Melanogenic Properties and Expression Profiles of Melanogenic Paracrine Molecules in Riehl’s Melanosis
Abstract
:1. Introduction
2. Results
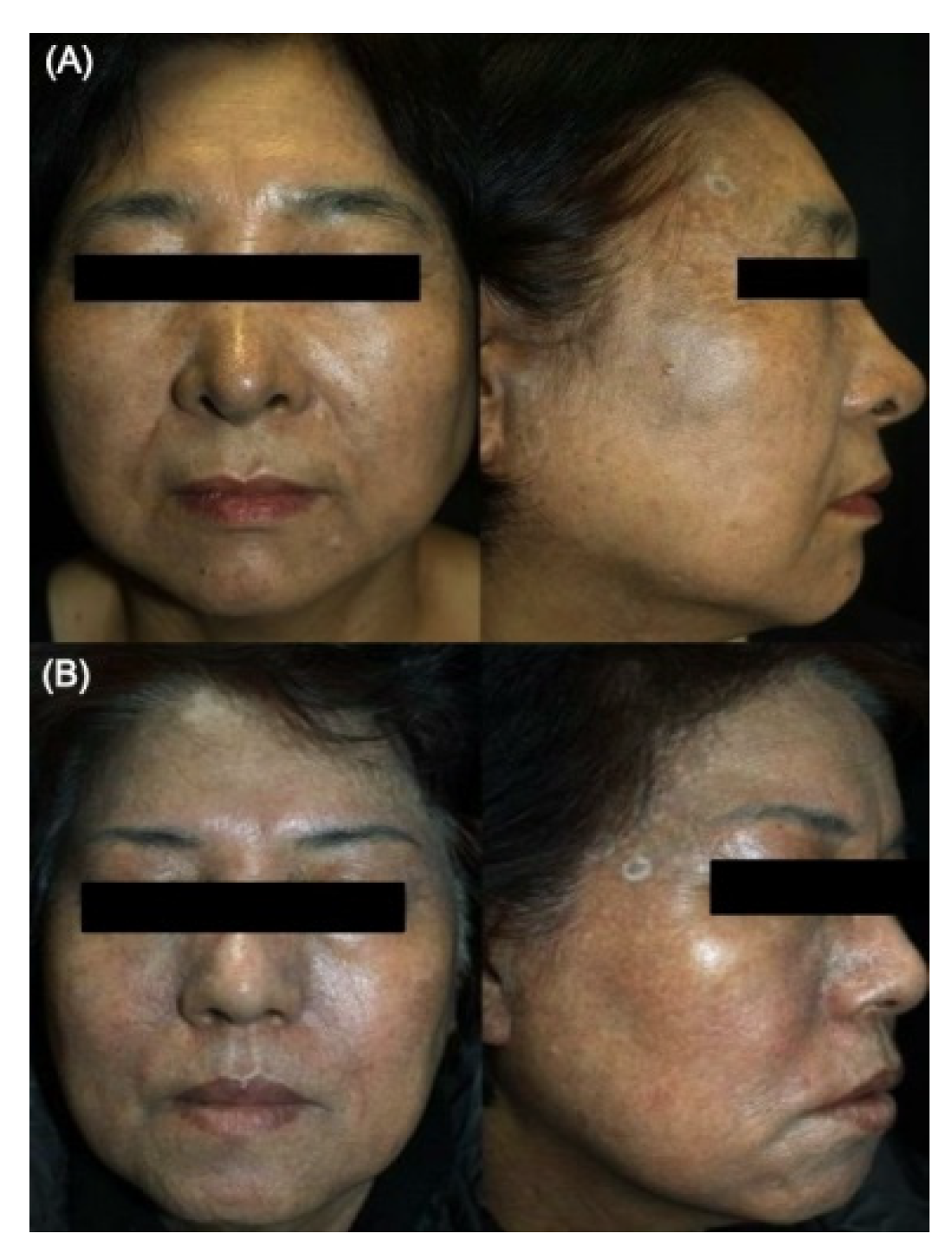
2.1. Comparative General Histopathological Patterns in Riehl’s Melanosis and Healthy Controls
2.2. Profiles of Melanin Pigmentation and Number of Melanocytes in Riehl’s Melanosis
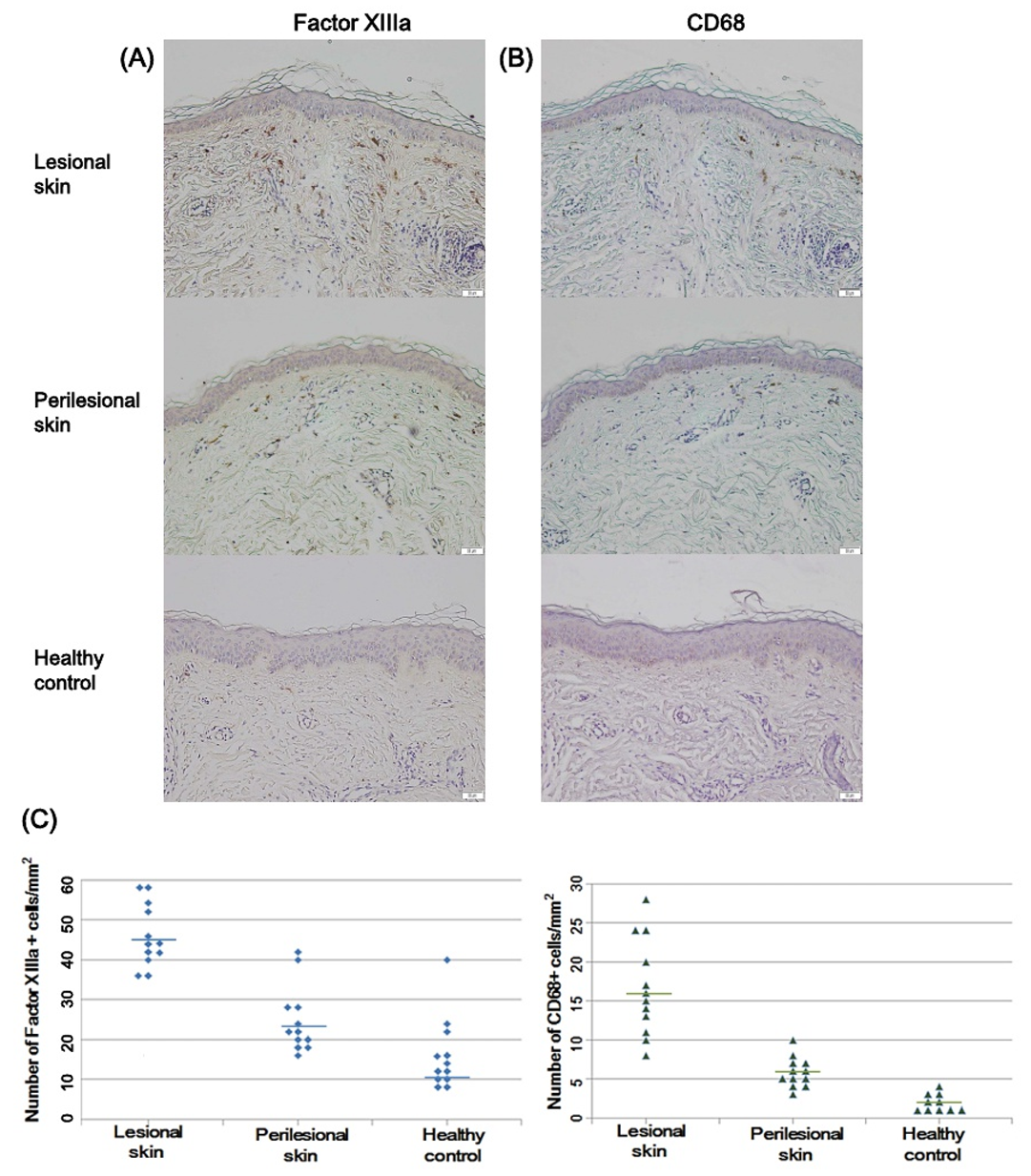
2.3. Factor XIIIa and CD68-Positive Cells in Riehl’s Melanosis
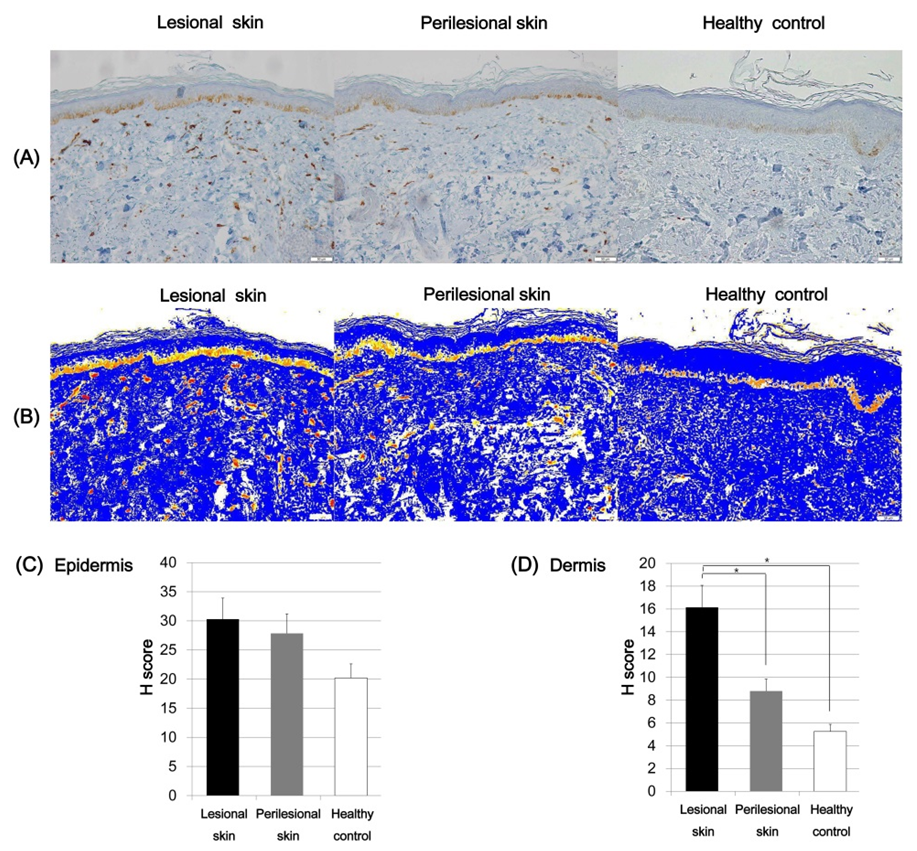
2.4. Expression of SCF and C-Kit in Riehl’s Melanosis
2.5. Expression of ET-1 in Riehl’s Melanosis
3. Discussion
4. Methods and Materials
4.1. Skin Biopsy Specimens
4.2. Skin Histology and Immunohistochemical Analyses
4.3. Quantitative and Semiquantitative Digital Analysis Methods
4.4. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Riehl, G. Über eine eigenartige Melanose. Wien. Klin Wochschr 1917, 25, 780–781. [Google Scholar]
- Woo, Y.R.; Kim, J.S.; Lim, J.H.; Choi, J.Y.; Kim, M.; Yu, D.S.; Park, Y.M.; Park, H.J. Acquired diffuse slate-grey facial dyspigmentation due to henna: An unrecognized cause of pigment contact dermatitis in Korean patients. Eur. J. Dermatol. 2018, 28, 644–648. [Google Scholar] [PubMed]
- Trattner, A.; Hodak, E.; David, M. Screening patch tests for pigmented contact dermatitis in Israel. Contact Dermat. 1999, 40, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, S.P.W.; Pandya, A.; Chandran, V.; Rodrigues, M.; Dlova, N.C.; Kang, H.Y.; Ramam, M.; Dayrit, J.F.; Goh, B.K.; Parsad, D. A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl’s melanosis. Int. J. Derm. 2019, 58, 263–272. [Google Scholar] [CrossRef]
- Kwon, H.H.; Ohn, J.; Suh, D.H.; Park, H.Y.; Choi, S.C.; Jung, J.Y.; Kwon, I.H.; Park, G.H. A pilot study for triple combination therapy with a low-fluence 1064 nm Q-switched Nd:YAG laser, hydroquinone cream and oral tranexamic acid for recalcitrant Riehl’s Melanosis. J. Dermatol. Treat. 2017, 28, 155–159. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, J.; Chen, J.Z.; Wu, Y.; Xu, T.H.; Zhu, X.; Liu, W.; Wei, H.C.; Gao, X.H.; Chen, H.D. A pilot study of intense pulsed light in the treatment of Riehl’s melanosis. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2011, 37, 119–122. [Google Scholar]
- Wang, L.; Xu, A.E. Four views of Riehl’s melanosis: Clinical appearance, dermoscopy, confocal microscopy and histopathology. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1199–1206. [Google Scholar] [CrossRef]
- Yada, Y.; Higuchi, K.; Imokawa, G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J. Biol. Chem. 1991, 266, 18352–18357. [Google Scholar]
- Hachiya, A.; Kobayashi, A.; Ohuchi, A.; Takema, Y.; Imokawa, G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J. Invest. Derm. 2001, 116, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Yada, Y.; Kimura, M.; Morisaki, N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. Biochem. J. 1996, 313, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Investig. 1994, 93, 2258–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unver, N.; Freyschmidt-Paul, P.; Horster, S.; Wenck, H.; Stab, F.; Blatt, T.; Elsasser, H.P. Alterations in the epidermal-dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. Br. J. Dermatol. 2006, 155, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, A.J.; Penneys, N.S. Factor XIIIa is expressed by fibroblasts in fibrovascular tumors. J. Cutan. Pathol. 1989, 16, 266–271. [Google Scholar] [CrossRef]
- Paragh, L.; Torocsik, D. Factor XIII Subunit A in the Skin: Applications in Diagnosis and Treatment. Biomed. Res. Int. 2017, 2017, 3571861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado, P.R.; Jardim Criado, R.F.; Sotto, M.N.; Pagliari, C.; Takakura, C.H.; Vasconcellos, C. Dermal dendrocytes FXIIIA+ phagocytizing extruded mast cell granules in drug-induced acute urticaria. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e105–e112. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.T.; Loncaric, A.; Krutzik, S.R.; Becker, T.C.; Modlin, R.L. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest. Derm. 2008, 128, 2225–2231. [Google Scholar] [CrossRef] [Green Version]
- Li, P.H.; Liu, L.H.; Chang, C.C.; Gao, R.; Leung, C.H.; Ma, D.L.; David Wang, H.M. Silencing Stem Cell Factor Gene in Fibroblasts to Regulate Paracrine Factor Productions and Enhance c-Kit Expression in Melanocytes on Melanogenesis. Int. J. Mol. Sci. 2018, 19, 1475. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Shibata, T.; Kwon, S.; Park, T.J.; Kang, H.Y. Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Sci. Rep. 2018, 8, 4235. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hartmann, K.; Eckes, B.; Krieg, T. Role of stem cell factor and monocyte chemoattractant protein-1 in the interaction between fibroblasts and mast cells in fibrosis. J. Dermatol. Sci. 2001, 26, 106–111. [Google Scholar] [CrossRef]
- Reber, L.; Da Silva, C.A.; Frossard, N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur. J. Pharmacol. 2006, 533, 327–340. [Google Scholar] [CrossRef]
- Grabbe, J.; Welker, P.; Dippel, E.; Czarnetzki, B.M. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Arch. Dermatol. Res. 1994, 287, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Longley Jr, B.J.; Morganroth, G.S.; Tyrrell, L.; Ding, T.G.; Anderson, D.M.; Williams, D.E.; Halaban, R. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N. Engl. J. Med. 1993, 328, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Kadono, S.; Manaka, I.; Kawashima, M.; Kobayashi, T.; Imokawa, G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J. Investig. Dermatol. 2001, 116, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demunter, A.; De Wolf-Peeters, C.; Degreef, H.; Stas, M.; van den Oord, J.J. Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001, 438, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Turnley, A.M.; Maxwell, G.D.; Kurihara, Y.; Kurihara, H.; Bartlett, P.F.; Murphy, M. Multiple roles for endothelin in melanocyte development: Regulation of progenitor number and stimulation of differentiation. Development 1996, 122, 3911–3919. [Google Scholar] [PubMed]
- Amiel, J.; Attie, T.; Jan, D.; Pelet, A.; Edery, P.; Bidaud, C.; Lacombe, D.; Tam, P.; Simeoni, J.; Flori, E.; et al. Heterozygous endothelin receptor B (EDNRB) mutations in isolated Hirschsprung disease. Hum. Mol. Genet. 1996, 5, 355–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teraki, E.; Tajima, S.; Manaka, I.; Kawashima, M.; Miyaclshi, M.; Imokawa, G. Role of endothelin-1 in hyperpigmentation in seborrhoeic keratosis. Br. J. Dermatol. 1996, 135, 918–923. [Google Scholar] [CrossRef]
- Sriwiriyanont, P.; Ohuchi, A.; Hachiya, A.; Visscher, M.O.; Boissy, R.E. Interaction between stem cell factor and endothelin-1: Effects on melanogenesis in human skin xenografts. Lab. Investig. 2006, 86, 1115–1125. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.E.; Jung, H.Y.; Jo, H.; Jeong, S.-w.; Lee, J.; Kim, C.H.; Kim, H.; Cho, D.; Park, H.J. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp. Dermatol. 2015, 24, 680–685. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A.; Di Maria, M.V.; Veve, R.; Bremnes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal growth factor receptor in non–small-cell lung carcinomas: Correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003, 21, 3798–3807. [Google Scholar] [CrossRef]



| Hematoxylin and Eosin | Riehl’s Melanosis | HC | p Value | |||
|---|---|---|---|---|---|---|
| L | PL | Riehl’s Melanosis, L vs. PL | Riehl’s Melanosis, L vs. HC | Riehl’s Melanosis, PL vs. HC | ||
| Epidermal change | ||||||
| Orthokeratosis | 1.42 ± 0.92 | 0.70 ± 0.23 | 0.20 ± 0.20 | 0.06 | 0.07 | 0.13 |
| Parakeratosis | 0.05 ± 0.04 | 0.08 ± 0.27 | 0.00 ± 0.00 | 0.68 | 0.33 | 0.47 |
| Epidermal thinning | 1.36 ± 0.98 | 0.50 ± 0.47 | 0.6 ± 0.79 | 0.07 | 0.14 | 0.57 |
| Cytoid body | 1.15 ± 1.12 | 0.08 ± 0.27 | 0.00 ± 0.00 | <0.001 * | <0.001 * | 0.47 |
| Rete ridge flattening | 1.63 ± 0.95 | 1.30 ± 0.74 | 0.40 ± 0.28 | 0.17 | 0.05 | 0.20 |
| Interface change | 2.10 ± 0.99 | 0.08 ± 0.27 | 0.00 ± 0.00 | <0.001 * | <0.001 * | 0.47 |
| Dermal change | ||||||
| Perivascular inflammation | 1.94 ± 0.77 | 1.66 ± 0.23 | 0.60 ± 0.24 | 0.40 | 0.001 * | 0.10 |
| Periadnexal inflammation | 1.06 ± 1.03 | 0.66 ± 0.33 | 0.20 ± 0.16 | 0.29 | 0.05 | 0.14 |
| Pigmentary incontinence | 1.89 ± 0.51 | 0.64 ± 0.27 | 0.00 ± 0.00 | 0.02 * | <0.001 * | 0.02 * |
| Solar elastosis | 0.63 ± 0.54 | 0.40 ± 0.09 | 0.20 ± 0.14 | 0.23 | 0.13 | 0.88 |
| Vascular proliferation | 1.73 ± 0.40 | 1.20 ± 0.36 | 0.80 ± 0.20 | 0.16 | 0.001 * | 0.17 |
| Riehl’s Melanosis | HC | p Value | |||
|---|---|---|---|---|---|
| L | PL | Riehl’s Melanosis, L vs. PL | Riehl’s Melanosis, L vs. HC | ||
| Pigmentation (PA/EA) | 0.23 ± 0.05 | 0.16 ± 0.03 | 0.19 ± 0.05 | 0.005 * | 0.06 |
| Melanocytes (MC/1R) | 17.81 ± 4.35 | 12.09 ± 4.07 | 10.45 ± 5.05 | 0.01 * | 0.01 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, Y.R.; Park, H.E.; Jeong, S.-W.; Park, H.J. Melanogenic Properties and Expression Profiles of Melanogenic Paracrine Molecules in Riehl’s Melanosis. Int. J. Mol. Sci. 2020, 21, 1695. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051695
Woo YR, Park HE, Jeong S-W, Park HJ. Melanogenic Properties and Expression Profiles of Melanogenic Paracrine Molecules in Riehl’s Melanosis. International Journal of Molecular Sciences. 2020; 21(5):1695. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051695
Chicago/Turabian StyleWoo, Yu Ri, Hyo Eun Park, Seo-Won Jeong, and Hyun Jeong Park. 2020. "Melanogenic Properties and Expression Profiles of Melanogenic Paracrine Molecules in Riehl’s Melanosis" International Journal of Molecular Sciences 21, no. 5: 1695. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051695





