Evaluation of the Genotoxic and Oxidative Damage Potential of Silver Nanoparticles in Human NCM460 and HCT116 Cells
Abstract
:1. Introduction
2. Results
2.1. Characterization of Ag-NPs
2.2. Morphology Changes of Ag-NPs Treated Cells
2.3. Cytotoxicity Analysis
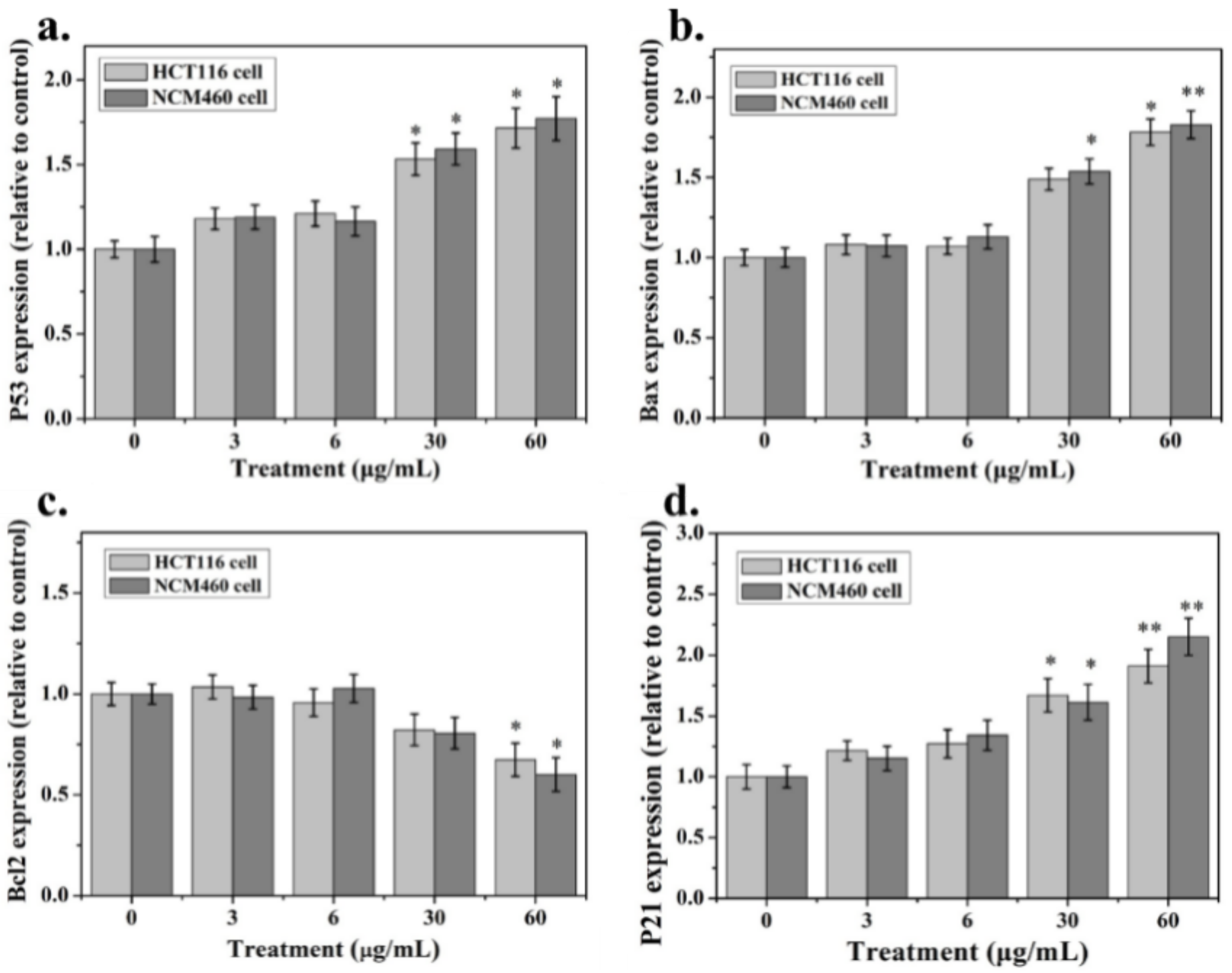
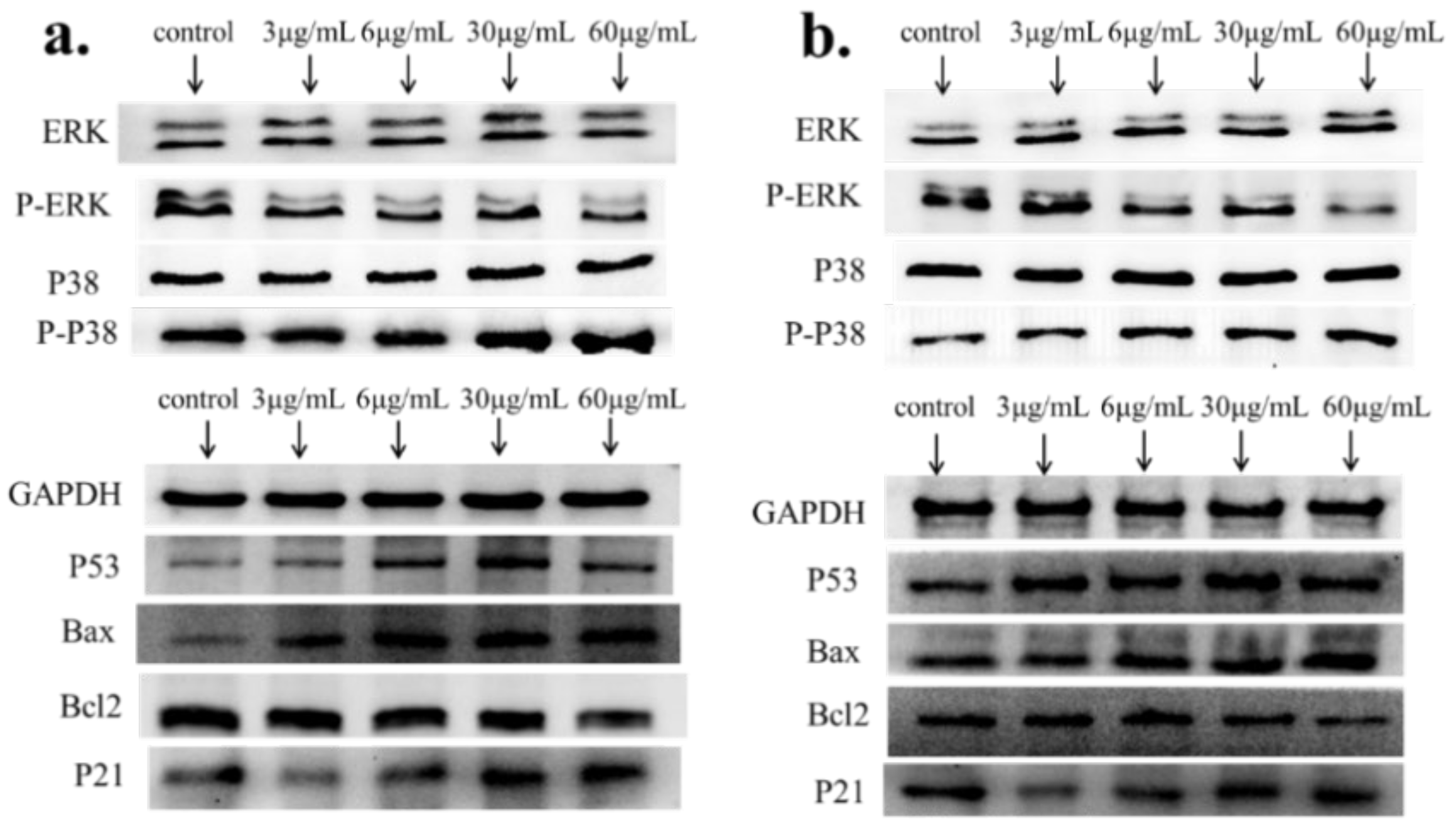
2.4. Apoptosis-Associated mRNA and Protein Expression
3. Discussion
4. Materials and Methods
4.1. Nanoparticles
4.2. Cell Culture and Nanoparticle Treatment
4.3. Cell Viability Assay
4.4. Intracellular Reactive Oxygen Species (ROS) Assay
4.5. LDH Release
4.6. RNA Isolation and Reverse-Transcriptase PCR Analysis
4.7. Western-Blotting Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hamzeh, M.; Sunahara, G.I. In vitro cytotoxicity and genotoxicity studies of titanium dioxide (TiO2) nanoparticles in Chinese hamster lung fibroblast cells. Toxicol. In Vitro 2013, 27, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Coto-García, A.M.; Sotelo-González, E.; Fernández-Argüelles, M.T.; Pereiro, R.; Costa-Fernández, J.M.; Sanz-Medel, A. Nanoparticles as fluorescent labels for optical imaging and sensing in genomics and proteomics. Anal. Bioanal. Chem. 2011, 399, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, B.K.; Hirn, S.; Kermanizadeh, A.; Kanase, N.; Fytianos, K.; Wenk, A.; Stone, V. Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol. Sci. 2012, 131, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Ding, Q.M.; Li, W. Preparation of Nano-Silver-Containing Polyethylene Composite Film and Ag Ion Migration into Food-Simulants. J. Nanosci. Nanotechnol. 2020, 20, 1613–1621. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.J.; Peng, Z.Y. Nano-silver-containing polyvinyl alcohol composite film for grape fresh-keeping. Mater. Express 2019, 9, 985–992. [Google Scholar] [CrossRef]
- Sayes, C.M.; Warheit, D.B. Characterization of nanomaterials for toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 660–670. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carriere, M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Kim, K.T.; Ryu, T.K. Stepwise embryonic toxicity of silver nanoparticles on Oryzias latipes. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, Z.R. Effect of Titanium Dioxide Nanoparticles on The Amount of Blood Cells and Liver Enzymes in Wistar Rats. J. Shahid Sadoughi Univ. Med. Sci. 2011, 19, 618–626. [Google Scholar]
- Ruckerl, R.; Ibald-Mulli, A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am. J. Respir. Crit. Care Med. 2006, 173, 432–441. [Google Scholar] [CrossRef]
- Veronesi, B.; Makwana, O.; Pooler, M.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles: VII. Degeneration of Dopaminergic Neurons in Apo E−/− Mice. Inhal. Toxicol. 2005, 17, 235–241. [Google Scholar] [CrossRef]
- Guildford, A.L.; Poletti, T.; Osbourne, L.H.; Di Cerbo, A.; Gatti, A.M.; Santin, M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J. R. Soc. Interface 2009, 6, 1213–1221. [Google Scholar] [CrossRef]
- Sarhan, O.M.M.; Hussein, R.M. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int. J. Nanomed. 2014, 9, 1505. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Jeong, H.J.; Yun, K.N. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int. J. Nanomed. 2012, 7, 3203. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, Z.; Ba, T. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver nanoparticle-induced autophagic-lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012, 32, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Huang, Y. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J. Appl. Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkaleli, M.; Erdem, A. Biotoxicity of TiO2 nanoparticles on Raphidocelis subcapitata microalgae exemplified by membrane deformation. Int. J. Env. Res. Public Health 2018, 15, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, V.D.; Prasad, S.V.; Banerjee, A. Health hazards of nanoparticles: Understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem. Toxicol. 2019, 42, 84–93. [Google Scholar] [CrossRef]
- Sahu, S.C.; Njoroge, J.; Bryce, S.M.; Yourick, J.J.; Sprando, R.L. Comparative genotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells evaluated by a flow cytometric in vitro micronucleus assay. J. Appl. Toxicol. 2014, 34, 1226–1234. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, T.; Zhang, B.; Gong, F.; Huang, Y.; Tang, M. Cytotoxicity and apoptosis induced by silver nanoparticles in human liver HepG2 cells in different dispersion media. J. Appl. Toxicol. 2016, 36, 352–360. [Google Scholar] [CrossRef]
- Wilson, C.L.; Natarajan, V.; Hayward, S.L.; Khalimonchuk, O.; Kidambi, S. Mitochondrial dysfunction and loss of glutamate uptake in primary astrocytes exposed to titanium dioxide nanoparticles. Nanoscale 2015, 7, 18477–18488. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Lin, Q.; Huang, P.; Wang, Y.; Li, J.; Zhang, L.; Cao, J. Rice Bioactive Peptide Binding with TLR4 To Overcome H2O2-Induced Injury in Human Umbilical Vein Endothelial Cells through NF-κB Signaling. J. Agric. Food Chem. 2018, 66, 440–448. [Google Scholar] [CrossRef]
- Natarajan, V.; Wilson, C.L.; Hayward, S.L.; Kidambi, S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. PLoS ONE 2015, 10, e0134541. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Madl, A.K.; Plummer, L.E.; Carosino, C.; Pinkerton, K.E. Nanoparticles, lung injury, and the role of oxidant stress. Annu. Rev. Physiol. 2014, 76, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ma, L.; Yan, T.; Han, X.; Xu, J.; Xu, J.; Xu, X. Activated mitochondrial apoptosis in hESCs after dissociation involving the PKA/p-p53/Bax signaling pathway. Exp. cell Res. 2018, 369, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrześniok, D.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin triggers the apoptosis of human triple-negative breast cancer MDA-MB-231 cells via the p53/Bax/Bcl-2 signaling pathway. Int. J. Oncol. 2018, 52, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- El-Deeb, N.M.; El-Sherbiny, I.M.; El-Aassara, M.R.; Hafez, E.E. Novel trend in colon cancer therapy using silver nanoparticles synthesized by honey bee. J. Nanomed. Nanotechnol. 2015, 6, 265. [Google Scholar] [CrossRef] [Green Version]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A two-faced genome guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Miethling-Graff, R.; Rumpker, R.; Richter, M.; Verano-Braga, T.; Kjeldsen, F.; Brewer, J. Exposure to silver nanoparticles induces size-and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Vitr. 2014, 28, 1280–1289. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nano silver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jia, M.X.; Wang, J.H.; Lu, J.L.; Deng, J.; Tang, J.X.; Liu, C. Association of MMP9-1562C/T and MMP13-77A/G Polymorphisms with Non-Small Cell Lung Cancer in Southern Chinese Population. Biomolecules 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Jia, M.X.; Deng, J.; Wang, J.H.; Lin, Q.L.; Tang, J.X.; Liu, X.Y. Down-regulation of microRNA-200b is a potential prognostic marker of lung cancer in southern-central Chinese population. Saudi J. Biol. Sci. 2019, 26, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jia, M.X.; Deng, J.; Wang, J.H.; Lin, Q.L.; Liu, C.; Su, W. Isolation, genetic identification and degradation characteristics of COD-degrading bacterial strain in slaughter wastewater. Saudi J. Biol. Sci. 2018, 25, 1800–1805. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Zhang, W.; He, T.; Shu, M.; Deng, J.; Wang, J.; Li, W.; Bai, J.; Lin, Q.; Luo, F.; et al. Evaluation of the Genotoxic and Oxidative Damage Potential of Silver Nanoparticles in Human NCM460 and HCT116 Cells. Int. J. Mol. Sci. 2020, 21, 1618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051618
Jia M, Zhang W, He T, Shu M, Deng J, Wang J, Li W, Bai J, Lin Q, Luo F, et al. Evaluation of the Genotoxic and Oxidative Damage Potential of Silver Nanoparticles in Human NCM460 and HCT116 Cells. International Journal of Molecular Sciences. 2020; 21(5):1618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051618
Chicago/Turabian StyleJia, Mingxi, Wenjing Zhang, Taojin He, Meng Shu, Jing Deng, Jianhui Wang, Wen Li, Jie Bai, Qinlu Lin, Feijun Luo, and et al. 2020. "Evaluation of the Genotoxic and Oxidative Damage Potential of Silver Nanoparticles in Human NCM460 and HCT116 Cells" International Journal of Molecular Sciences 21, no. 5: 1618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051618




