Altered Levels of Sphingosine, Sphinganine and Their Ceramides in Atopic Dermatitis Are Related to Skin Barrier Function, Disease Severity and Local Cytokine Milieu
Abstract
:1. Introduction
2. Results
2.1. Biophysical Parameters
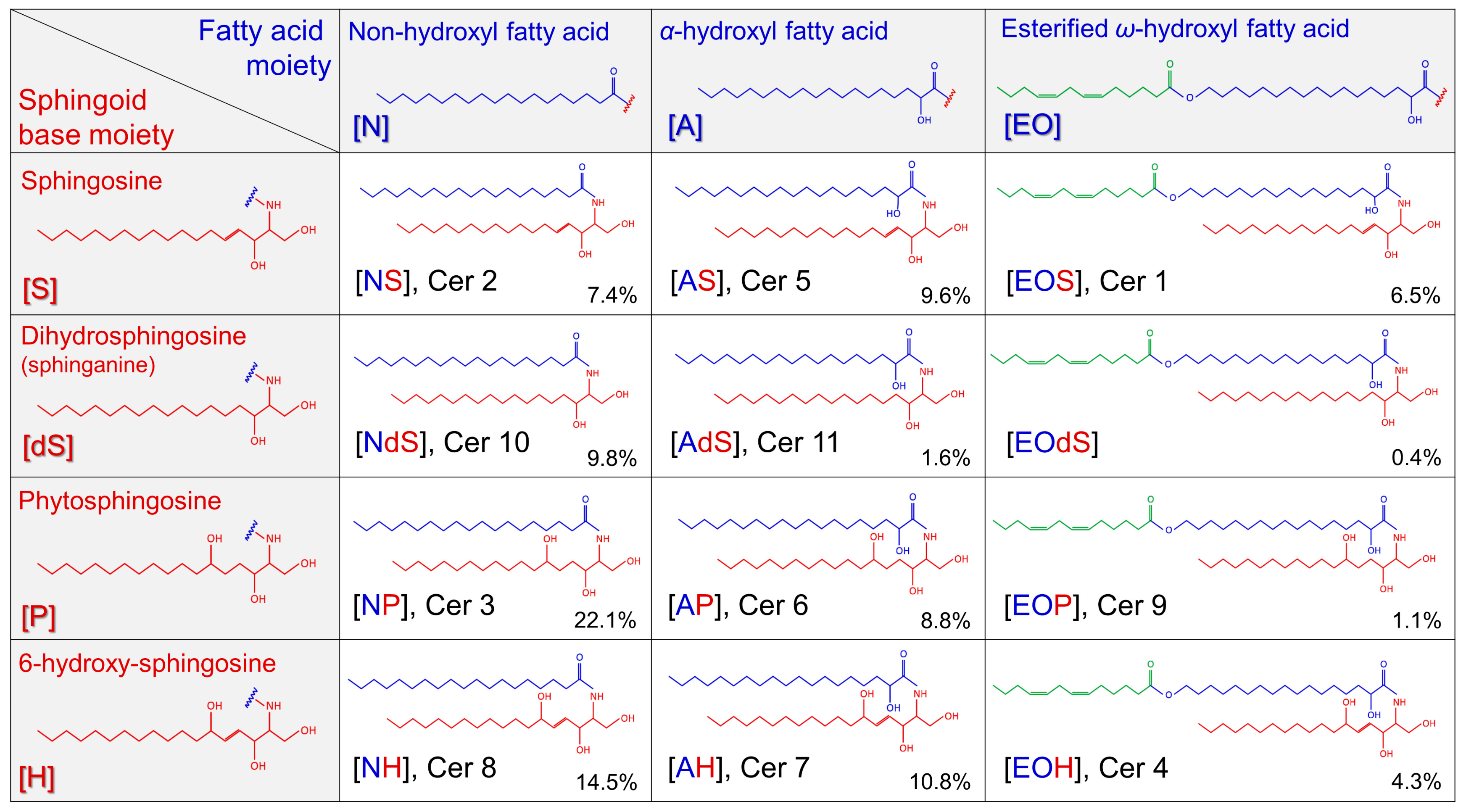
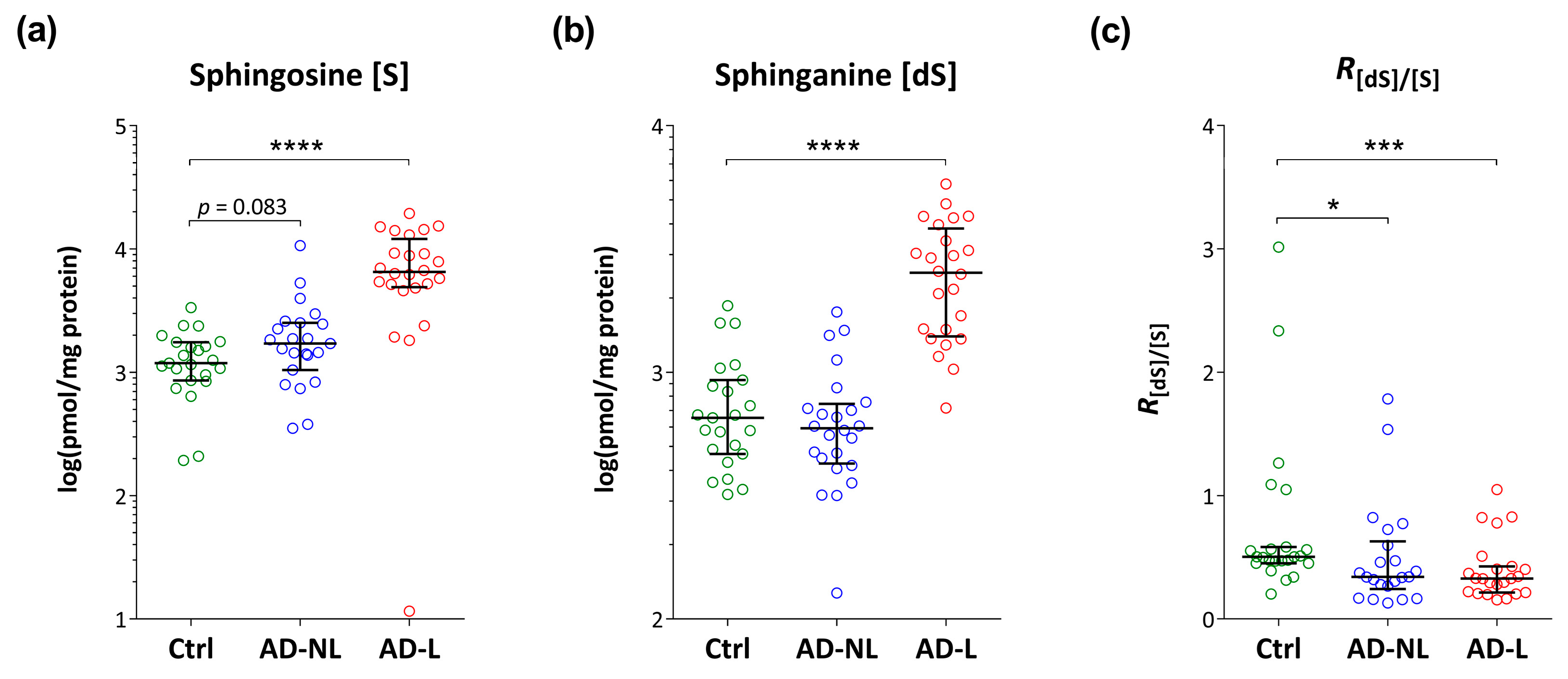
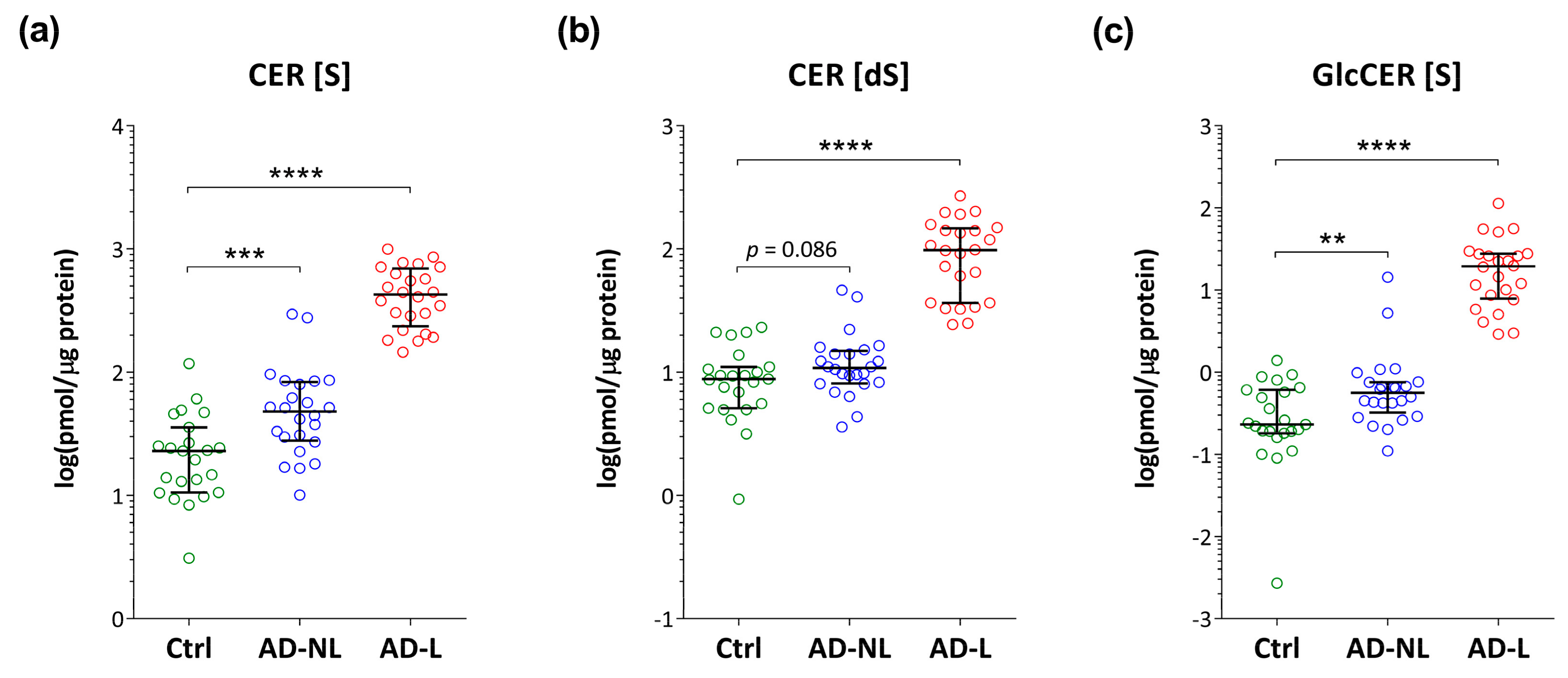
2.2. Sphingoid Bases and Their Ceramides in Healthy and AD Skin
2.3. Association of Ceramide Markers with Disease Severity (SCORAD) and Skin Barrier Function (TEWL)
2.4. Association of Lipid Biomarkers with Immunological Markers
3. Discussion
4. Materials and Methods
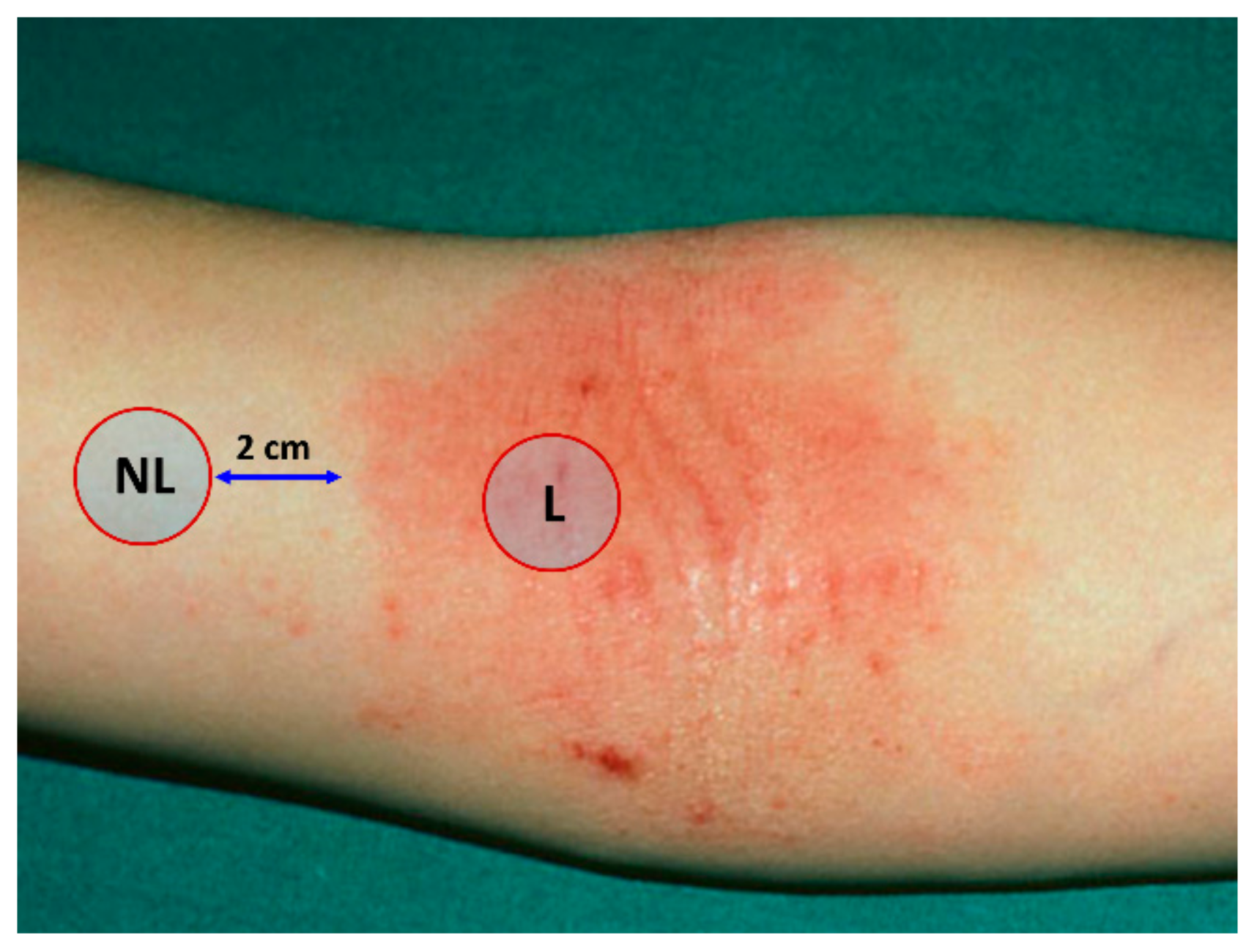
4.1. Study Population
4.2. Sampling of the Stratum Corneum
4.3. Measurements of TEWL And pH
4.4. Ceramide Analysis
4.4.1. Chemicals
4.4.2. Extraction from the Tapes and Processing
4.4.3. LC–MS/MS Analysis
4.5. Cytokine Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| aSMase | acid sphingomylinase |
| CER CER (S) CER (dS) | ceramides d18:1 ceramides d18:0 ceramides |
| FA FFA GBA GBA1 GlcCER LC MS (S) (dS) SB SC SCORAD TEWL | fatty acid free fatty acid β-glucosylcerebrosidase lysosomal glucosylceramide-β-glucosidase glucosyl ceramide liquid chromatography mass spectrometry sphingosine (d18:1) sphinganine (d18:0) sphingoid base stratum corneum scoring atopic dermatitis trans-epidermal water loss |
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Israel, A.; Zhang, N.; Leonard, A.; Wen, H.C.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; et al. Early-onset pediatric atopic dermatitis is characterized by T(H)2/T(H)17/T(H)22-centered inflammation and lipid alterations. J. Allergy Clin. Immunol. 2018, 141, 2094–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, P.M.; Leung, D.Y.M.; Guttman-Yassky, E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann. Allergy Asthma. Immunol. 2018, 120, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manti, S.; Amorini, M.; Cuppari, C.; Salpietro, A.; Porcino, F.; Leonardi, S.; Giudice, M.M.D.; Marseglia, G.; Caimmi, D.P.; Salpietro, C. Filaggrin mutations and Molluscum contagiosum skin infection in patients with atopic dermatitis. Ann. Allergy Asthma. Immunol. 2017, 119, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, C.; Manti, S.; Salpietro, A.; Valenti, S.; Capizzi, A.; Arrigo, T.; Salpietro, C.; Leonardi, S. HMGB1 levels in children with atopic eczema/dermatitis syndrome (AEDS). Pediatr. Allergy Immunol. 2016, 27, 99–102. [Google Scholar] [CrossRef]
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the barrier abnormality in atopic dermatitis: Outside-inside-outside pathogenic mechanisms. J. Allergy Clin. Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Allergy Asthma. Rep. 2009, 9, 265–272. [Google Scholar] [CrossRef]
- van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad Derm. 2014, 71, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Epidermal lipids, barrier function, and desquamation. J. Invest. Dermatol. 1983, 80, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Skin barrier function. Curr. Allergy Asthma. Rep. 2008, 8, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Rabionet, M.; Gorgas, K.; Sandhoff, R. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta 2014, 1841, 422–434. [Google Scholar] [CrossRef]
- Danso, M.; Boiten, W.; van Drongelen, V.; Gmelig Meijling, K.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J. Derm. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the ceramide profile of atopic dermatitis patients. J. Invest Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef] [Green Version]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- van Smeden, J.; Janssens, M.; Kaye, E.C.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol 2014, 23, 45–52. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Masukawa, Y.; Narita, H.; Shimizu, E.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; Kitahara, T.; et al. Characterization of overall ceramide species in human stratum corneum. J. Lipid Res. 2008, 49, 1466–1476. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.E.; Boudreau, R.M.; Couch, C.; Becker, K.A.; Edwards, M.J.; Caldwell, C.C.; Gulbins, E.; Seitz, A. Sphingosine’s role in epithelial host defense: A natural antimicrobial and novel therapeutic. Biochimie 2017, 141, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Blanchette, D.R.; Dawson, D.V.; Brogden, K.A.; Wertz, P.W. Sphingoid bases are taken up by Escherichia coli and Staphylococcus aureus and induce ultrastructural damage. Skin Pharmacol. Phys. 2013, 26, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loiseau, N.; Obata, Y.; Moradian, S.; Sano, H.; Yoshino, S.; Aburai, K.; Takayama, K.; Sakamoto, K.; Holleran, W.M.; Elias, P.M.; et al. Altered sphingoid base profiles predict compromised membrane structure and permeability in atopic dermatitis. J. Dermatol. Sci. 2013, 72, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihara, A. Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog. Lipid Res. 2016, 63, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Strum, J.C.; Ghosh, S.; Bell, R.M. Lipid second messengers. A role in cell growth regulation and cell cycle progression. Adv. Exp. Med. Biol. 1997, 407, 421–431. [Google Scholar]
- Agrawal, K.; Sivamani, R.K.; Newman, J.W. Noninvasive profiling of sweat-derived lipid mediators for cutaneous research. Skin Res. Technol. 2019, 25, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Arikawa, J.; Ishibashi, M.; Kawashima, M.; Takagi, Y.; Ichikawa, Y.; Imokawa, G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Invest Dermatol. 2002, 119, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.P.; Zhao, M.T.; Jia, Z.X.; Zhang, J.L.; Jiao, L.; Ma, L. Skin Ceramide Profile in Children With Atopic Dermatitis. Dermatitis 2018, 29, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Invest Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Fang, H.; Dang, E.; Wang, G. The role of ceramides in skin homeostasis and inflammatory skin diseases. J. Dermatol. Sci. 2019. [Google Scholar] [CrossRef]
- Owczarek, W.; Paplinska, M.; Targowski, T.; Jahnz-Rozyk, K.; Paluchowska, E.; Kucharczyk, A.; Kasztalewicz, B. Analysis of eotaxin 1/CCL11, eotaxin 2/CCL24 and eotaxin 3/CCL26 expression in lesional and non-lesional skin of patients with atopic dermatitis. Cytokine 2010, 50, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; He, H.; Pavel, A.B.; Czarnowicki, T.; Lefferdink, R.; Erickson, T.; Canter, T.; Puar, N.; Rangel, S.M.; Malik, K.; et al. The blood proteomic signature of early-onset pediatric atopic dermatitis shows systemic inflammation and is distinct from adult long-standing disease. J. Am. Acad Dermatol. 2019, 81, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; Oyoshi, M.K.; Le, Y.; Bianchi, T.; Koduru, S.; Mathias, C.B.; Kumar, L.; Le Bras, S.; Young, D.; Collins, M.; et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J. Clin. Investig. 2009, 119, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Suarez-Farinas, M.; Chiricozzi, A.; Nograles, K.E.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Lin, P.; Bergman, R.; Bowcock, A.M.; et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J. Allergy Clin. Immunol. 2009, 124, 1235–1244.e58. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.O.; van Drongelen, V.; Mulder, A.; van Esch, J.; Scott, H.; van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Invest Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanoh, H.; Ishitsuka, A.; Fujine, E.; Matsuhaba, S.; Nakamura, M.; Ito, H.; Inagaki, N.; Banno, Y.; Seishima, M. IFN-gamma Reduces Epidermal Barrier Function by Affecting Fatty Acid Composition of Ceramide in a Mouse Atopic Dermatitis Model. J. Immunol. Res. 2019, 2019, 3030268. [Google Scholar] [CrossRef] [Green Version]
- Sawada, E.; Yoshida, N.; Sugiura, A.; Imokawa, G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: An implication for the disrupted barrier mechanism in atopic dermatitis. J. Dermatol. Sci. 2012, 68, 25–35. [Google Scholar] [CrossRef]
- Tawada, C.; Kanoh, H.; Nakamura, M.; Mizutani, Y.; Fujisawa, T.; Banno, Y.; Seishima, M. Interferon-gamma decreases ceramides with long-chain fatty acids: Possible involvement in atopic dermatitis and psoriasis. J. Invest. Dermatol. 2014, 134, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Boiten, W.; Absalah, S.; Vreeken, R.; Bouwstra, J.; van Smeden, J. Quantitative analysis of ceramides using a novel lipidomics approach with three dimensional response modelling. Biochim. Biophys. Acta 2016, 1861, 1652–1661. [Google Scholar] [CrossRef]
- van Smeden, J.; Boiten, W.A.; Hankemeier, T.; Rissmann, R.; Bouwstra, J.A.; Vreeken, R.J. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim. Biophys. Acta 2014, 1841, 70–79. [Google Scholar] [CrossRef]
- van Smeden, J.; Hoppel, L.; van der Heijden, R.; Hankemeier, T.; Vreeken, R.J.; Bouwstra, J.A. LC/MS analysis of stratum corneum lipids: Ceramide profiling and discovery. J. Lipid Res. 2011, 52, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffet, G.P.; Genette, A.; Gamboa, B.; Auroy, V.; Voegel, J.J. Determination of fatty acid and sphingoid base composition of eleven ceramide subclasses in stratum corneum by UHPLC/scheduled-MRM. Metabolomics 2018, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.X.; Zhang, J.L.; Shen, C.P.; Ma, L. Profile and quantification of human stratum corneum ceramides by normal-phase liquid chromatography coupled with dynamic multiple reaction monitoring of mass spectrometry: Development of targeted lipidomic method and application to human stratum corneum of different age groups. Anal. Bioanal. Chem. 2016, 408, 6623–6636. [Google Scholar] [PubMed]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suarez-Farinas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanifin, J.M. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. Suppl. 1980, 92, 44–47. [Google Scholar]
- Kunz, B.; Oranje, A.P.; Labreze, L.; Stalder, J.F.; Ring, J.; Taieb, A. Clinical validation and guidelines for the SCORAD index: Consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997, 195, 10–19. [Google Scholar] [CrossRef]
- McAleer, M.A.; Jakasa, I.; Hurault, G.; Sarvari, P.; McLean, W.H.I.; Tanaka, R.J.; Kezic, S.; Irvine, A.D. Systemic and stratum corneum biomarkers of severity in infant atopic dermatitis include markers of innate and T helper cell-related immunity and angiogenesis. Br. J. Dermatol 2019, 180, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Groener, J.E.; Poorthuis, B.J.; Kuiper, S.; Helmond, M.T.; Hollak, C.E.; Aerts, J.M. HPLC for simultaneous quantification of total ceramide, glucosylceramide, and ceramide trihexoside concentrations in plasma. Clin. Chem. 2007, 53, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Gold, H.; Mirzaian, M.; Dekker, N.; Joao Ferraz, M.; Lugtenburg, J.; Codee, J.D.; van der Marel, G.A.; Overkleeft, H.S.; Linthorst, G.E.; Groener, J.E.; et al. Quantification of globotriaosylsphingosine in plasma and urine of fabry patients by stable isotope ultraperformance liquid chromatography-tandem mass spectrometry. Clin. Chem. 2013, 59, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Whitley, E.; Ball, J. Statistics review 4: Sample size calculations. Crit. Care 2002, 6, 335–341. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toncic, R.J.; Jakasa, I.; Hadzavdic, S.L.; Goorden, S.M.; Vlugt, K.J.G.-v.d.; Stet, F.S.; Balic, A.; Petkovic, M.; Pavicic, B.; Zuzul, K.; et al. Altered Levels of Sphingosine, Sphinganine and Their Ceramides in Atopic Dermatitis Are Related to Skin Barrier Function, Disease Severity and Local Cytokine Milieu. Int. J. Mol. Sci. 2020, 21, 1958. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21061958
Toncic RJ, Jakasa I, Hadzavdic SL, Goorden SM, Vlugt KJG-vd, Stet FS, Balic A, Petkovic M, Pavicic B, Zuzul K, et al. Altered Levels of Sphingosine, Sphinganine and Their Ceramides in Atopic Dermatitis Are Related to Skin Barrier Function, Disease Severity and Local Cytokine Milieu. International Journal of Molecular Sciences. 2020; 21(6):1958. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21061958
Chicago/Turabian StyleToncic, Ruzica Jurakic, Ivone Jakasa, Suzana Ljubojevic Hadzavdic, Susan MI Goorden, Karen JM Ghauharali-van der Vlugt, Femke S Stet, Anamaria Balic, Mikela Petkovic, Borna Pavicic, Kristina Zuzul, and et al. 2020. "Altered Levels of Sphingosine, Sphinganine and Their Ceramides in Atopic Dermatitis Are Related to Skin Barrier Function, Disease Severity and Local Cytokine Milieu" International Journal of Molecular Sciences 21, no. 6: 1958. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21061958




