Endothelial Nitric Oxide Mediates the Anti-Atherosclerotic Action of Torenia concolor Lindley var. Formosama Yamazaki
Abstract
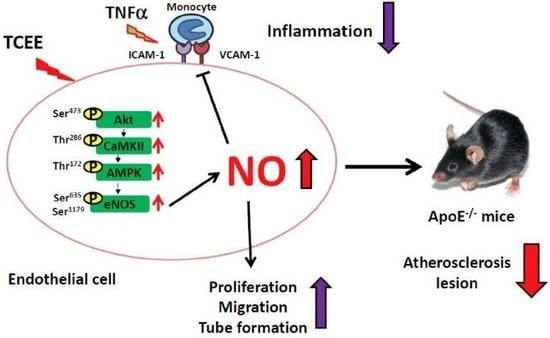
:1. Introduction
2. Results
2.1. TCEE Increases NO Production by Increasing the Phosphorylation of eNOS in ECs
2.2. Activation of the Akt-CaMKII-AMPK Signaling Pathway Is Essential for the TCEE-Induced Phosphorylation of eNOS and NO Production
2.3. The Akt-CaMKII-AMPK Signaling Plays a Key Role in the TCEE-Mediated Promotion of EC Proliferation, Migration, and Tube Formation
2.4. Activation of eNOS Is Essential for the Anti-Inflammatory Effects of TCEE
2.5. TCEE Ameliorates Inflammation and Atherosclerosis in ApoE−/− Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. TC Extraction
4.4. Cell Viability Assay
4.5. Protein Extraction and Western Blot Analysis
4.6. Determination of Nitrite Production
4.7. Cell Proliferation Assay
4.8. Cell Migration Assay
4.9. Matrigel Angiogenesis Assay
4.10. In Vitro Mononuclear-Endothelial Cell Adhesion Assay
4.11. Serum Lipid Profile Analysis
4.12. Measurement of Inflammatory Cytokines
4.13. Mice
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ando, J.; Kamiya, A. Blood flow and vascular endothelial cell function. Front. Med. Biol. Eng. 1993, 5, 245–264. [Google Scholar]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar]
- Kuhlencordt, P.J.; Rosel, E.; Gerszten, R.E.; Morales-Ruiz, M.; Dombkowski, D.; Atkinson, W.J.; Han, F.; Preffer, F.; Rosenzweig, A.; Sessa, W.C.; et al. Role of endothelial nitric oxide synthase in endothelial activation: Insights from eNOS knockout endothelial cells. Am. J. Physiol. Cell Physiol. 2004, 286, C1195–C1202. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.B.; Yang, Y.B.; Song, Y.L.; Zhang, Y.C.; Li, Y.R. Lipoic acid attenuates the expression of adhesion molecules by increasing endothelial nitric-oxide synthase activity. Mol. Biol. Rep. 2013, 40, 377–382. [Google Scholar] [CrossRef]
- Masseau, I.; Bowles, D.K. Carotid endothelial VCAM-1 is an early marker of carotid atherosclerosis and predicts coronary artery disease in swine. J. Biomed. Sci. Eng. 2015, 8, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathouska, J.; Nemeckova, I.; Zemankova, L.; Strasky, Z.; Jezkova, K.; Varejckova, M.; Nachtigal, P. Cell adhesion molecules and eNOS expression in aorta of normocholesterolemic mice with different predispositions to atherosclerosis. Heart Vessels 2015, 30, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Sessa, W.C. Endothelial NOS: Perspective and recent developments. Br. J. Pharmacol. 2019, 176, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Haehling, S.; Anker, S.D.; Bassenge, E. Statins and the role of nitric oxide in chronic heart failure. Heart Fail. Rev. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- Tousoulis, D.; Charakida, M.; Stefanadis, C. Endothelial function and inflammation in coronary artery disease. Heart 2006, 92, 441–444. [Google Scholar] [CrossRef]
- Tao, C.; Shkumatov, A.A.; Alexander, S.T.; Ason, B.L.; Zhou, M. Stigmasterol accumulation causes cardiac injury and promotes mortality. Commun. Biol. 2019, 2, 20. [Google Scholar] [CrossRef]
- Sudhahar, V.; Kumar, S.A.; Sudharsan, P.T.; Varalakshmi, P. Protective effect of lupeol and its ester on cardiac abnormalities in experimental hypercholesterolemia. Vascul. Pharmacol. 2007, 46, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Su, K.H.; Kou, Y.R.; Guo, B.C.; Lee, K.I.; Wei, J.; Lee, T.S. Role of transient receptor potential vanilloid 1 in regulating erythropoietin-induced activation of endothelial nitric oxide synthase. Acta Physiol. (Oxf.) 2017, 219, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.C.; Kou, Y.R.; Shyue, S.K.; Su, K.H.; Wei, J.; Cheng, L.C.; Yu, Y.B.; Pan, C.C.; Lee, T.S. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc. Res. 2011, 91, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.F.; Ching, L.C.; Kou, Y.R.; Lin, S.J.; Wei, J.; Shyue, S.K.; Lee, T.S. Activation of TRPV1 prevents oxLDL-induced lipid accumulation and TNF-α-induced inflammation in macrophages: Role of liver X receptor α. Mediat. Inflamm. 2013, 2013, 925171. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.H.; Lin, A.H.; Lee, H.F.; Ko, H.K.; Lee, T.S.; Kou, Y.R. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediat. Inflamm. 2014, 2014, 651890. [Google Scholar] [CrossRef]
- Ye, T.; Meng, X.; Wang, R.; Zhang, C.; He, S.; Sun, G.; Sun, X. Gastrodin alleviates cognitive dysfunction and depressive-like behaviors by inhibiting ER stress and NLRP3 inflammasome activation in db/db mice. Int. J. Mol. Sci. 2018, 19, 3977. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yao, X.; Xu, J.; Wu, Y.; Yang, Y.; Jin, Y.; Xie, H.; Liu, Y.; Yang, Y.; Zheng, X. One new phenolic compound from Castanea mollissima Shells and its suppression of hepatoma cell proliferation and inflammation by inhibiting NF-κB pathway. Int. J. Mol. Sci. 2019, 20, 466. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Yang, L.; Zhou, W.; Tang, X.; Wang, Y.; Ma, Z.; Gao, S.; Gao, Y. Study on cardiotoxicity and mechanism of “Fuzi” extracts based on metabonomics. Int. J. Mol. Sci. 2018, 19, 3506. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.Y.; Park, S.J.; Park, Y.J. The anticancer properties of cordycepin and their underlying mechanisms. Int. J. Mol. Sci. 2018, 19, 3027. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Cheng, H.Y.; Huang, T.H.; Huang, H.W.; Lee, Y.H.; Peng, W.H. Analgesic and anti-inflammatory activities of Torenia concolor Lindley var. formosana Yamazaki and betulin in mice. Am. J. Chin. Med. 2009, 37, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Hu, J.C.; Li, P.Y.; Huang, G.J.; Kuo, Y.H.; Chao, C.Y. Torenia concolor Lindley var. formosana Yamazaki extracts improve inflammatory response and lipid accumulation via PPARs activation. Biomedicine (Taipei) 2017, 7, 29–36. [Google Scholar]
- Lee, W.; Yang, E.J.; Ku, S.K.; Song, K.S.; Bae, J.S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.W.; Ho, L.K.; Chang, Y.S. Studies on the chemical constituents of Torenia concolor Lindley. J. Agric. For. 2014, 54, 69–76. [Google Scholar]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in LPS-stimulated macrophages and endotoxin-shocked mice through an AMPK/AKT/Nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.C.; Wei, J.; Su, K.H.; Chiang, A.N.; Zhao, J.F.; Chen, H.Y.; Shyue, S.K.; Lee, T.S. Transient receptor potential vanilloid type 1 is vital for (-)-epigallocatechin-3-gallate mediated activation of endothelial nitric oxide synthase. Mol. Nutr. Food Res. 2015, 59, 646–657. [Google Scholar] [CrossRef]
- Ching, L.C.; Zhao, J.F.; Su, K.H.; Shyue, S.K.; Hsu, C.P.; Lu, T.M.; Lin, S.J.; Lee, T.S. Activation of transient receptor potential vanilloid 1 decreases endothelial nitric oxide synthase phosphorylation at Thr497 by protein phosphatase 2B-dependent dephosphorylation of protein kinase C. Acta Physiol. (Oxf.) 2013, 209, 124–135. [Google Scholar] [CrossRef]
- Tran, J.; Magenau, A.; Rodriguez, M.; Rentero, C.; Royo, T.; Enrich, C.; Thomas, S.R.; Grewal, T.; Gaus, K. Activation of endothelial nitric oxide (eNOS) occurs through different membrane domains in endothelial cells. PLoS ONE 2016, 11, e0151556. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [PubMed]
- Hsu, W.H.; Lee, B.H.; Lu, I.J.; Pan, T.M. Ankaflavin and monascin regulate endothelial adhesion molecules and endothelial NO synthase (eNOS) expression induced by tumor necrosis factor-α (TNF-α) in human umbilical vein endothelial cells (HUVECs). J. Agric. Food Chem. 2012, 60, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhon, H.H.; Hong, F.F.; Yang, S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes. Care 2009, 32 (Suppl. S2), S314–S321. [Google Scholar] [CrossRef] [Green Version]
- Fraga-Silva, R.A.; Montecucco, F.; Costa-Fraga, F.P.; Nencioni, A.; Caffa, I.; Bragina, M.E.; Mach, F.; Raizada, M.K.; Santos, R.A.S.; da Silva, R.F.; et al. Diminazene enhances stability of atherosclerotic plaques in ApoE-deficient mice. Vascul. Pharmacol. 2015, 74, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhong, Z.; Yuan, J.; Chen, X.; Huang, Z.; Wu, Z. Resveratrol improves endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2019, 67, 63–71. [Google Scholar] [CrossRef]
- Carrizzo, A.; Ambrosio, M.; Damato, A.; Madonna, M.; Storto, M.; Capocci, L.; Campiglia, P.; Sommella, E.; Trimarco, V.; Rozza, F.; et al. Morus alba extract modulates blood pressure homeostasis through eNOS signaling. Mol. Nutr. Food Res. 2016, 60, 2304–2311. [Google Scholar] [CrossRef]
- Zhao, J.F.; Leu, S.J.; Shyu, S.K.; Su, K.H.; Wei, J.; Lee, T.S. Novel effect of paeonol on the formation of foam cells: Promotion of LXRα-ABCA1-dependent cholesterol efflux in macrophages. Am. J. Chin. Med. 2013, 41, 1079–1096. [Google Scholar] [CrossRef]
- Su, K.H.; Lin, S.J.; Wei, J.; Lee, K.I.; Zhao, J.F.; Shyue, S.K.; Lee, T.S. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol. (Oxf.) 2014, 212, 191–204. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Fauler, G.; Hoffmann, M.M.; Lütjohann, D.; Winkelmann, B.R.; Boehm, B.O.; März, W. The associations of cholesterol metabolism and plasma plant sterols with all-cause and cardiovascular mortality. J. Lipid Res. 2010, 51, 2384–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Förstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Freeman, B.A. Interactions between nitric oxide and lipid oxidation pathways: Implications for vascular disease. Circ. Res. 2001, 88, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Aspects. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef] [PubMed]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.-C.; Guo, B.-C.; Chen, C.-H.; Chang, C.-J.; Yeh, T.-S.; Lee, T.-S. Endothelial Nitric Oxide Mediates the Anti-Atherosclerotic Action of Torenia concolor Lindley var. Formosama Yamazaki. Int. J. Mol. Sci. 2020, 21, 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041532
Cheng L-C, Guo B-C, Chen C-H, Chang C-J, Yeh T-S, Lee T-S. Endothelial Nitric Oxide Mediates the Anti-Atherosclerotic Action of Torenia concolor Lindley var. Formosama Yamazaki. International Journal of Molecular Sciences. 2020; 21(4):1532. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041532
Chicago/Turabian StyleCheng, Li-Ching, Bei-Chia Guo, Chia-Hui Chen, Chi-Jen Chang, Ta-Sen Yeh, and Tzong-Shyuan Lee. 2020. "Endothelial Nitric Oxide Mediates the Anti-Atherosclerotic Action of Torenia concolor Lindley var. Formosama Yamazaki" International Journal of Molecular Sciences 21, no. 4: 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21041532