Sexual Dimorphism in Doxorubicin-induced Systemic Inflammation: Implications for Hepatic Cytochrome P450 Regulation
Abstract
:1. Introduction
2. Results
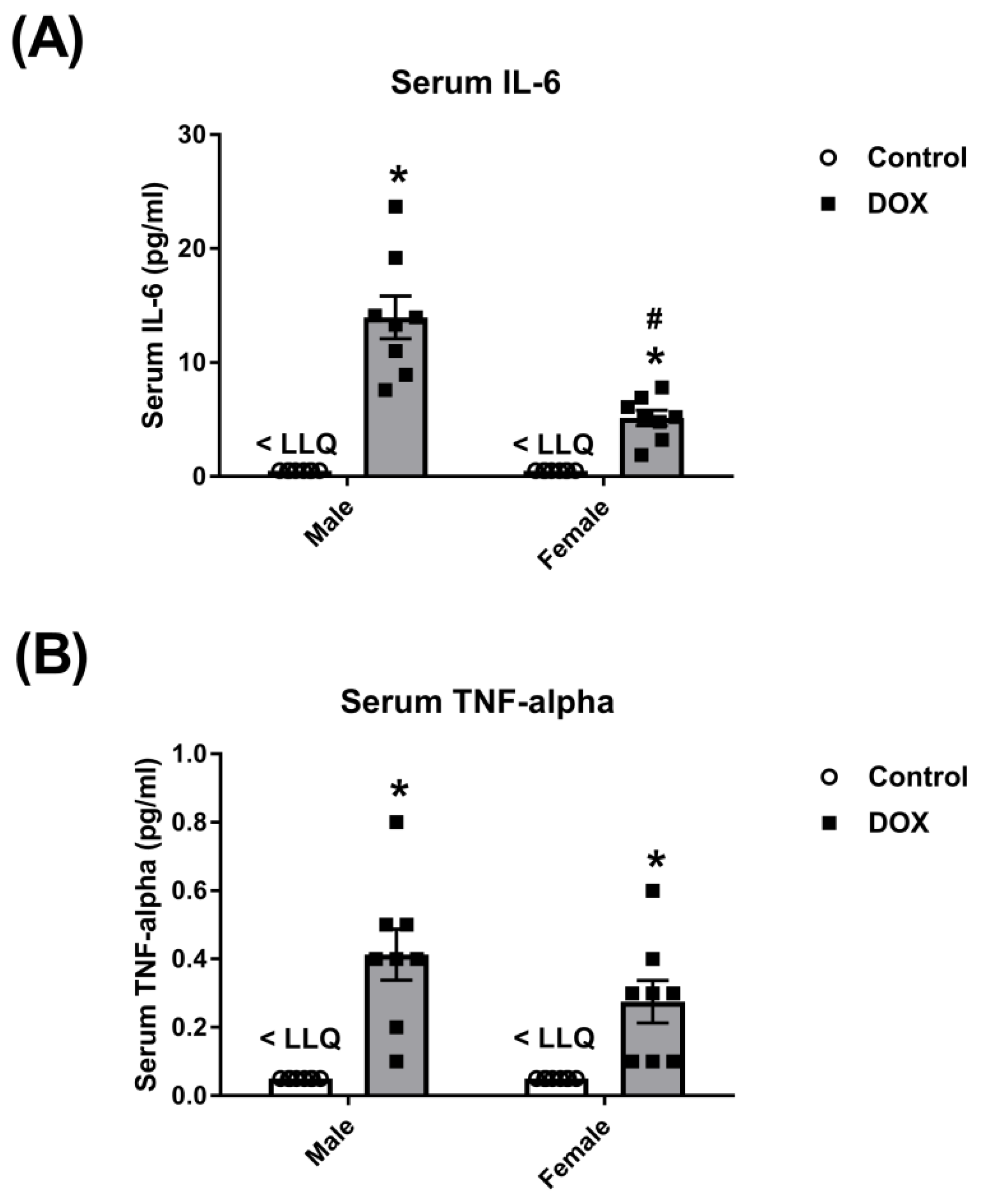
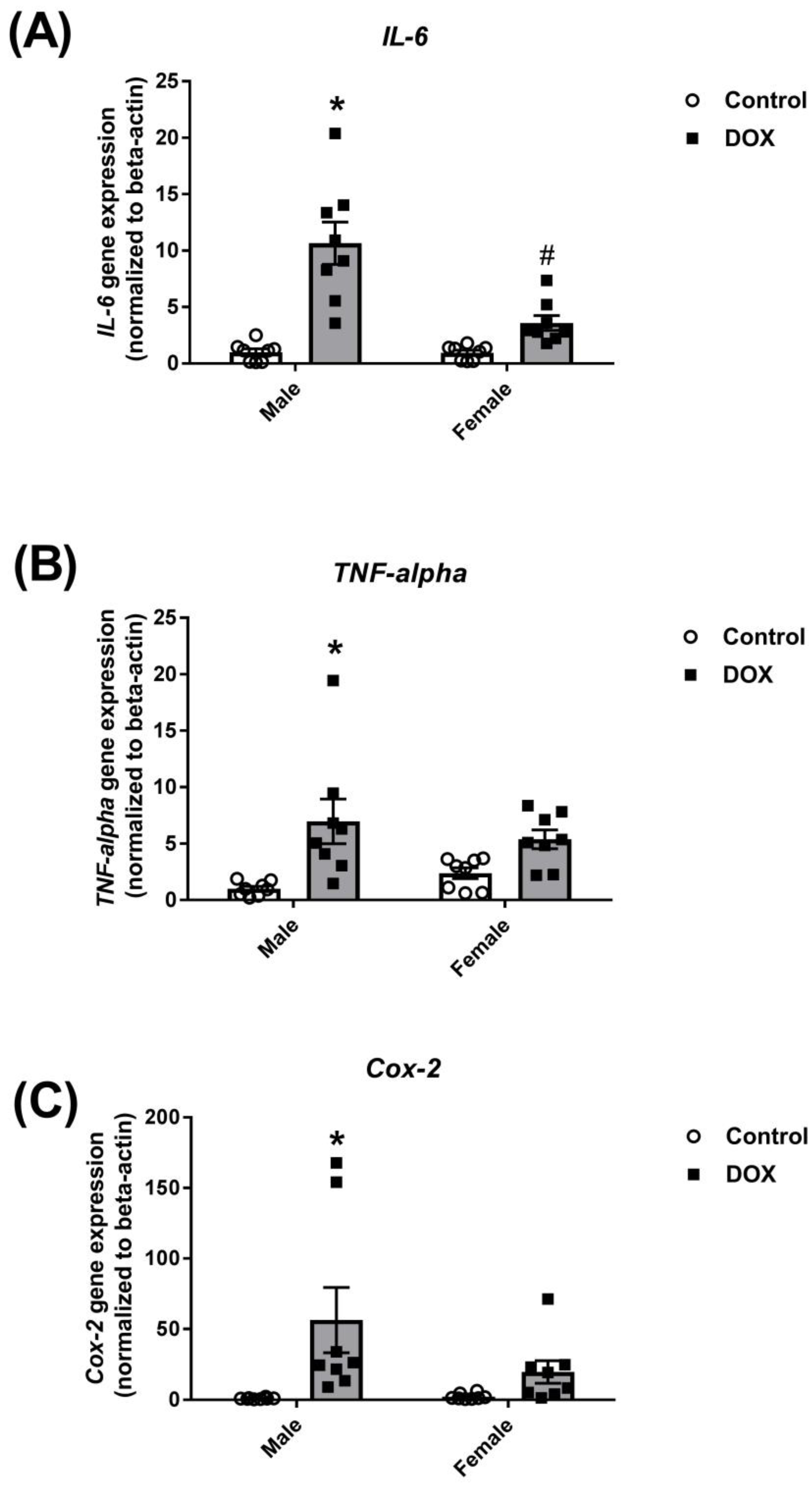
2.1. Effect of DOX Treatment on Inflammatory Markers
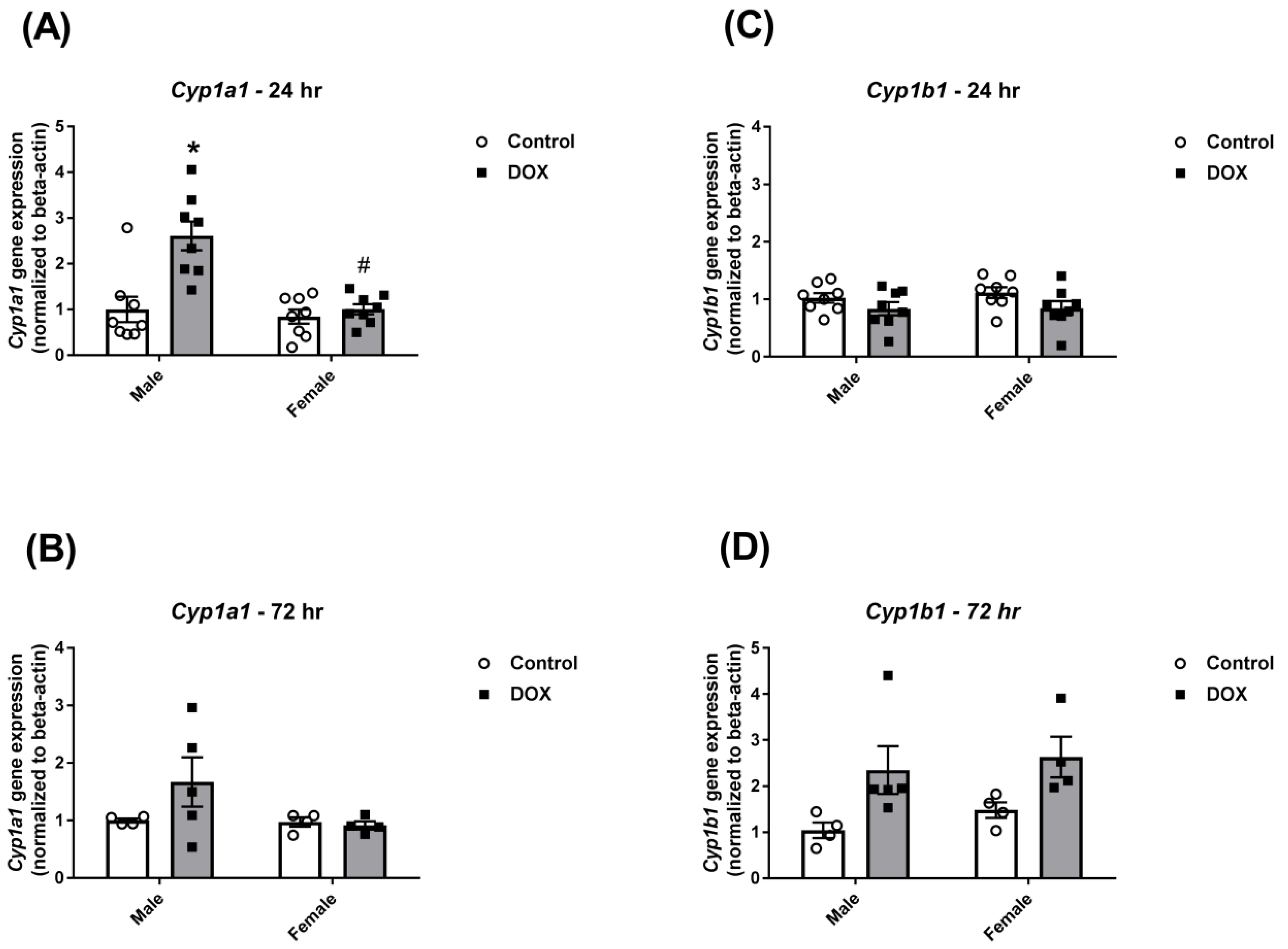
2.2. Effect of DOX Treatment on Cyp1 Family Expression
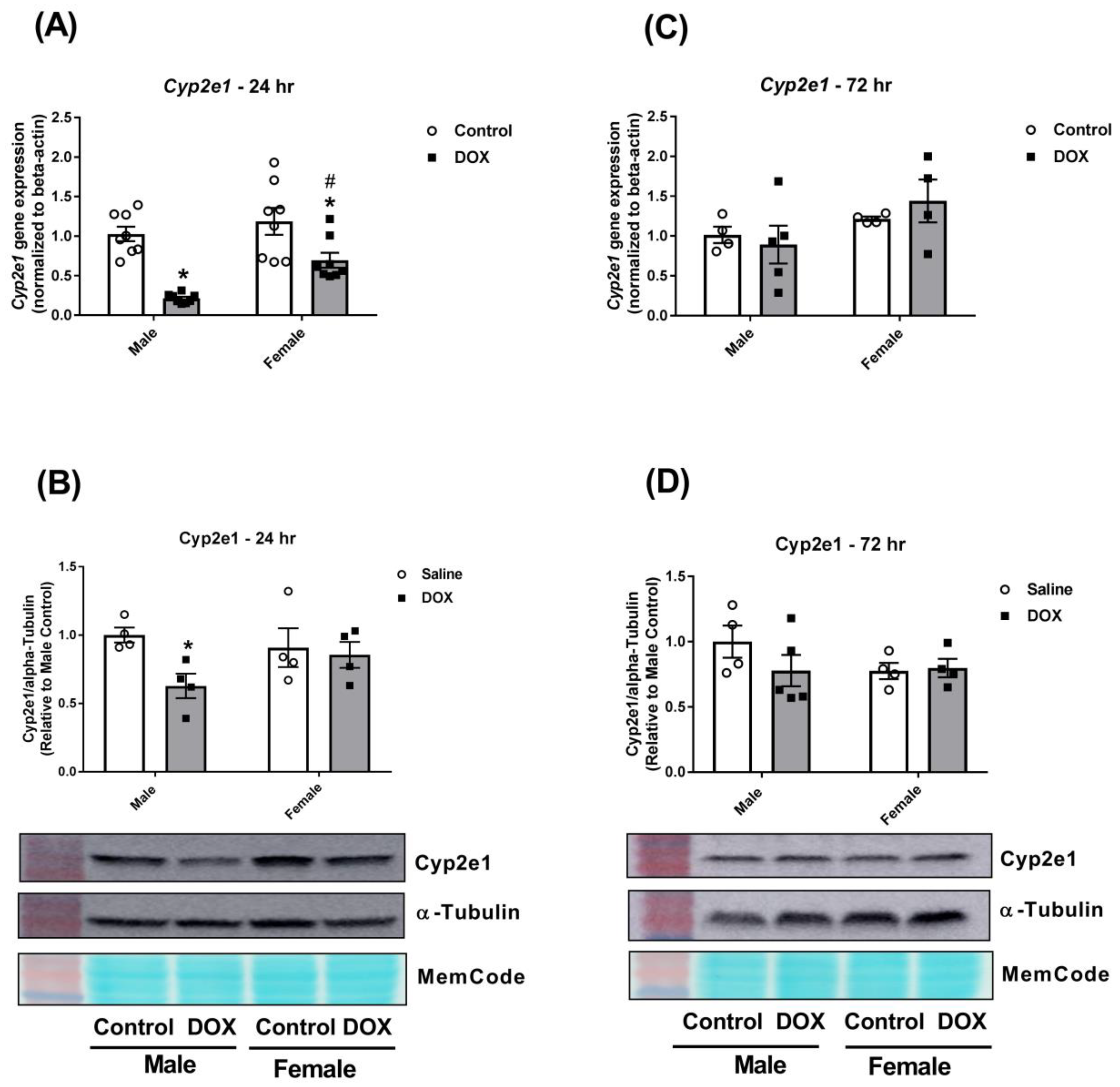
2.3. Effect of DOX Treatment on Cyp2 Family Expression
2.4. Effect of DOX Treatment on Cyp4a Sub-family Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measurement of Inflammatory Markers
4.3. RNA Extraction and Real-time PCR
4.4. Protein Extraction and Western Blotting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon Receptor |
| Cox-2 | Cyclooxygenase-2 |
| CYP | Cytochrome P450 |
| DOX | Doxorubicin |
| IL-6 | Interleukin-6 |
| TNF-alpha | Tumor Necrosis Factor-alpha |
References
- Lipshultz, S.E.; Giantris, A.L.; Lipsitz, S.R.; Kimball Dalton, V.; Asselin, B.L.; Barr, R.D.; Clavell, L.A.; Hurwitz, C.A.; Moghrabi, A.; Samson, Y.; et al. Doxorubicin administration by continuous infusion is not cardioprotective: The Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J. Clin. Oncol. 2002, 20, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Damodar, G.; Smitha, T.; Gopinath, S.; Vijayakumar, S.; Rao, Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann. Med. Health Sci. Res. 2014, 4, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective Effect of Boswellic Acids against Doxorubicin-Induced Hepatotoxicity: Impact on Nrf2/HO-1 Defense Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 8296451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacevic, V.; Dragojevic-Simic, V.; Tatomirovic, Z.; Dobric, S.; Bokonjic, D.; Kovacevic, A.; Nepovimova, E.; Valis, M.; Kuca, K. The Efficacy of Amifostine against Multiple-Dose Doxorubicin-Induced Toxicity in Rats. Int. J. Mol. Sci. 2018, 19, 2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zordoky, B.N.; Anwar-Mohamed, A.; Aboutabl, M.E.; El-Kadi, A.O.S. Acute doxorubicin toxicity differentially alters cytochrome P450 expression and arachidonic acid metabolism in rat kidney and liver. Drug Metab. Dispos. 2011, 39, 1440–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, K.; Sial, A.A.; Baig, M.T.; Ansari, S.H.; Adil, S.O.; Shamsi, T.S. Detection of the Incidence of Infections and Acute Biochemical Changes in Diffused Large B-Cell Lymphoma Patients Treated with Cyclophosphamide, Doxorubicin, Vincristine and Prednisone (CHOP) with and without Rituximab. Curr. Drug Saf. 2018, 13, 102–106. [Google Scholar] [CrossRef]
- Shen, R.L.; Pontoppidan, P.E.; Rathe, M.; Jiang, P.; Hansen, C.F.; Buddington, R.K.; Heegaard, P.M.; Muller, K.; Sangild, P.T. Milk diets influence doxorubicin-induced intestinal toxicity in piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G324–G333. [Google Scholar] [CrossRef] [Green Version]
- Carron, P.L.; Padilla, M.; Maurizi Balzan, J. Nephrotic syndrome and acute renal failure during pegylated liposomal doxorubicin treatment. Hemodial. Int. 2014, 18, 846–847. [Google Scholar] [CrossRef]
- Mohamed, N.; Goldstein, J.; Schiff, J.; John, R. Collapsing glomerulopathy following anthracycline therapy. Am. J. Kidney Dis. 2013, 61, 778–781. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Qi, H.; Wang, C.; Wang, C.; Zhang, J.; Dong, L. Doxorubicin-Induced Systemic Inflammation Is Driven by Upregulation of Toll-Like Receptor TLR4 and Endotoxin Leakage. Cancer Res. 2016, 76, 6631–6642. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.K.; Seelig, D.M.; Sharkey, L.C.; Zordoky, B.N. Sex-dependent alteration of cardiac cytochrome P450 gene expression by doxorubicin in C57Bl/6 mice. Biol. Sex Differ. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.L.; Yang, J.J.; Zhang, H.S. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed. Pharmacother. 2019, 109, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Del Pizzo, M.; Marzocco, S.; Sorrentino, R.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 2016, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauter, K.A.; Wood, L.J.; Wong, J.; Iordanov, M.; Magun, B.E. Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer Biol. Ther. 2011, 11, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar-mohamed, A.; Zordoky, B.N.; Aboutabl, M.E.; El-Kadi, A.O. Alteration of cardiac cytochrome P450-mediated arachidonic acid metabolism in response to lipopolysaccharide-induced acute systemic inflammation. Pharmacol. Res. 2010, 61, 410–418. [Google Scholar] [CrossRef]
- Aitken, A.E.; Morgan, E.T. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab. Dispos. 2007, 35, 1687–1693. [Google Scholar] [CrossRef]
- Barclay, T.B.; Peters, J.M.; Sewer, M.B.; Ferrari, L.; Gonzalez, F.J.; Morgan, E.T. Modulation of cytochrome P-450 gene expression in endotoxemic mice is tissue specific and peroxisome proliferator-activated receptor-alpha dependent. J. Pharmacol. Exp. Ther. 1999, 290, 1250–1257. [Google Scholar]
- Fukuno, S.; Nagai, K.; Yamamoto, K.; Tanimura, T.; Nabe, T.; Konishi, H. Pharmacokinetic interference of doxorubicin with tolbutamide due to reduced metabolic clearance with increased serum unbound fraction in rats. Biopharm. Drug Dispos. 2019, 40, 225–233. [Google Scholar] [CrossRef]
- Chen, Z.B.; Zhi, A.Y.; Lin, F.Y.; Li, D.; Yu, X.G.; Chen, W.H.; Hu, L.F. Pharmacokinetic of four probe drugs in adriamycin-induced nephropathy rat. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1439–1447. [Google Scholar]
- Elkiran, T.; Harputluoglu, H.; Yasar, U.; Babaoglu, M.O.; Dincel, A.K.; Altundag, K.; Ozisik, Y.; Guler, N.; Bozkurt, A. Differential alteration of drug-metabolizing enzyme activities after cyclophosphamide/adriamycin administration in breast cancer patients. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; El-Kadi, A.O. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol. Ther. 2010, 125, 446–463. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Anwar-Mohamed, A.; Aboutabl, M.E.; El-Kadi, A.O. Acute doxorubicin cardiotoxicity alters cardiac cytochrome P450 expression and arachidonic acid metabolism in rats. Toxicol. Appl. Pharmacol. 2010, 242, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, B.; Choudhury, A.I.; Horley, N.; Bruce, M.; Tomlinson, S.R.; Roberts, R.A.; Gray, T.J.; Barrett, D.A.; Shaw, P.N.; Kendall, D.; et al. Peroxisome proliferator activated receptor alpha regulates a male-specific cytochrome P450 in mouse liver. Arch. Biochem. Biophys. 2004, 429, 231–236. [Google Scholar] [CrossRef]
- Sun, F.; Zhu, J.; Lu, S.; Zhen, Z.; Wang, J.; Huang, J.; Ding, Z.; Zeng, M.; Sun, X. An inflammation-based cumulative prognostic score system in patients with diffuse large B cell lymphoma in rituximab era. BMC Cancer 2018, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2019, 234, 4959–4969. [Google Scholar] [CrossRef]
- Abdelgawad, I.Y.; Grant, M.K.O.; Zordoky, B.N. Leveraging the Cardio-Protective and Anticancer Properties of Resveratrol in Cardio-Oncology. Nutrients 2019, 11, 627. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.K.O.; Seelig, D.M.; Sharkey, L.C.; Choi, W.S.V.; Abdelgawad, I.Y.; Zordoky, B.N. Sexual dimorphism of acute doxorubicin-induced nephrotoxicity in C57Bl/6 mice. PLoS ONE 2019, 14, e0212486. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Radin, M.J.; Heller, L.; Tobias, A.; Matise, I.; Apple, F.S.; McCune, S.A.; Sharkey, L.C. The interplay between genetic background and sexual dimorphism of doxorubicin-induced cardiotoxicity. Cardiooncology 2016, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, G.R.; Lee, T.; Moland, C.L.; Vijay, V.; Herman, E.H.; Lewis, S.M.; Davis, K.J.; Muskhelishvili, L.; Kerr, S.; Fuscoe, J.C.; et al. Sex-related differential susceptibility to doxorubicin-induced cardiotoxicity in B6C3F1 mice. Toxicol. Appl. Pharmacol. 2016, 310, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Meiners, B.; Shenoy, C.; Zordoky, B.N. Clinical and preclinical evidence of sex-related differences in anthracycline-induced cardiotoxicity. Biol. Sex Differ. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Knapton, A.; Lipshultz, S.E.; Cochran, T.R.; Hiraragi, H.; Herman, E.H. Sex-related differences in mast cell activity and doxorubicin toxicity: A study in spontaneously hypertensive rats. Toxicol. Pathol. 2014, 42, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; El-Kadi, A.O. Induction of several cytochrome P450 genes by doxorubicin in H9c2 cells. Vasc. Pharmacol. 2008, 49, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Palmeri, M.; Russell, K.S.; Russell, R.R. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc. Res. 2011, 90, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asnani, A.; Zheng, B.; Liu, Y.; Wang, Y.; Chen, H.H.; Vohra, A.; Chi, A.; Cornella-Taracido, I.; Wang, H.; Johns, D.G.; et al. Highly potent visnagin derivatives inhibit Cyp1 and prevent doxorubicin cardiotoxicity. JCI Insight 2018, 3, e96753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharavi, N.; El-Kadi, A.O. Down-regulation of aryl hydrocarbon receptor-regulated genes by tumor necrosis factor-alpha and lipopolysaccharide in murine hepatoma Hepa 1c1c7 cells. J. Pharm. Sci. 2005, 94, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; El-Kadi, A.O. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr. Drug Metab. 2009, 10, 164–178. [Google Scholar] [CrossRef]
- Malaplate-Armand, C.; Ferrari, L.; Masson, C.; Siest, G.; Batt, A.M. Astroglial CYP1B1 up-regulation in inflammatory/oxidative toxic conditions: IL-1beta effect and protection by N-acetylcysteine. Toxicol. Lett. 2003, 138, 243–251. [Google Scholar] [CrossRef]
- Deng, Y.; Theken, K.N.; Lee, C.R. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J. Mol. Cell. Cardiol. 2010, 48, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Hiratsuka, M.; Matsuura, T.; Watanabe, E.; Sato, M.; Suzuki, Y. Sex and strain differences in constitutive expression of fatty acid omega-hydroxylase (CYP4A-related proteins) in mice. J. Biochem. 1996, 119, 340–345. [Google Scholar] [CrossRef]
- Carlson, A.; Alderete, K.S.; Grant, M.K.O.; Seelig, D.M.; Sharkey, L.C.; Zordoky, B.N.M. Anticancer effects of resveratrol in canine hemangiosarcoma cell lines. Vet. Comp. Oncol. 2018, 16, 253–261. [Google Scholar] [CrossRef] [PubMed]


| 24 h Post-DOX | ||||||
| DOX Effect | Sex Effect | Interaction | ||||
| F (1, 28) | p Value | F (1, 28) | p Value | F (1, 28) | p Value | |
| Cyp1a1 | 14.75 | 0.0006 | 14.61 | 0.0007 | 9.855 | 0.004 |
| Cyp1b1 | ns | ns | ns | |||
| Cyp2c29 | 82.54 | <0.0001 | ns | 5.58 | 0.0254 | |
| Cyp2e1 | 36.63 | <0.0001 | 8.817 | 0.0061 | ns | |
| Cyp4a10 | 114.9 | <0.0001 | 20.01 | 0.0001 | 11.67 | 0.002 |
| Cyp4a12 | 11.42 | 0.0022 | 229.9 | <0.0001 | 11.45 | 0.0021 |
| 72 h Post-DOX | ||||||
| DOX Effect | Sex Effect | Interaction | ||||
| F (1, 13) | p Value | F (1, 13) | p Value | F (1, 13) | p Value | |
| Cyp1a1 | ns | ns | ns | |||
| Cyp1b1 | 9.828 | 0.0079 | ns | ns | ||
| Cyp2c29 | 8.005 | 0.0142 | ns | 7.136 | 0.0192 | |
| Cyp2e1 | ns | ns | ns | |||
| Cyp4a10 | 6.711 | 0.0224 | ns | ns | ||
| Cyp4a12 | 153.1 | <0.0001 | 202.6 | <0.0001 | 151.4 | <0.0001 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3’) |
|---|---|---|
| IL-6 | CCA GAG ATA CAA AGA AAT GAT GG | ACT CCA GAA GAC CAG AGG AAA T |
| TNF-alpha | CCA GAC CCT CAC ACT CAG ATC A | CAC TTG GTG GTT TGC TAC GAC |
| Cox-2 | CTG GTG CCT GGT CTG ATG ATG | GGC AAT GCG GTT CTG ATA CTG |
| Cyp1a1 | GGT TAA CCA TGA CCG GGA ACT | TGC CCA AAC CAA AGA GAG TGA |
| Cyp1b1 | AAT GAG GAG TTC GGG CGC ACA | GGC GTG TGG AAT GGT GAC AGG |
| Cyp2c29 | TGG TCC ACC CAA AAG AAA TTG A | GCA GAG AGG CAA ATC CAT TCA |
| Cyp2e1 | CCC AAG TCT TTA ACC AAG TTG GC | CTT CCA TGT GGG TCC ATT ATT GA |
| Cyp4a10 | GTG CTG AGG TGG ACA CAT TCA T | TGT GGC CAG AGC ATA GAA GAT C |
| Cyp4a12 | TGA CCC CAG CTT TCC ACT ATG | TTG TTC AGG TCC TCA ACT GCC |
| Beta-actin | TAT TGG CAA CGA GCG GTT CC | GGC ATA GAG GTC TTT ACG GAT GTC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, M.K.O.; Abdelgawad, I.Y.; Lewis, C.A.; Zordoky, B.N. Sexual Dimorphism in Doxorubicin-induced Systemic Inflammation: Implications for Hepatic Cytochrome P450 Regulation. Int. J. Mol. Sci. 2020, 21, 1279. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041279
Grant MKO, Abdelgawad IY, Lewis CA, Zordoky BN. Sexual Dimorphism in Doxorubicin-induced Systemic Inflammation: Implications for Hepatic Cytochrome P450 Regulation. International Journal of Molecular Sciences. 2020; 21(4):1279. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041279
Chicago/Turabian StyleGrant, Marianne K.O., Ibrahim Y. Abdelgawad, Christine A. Lewis, and Beshay N. Zordoky. 2020. "Sexual Dimorphism in Doxorubicin-induced Systemic Inflammation: Implications for Hepatic Cytochrome P450 Regulation" International Journal of Molecular Sciences 21, no. 4: 1279. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041279





