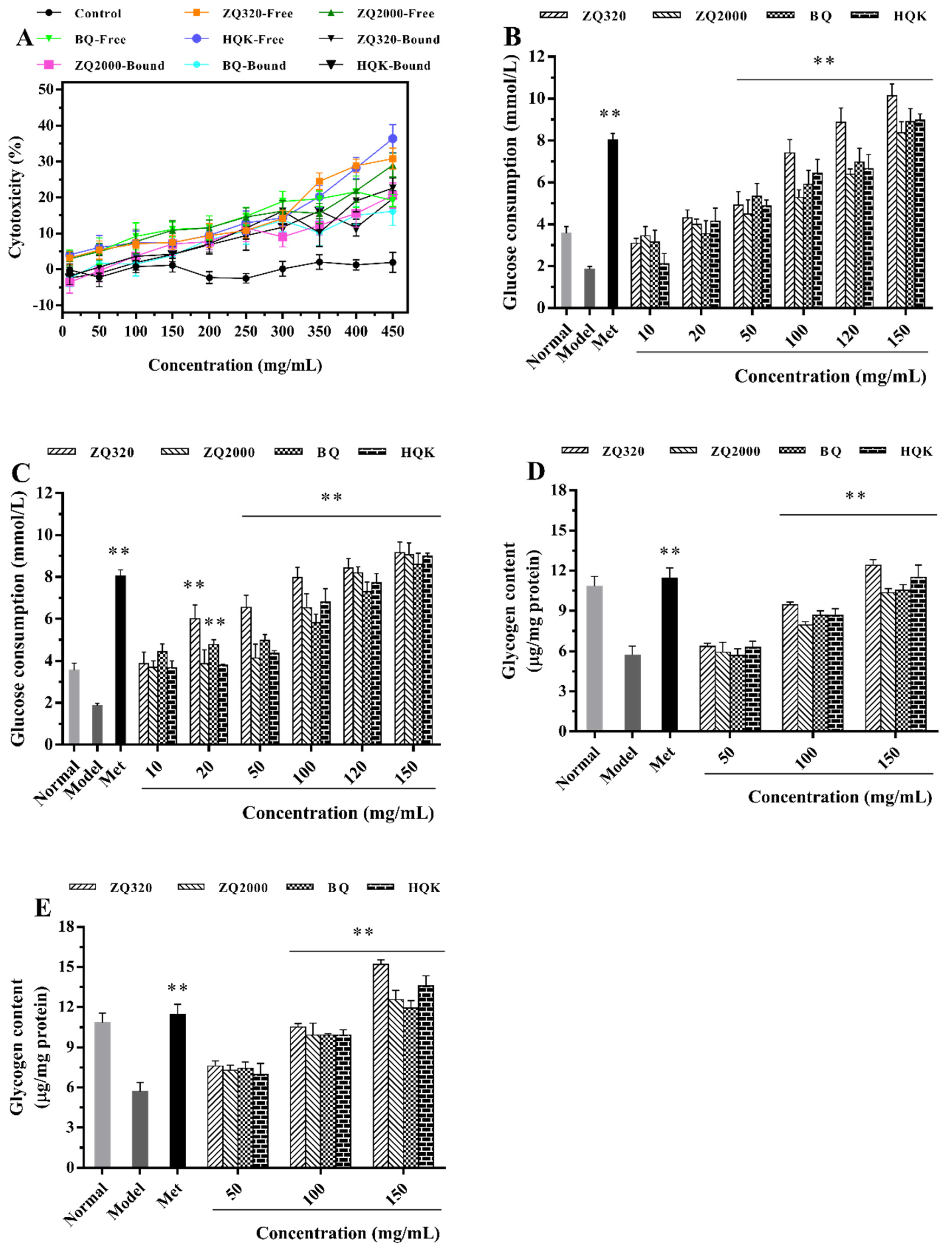
2.5. Cell-based Hypoglycemic Activity of Highland Barley Varieties
2.5.1. Glucose Consumption Activity
2.5.2. Glycogen Accumulation Capacity
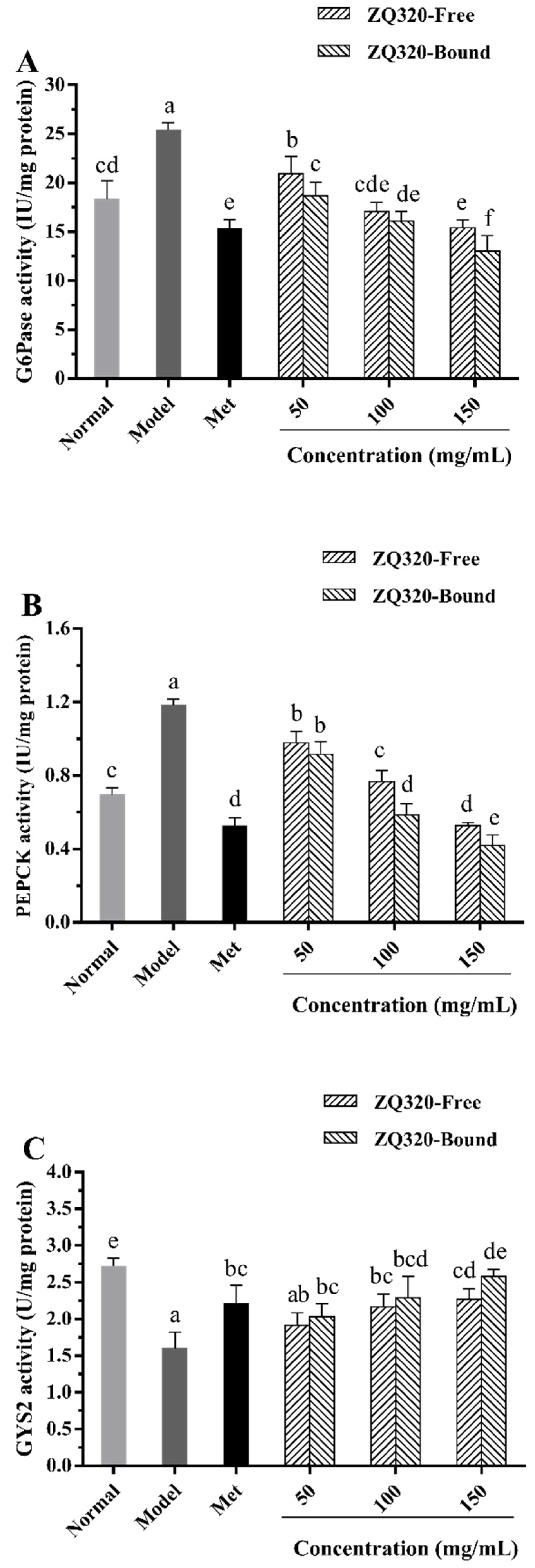
2.5.3. Glucose-6-phosphatase (G6Pase), Phosphoenolpyruvate Carboxykinase (PEPCK), and Glycogen Synthase 2 (GYS2) Activities
2.6. Effects of Highland Barley on the Gene Expression Levels
3. Materials and Methods
3.1. Materials and Reagents
3.2. Determination of Moisture Content
3.3. Extraction of Free and Bound Phenolics
3.4. Determination of Phenolics, Flavonoids, and Anthocyanins
3.5. Identification of Phenolic Profiles
3.6. Chemical-Based Hypoglycemic Activity Assays
3.7. Cell-Based Hypoglycemic Activity Assays
3.7.1. Cytotoxicity Assay
3.7.2. Glucose Consumption Assay
3.7.3. Glycogen Accumulation Assay
3.7.4. G6Pase, PEPCK, and GYS2 Activity Assays
3.8. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| FBS | Fetal bovine serum |
| DW | Dry weight |
| HPLC-PAD | High performance liquid chromatography-photodiode array detector |
| GAE | Gallic acid equivalents |
| CE | Catechin equivalents |
| pNPG | p-nitrophenyl-α-D-glucopyranoside |
| DMEM | Dulbecco’s modified eagle medium |
| CC50 | Median cytotoxic concentration |
| IC50 | Median inhibition dose |
| IR | Insulin resistance |
| G6Pase | Glucose-6-phosphatase |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| GYS2 | Glycogen synthase 2 |
| RT-qPCR | Quantitative real-time polymerase chain reaction |
| IRS-1 | Insulin receptor substrate-1 |
| PI3K | Phosphatidylinositol 3 kinase |
| Akt | Serine/threonine kinase |
| GSK3β | Glycogen synthase kinase 3β |
| GLUT4 | Glucose transporter type 4 |
References
- Sonnett, T.E.; Levien, T.L.; Gates, B.J.; Robinson, J.D.; Campbell, R.K. Diabetes mellitus, inflammation, obesity: Proposed treatment pathways for current and future therapies. Ann. Pharmacother. 2010, 44, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, F.G.; Stringuetta Belik, F.; Oliveira, E.S.V.R.; Martin, L.C.; Hueb, J.C.; Goncalves Rde, S.; Caramori, J.C.; Barreti, P.; Franco, R.J. Inflammation, diabetes, and chronic kidney disease: Role of aerobic capacity. Exp. Diabetes Res. 2012, 2012, 750286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. BBA-Mol. Basis. Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef] [Green Version]
- Sekar, V.; Mani, S.; Malarvizhi, R.; Nithya, P.; Vasanthi, H.R. Antidiabetic effect of mangiferin in combination with oral hypoglycemic agents metformin and gliclazide. Phytomedicine 2019, 59, 152901. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef]
- Zhang, L.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Ouyang, Z.; Duan, J. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF-β/Smads signaling pathway. Biomed. Pharmacother. 2019, 112, 108675. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef]
- Jood, S.; Kalra, S. Chemical composition and nutritional characteristics of some hull less and hulled barley cultivars grown in India. Nahrung 2001, 45, 35–39. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Qiao, Z.; Cao, X.; Luo, Q.; Zhao, J.; Wang, F.; Zhang, W. Assessment of β-glucans, phenols, flavor and volatile profiles of hulless barley wine originating from highland areas of China. Food Chem. 2019, 293, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.; Liu, R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Xia, X.; Li, G.; Ding, Y.; Ren, T.; Zheng, J.; Kan, J. Effect of whole grain Qingke (Tibetan Hordeum vulgare L. Zangqing 320) on the serum lipid levels and intestinal microbiota of rats under high-fat diet. J. Agric. Food Chem. 2017, 65, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J.G. Barley β-glucan reduces plasma glucose and insulin responses compared with resistant starch in men. Nutr. Res. 2006, 26, 644–650. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Schwarz, P.; Shetty, K. Phenolic linked anti-hyperglycemic bioactives of barley (Hordeum vulgare L.) cultivars as nutraceuticals targeting type 2 diabetes. Ind. Crops Prod. 2017, 107, 509–517. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.Z.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Antioxidant, α-glucosidase and xanthine oxidase inhibitory activity of bioactive compounds from maize (Zea mays L.). Chem. Biol. Drug. Des. 2014, 83, 119–125. [Google Scholar] [CrossRef]
- Giovannini, L.; Bianchi, S. Role of nutraceutical SIRT1 modulators in AMPK and mTOR pathway: Evidence of a synergistic effect. Nutrition 2017, 34, 82–96. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Me. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Gao, D.M.; Mohamed, S.; Chen, J.; Zhang, J.; Zhou, X.Y.; Zhou, N.J.; Xie, J.; Jiang, H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012, 518, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Balasubramanian, K.; Rajalakshmi, M.; Eliza, J.; Selvaraj, J. Insulin mimetic impact of catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on streptozotocin-induced diabetic Wistar rats. Phytomedicine 2010, 17, 28–36. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Liu, Y.; Zhang, M.; Zhang, R.; Abbasi, A.M.; You, L.; Li, T.; Liu, R.H. Comparative assessment of phytochemical profile, antioxidant capacity and anti-proliferative activity in different varieties of brown rice (Oryza sativa L.). LWT-Food Sci. Technol. 2018, 96, 19–25. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Xie, H.; Ju, X.; Liu, R.H. Phytochemical profiles and antioxidant activity of adlay varieties. J. Agric. Food Chem. 2013, 61, 5103–5113. [Google Scholar] [CrossRef]
- He, X.; Liu, D.; Liu, R.H. Sodium borohydride/chloranil-based assay for quantifying total flavonoids. J. Agric. Food Chem. 2008, 56, 9337–9344. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Nguyen, T.H.; Nguyen, M.T.; Doan, C.T.; Tran, T.N.; Lin, Z.H.; Nguyen, Q.V.; Kuo, Y.H.; Nguyen, A.D. Novel potent hypoglycemic compounds from Euonymus laxiflorus Champ. and their effect on reducing plasma glucose in an ICR mouse model. Molecules 2018, 23, 1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure-activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, S.-Y.; Ma, D.; Zhang, J.; Lee, W. The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS ONE 2011, 6, e17343. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [Green Version]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Zhang, L.; Zheng, X. Mulberry anthocyanin extract regulates glucose metabolism by promotion of glycogen synthesis and reduction of gluconeogenesis in human HepG2 cells. Food Funct. 2016, 7, 425–433. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.-K.; Jeong, K.-S.; Choi, M.-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.G.; Wang, H.Y.; Wei, Z.G.; Zhang, Y.Q. Therapeutic effects of ethanolic extract from the green cocoon shell of silkworm Bombyx mori on type 2 diabetic mice and its hypoglycaemic mechanism. Toxicol. Res. 2019, 8, 407–420. [Google Scholar] [CrossRef]
- Jeong, O.; Kim, H.S. Dietary chokeberry and dried jujube fruit attenuates high-fat and high-fructose diet-induced dyslipidemia and insulin resistance via activation of the IRS-1/PI3K/Akt pathway in C57BL/6J mice. Nutr. Metab. 2019, 16, 38. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem-Biol. Interact. 2019, 305, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Achour, M.; Saguem, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability and metabolism of rosemary infusion polyphenols using Caco-2 and HepG2 cell model systems. J. Sci. Food Agric. 2018, 98, 3741–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Tao, W.; Huang, H.; Ye, X.; Sun, P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chem. 2019, 279, 321–327. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Murota, K.; Shimizu, S.; Chujo, H.; Moon, J.H.; Terao, J. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line Caco-2. Arch. Biochem. Biophys. 2000, 384, 391–397. [Google Scholar] [CrossRef]
- Kanazawa, K.; Uehara, M.; Yanagitani, H.; Hashimoto, T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch. Biochem. Biophys. 2006, 455, 197–203. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Dang, B.; Fan, M.T. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef] [Green Version]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The alpha-amylase and alpha-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Felice, D.L.; Sun, J.; Liu, R.H. A modified methylene blue assay for accurate cell counting. J. Funct. Foods 2009, 1, 109–118. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Qiu, J.; Li, Z. The analysis of fagopyritols from tartary buckwheat and their anti-diabetic effects in KK-Ay type 2 diabetic mice and HepG2 cells. J. Funct. Foods 2018, 50, 137–146. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, D.; Bao, T.; Xie, J.; Chen, W. A simple and rapid method for the preparation of pure delphinidin-3-O-sambubioside from Roselle and its antioxidant and hypoglycemic activity. J. Funct. Foods 2017, 39, 9–17. [Google Scholar] [CrossRef]

| Varieties | Moisture Content (%) | Fractions | Total Anthocyanin Content (%) | CC50 of Cytotoxicity (mg/mL) |
|---|---|---|---|---|
| ZQ320 A/White | 12.66 ± 0.05b | Free | 0.42 ± 0.08b (100) B | > 600.0 |
| Bound | ND C | 538.2 ± 8.4 | ||
| Total | 0.42 ± 0.08b | - | ||
| ZQ2000/White | 11.95 ± 0.05d | Free | 0.47 ± 0.09b (100) | > 600.0 |
| Bound | ND | 384.5 ± 17.5 | ||
| Total | 0.47 ± 0.09b | - | ||
| BQ/Blue | 14.44 ± 0.03a | Free | 0.86 ± 0.02b (100) | > 600.0 |
| Bound | ND | 556.4 ± 29.8 | ||
| Total | 0.86 ± 0.02b | - | ||
| HQK/Black | 12.79 ± 0.10c | Free | 9.24 ± 0.79a (100) | > 600.0 |
| Bound | ND | 349.1 ± 27.8 | ||
| Total | 9.24 ± 0.79a | - |
| Varieties | Fractions | Phenolic acids (mg/100 g DW) | Flavonoids (mg/100 g DW) | |||||
|---|---|---|---|---|---|---|---|---|
| Gallic acid | Protocatechuic acid | Chlorogenic acid | Ferulic acid | Naringin | Catechin | Quercetin | ||
| ZQ320 A | Free | 0.47 ± 0.00b (100) B | 1.89 ± 0.01d (100) | 0.97 ± 0.04d (49.36) | 0.10 ± 0.00c (0.20) | 0.13 ± 0.00f (1.54) | 3.07 ± 0.08c (100) | ND |
| Bound | ND C | ND | 0.99 ± 0.02d (50.64) | 50.06 ± 1.46b (99.80) | 8.31 ± 0.37c (98.46) | ND | 2.21 ± 0.03b (100) | |
| Total | 0.47 ± 0.00b | 1.89 ± 0.01d | 1.96 ± 0.02a | 50.16 ± 1.45b | 8.44 ± 0.37c | 3.07 ± 0.08c | 2.21 ± 0.03b | |
| ZQ2000 | Free | 1.38 ± 0.02a (100) | 1.53 ± 0.04e (100) | 0.99 ± 0.04d (53.51) | 0.13 ± 0.00c (0.24) | 0.16 ± 0.01f (1.85) | 2.47 ± 0.01d (100) | ND |
| Bound | ND | ND | 0.86 ± 0.02e (46.49) | 54.52 ± 0.78a (99.76) | 8.38 ± 0.10c (98.15) | ND | 3.00 ± 0.08a (100) | |
| Total | 1.38 ± 0.02a | 1.53 ± 0.04e | 1.85 ± 0.06b | 54.65 ± 0.77a | 8.54 ± 0.11c | 2.47 ± 0.01d | 3.00 ± 0.08a | |
| BQ | Free | 0.13 ± 0.01c (100) | 3.21 ± 0.13b (100) | 0.97 ± 0.03d (100) | ND | 0.63 ± 0.04e (6.41) | 3.10 ± 0.04c (65.72) | ND |
| Bound | ND | ND | ND | 51.18 ± 1.53b (100) | 9.21 ± 0.26b (93.59) | 1.62 ± 0.02g (34.28) | 1.40 ± 0.03d (100) | |
| Total | 0.13 ± 0.01c | 3.21 ± 0.13b | 0.97 ± 0.03d | 51.18 ± 1.53b | 9.84 ± 0.23a | 4.72 ± 0.01a | 1.40 ± 0.03d | |
| HQK | Free | 0.16 ± 0.02c (100) | 2.69 ± 0.07c (64.57) | 1.23 ± 0.01c (100) | ND | 0.24 ± 0.04ef (3.30) | 1.89 ± 0.16f (47.35) | ND |
| Bound | ND | 1.48 ± 0.02e (35.43) | ND | 51.13 ± 2.76b (100) | 7.08 ± 0.30d (96.70) | 2.11 ± 0.07e (52.65) | 1.81 ± 0.04c (100) | |
| Total | 0.16 ± 0.02c | 4.17 ± 0.06a | 1.23 ± 0.01c | 51.13 ± 2.76b | 7.32 ± 0.32d | 4.00 ± 0.16b | 1.81 ± 0.04c | |
| Varieties | Fractions | IC50 of α-glucosidase Inhibition (mg/mL) | IC50 of α-amylase Inhibition (mg/mL) |
|---|---|---|---|
| ZQ320 A/White | Free | 176.0 ± 10.1 | ND B |
| Bound | 20.29 ± 0.53 | 9.48 ± 1.04 | |
| ZQ2000/White | Free | 164.1 ± 22.1 | ND |
| Bound | 27.05 ± 1.09 | 11.13 ± 0.34 | |
| BQ/Blue | Free | 90.10 ± 5.37 | ND |
| Bound | 43.31 ± 0.46 | 69.76 ± 5.02 | |
| HQK/Black | Free | 80.43 ± 6.00 | ND |
| Bound | 62.99 ± 2.82 | 105.4 ± 1.7 | |
| Acarbose | 4.62 ± 0.68 | 3.36 ± 0.47 | |
| Ferulic acid | 10.80 ± 0.90 | 7.61 ± 0.83 | |
| Naringin | 17.06 ± 0.62 | 49.75 ± 0.47 | |
| Catechin | 8.04 ± 0.50 | 7.04 ± 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties. Int. J. Mol. Sci. 2020, 21, 1175. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041175
Deng N, Zheng B, Li T, Liu RH. Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties. International Journal of Molecular Sciences. 2020; 21(4):1175. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041175
Chicago/Turabian StyleDeng, Na, Bisheng Zheng, Tong Li, and Rui Hai Liu. 2020. "Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties" International Journal of Molecular Sciences 21, no. 4: 1175. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041175