Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats
Abstract
:1. Introduction
2. Results
2.1. Behavioural Studies
Effect of Prenatal and Postnatal Fluoride Exposure on the Intensity of Morphine Withdrawal Signs Observed as Jumps
2.2. Ex Vivo Neurochemical Studies—the Analysis of the Concentration of Dopamine and Its Metabolites
2.3. The Analysis of Gene Expression of Dopamine Receptors in the Striatum, the Hippocampus, the Prefrontal Cortex, and the Cerebellum
2.4. The Analysis of the Protein Expression of Dopamine Receptors
2.5. Immunohistochemical Analysis of Dopamine Receptors
2.5.1. IHC for Dopamine Receptor D1
2.5.2. IHC for Dopamine Receptor D2
3. Discussion
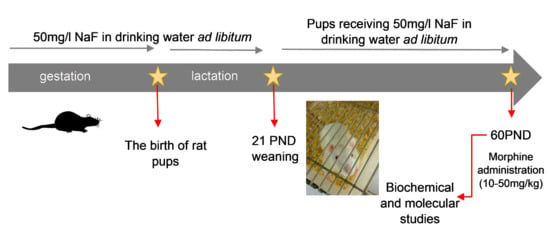
4. Materials and Methods
4.1. Fluoride Toxicity Procedure
4.2. The Procedure of Morphine Dependence
- (1)
- saline group—rats receiving 0.9% NaCl (n = 6);
- (2)
- saline + naloxone group—rats receiving 0.9% NaCl from the prenatal period until adulthood; on the last day of the experiment, they received saline with naloxone (n = 6); these animals were excluded from molecular studies;
- (3)
- fluoride group—rats treated with fluoride (NaF) from the prenatal period until adulthood and receiving 0.9% NaCl instead of morphine. They received naloxone on the last day of the experiment (n = 6);
- (4)
- morphine group—rats receiving 0.9% NaCl from the prenatal period until adulthood, and then procedure of morphine dependence was performed (n = 6);
- (5)
- fluoride + morphine group—rats treated with fluoride (NaF) from the prenatal period until adulthood, F-treated, and then the procedure of morphine dependence was performed (n = 6).
4.3. Ex Vivo Biochemical Studies—the Analysis of the Concentration of Dopamine and Its Metabolites in the Striatum, the Hippocampus, the Prefrontal Cortex, and the Cerebellum by the HPLC-ED Method
4.4. The Analysis of Gene Expression in the Striatum, the Hippocampus, the Prefrontal Cortex, and the Cerebellum by Real-Time Quantitative Reverse Transcription PCR (RQ-PCR)
4.5. The Analysis of Protein Expression in the Striatum, the Hippocampus, the Prefrontal Cortex, and the Cerebellum by the Western Blotting Method
4.6. Immunohistochemical Analysis of D1 and D2 Receptors in the Hippocampus, the Striatum, the Prefrontal Cortex, and the Cerebellum
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3-MT | 3-methoxytyramine |
| CaMKIIg | Ca2 + /CaM dependent protein, kinase II gamma |
| COMT | catechol-O-methyl transferase |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| D1R | dopamine receptor D1 |
| D2R | dopamine receptor D2 |
| ERK | extracellular signal- regulated kinases |
| GABA | gamma-aminobutyric acid |
| GCL | granular cell layer |
| GSH | reduced glutathione |
| H2O2 | hydrogen peroxide |
| HPLC-ED | high-performance liquid chromatography with electrochemical detection |
| HVA | homovanillic acid |
| IHC | immunohistochemical analysis |
| IQ | intelligence quotient |
| MAO-B | monoaminooxidase-B |
| MCL | molecular cell layer |
| PCL | polymorphic cell layer |
| PND | postnatal day |
| PyrCL | pyramidal cell layer |
| qRT-PCR | real-time quantitative reverse transcription PCR |
| WM | white matter |
References
- Dec, K.; Łukomska, A.; Maciejewska, D.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Chlubek, D.; Wąsik, A.; Gutowska, I. The Influence of Fluorine on the Disturbances of Homeostasis in the Central Nervous System. Biol. Trace Elem. Res. 2017, 177, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Han, Y.-E.E.; Favorov, O.; Tommerdahl, M.; Whitsel, B.; Lee, C.J. Fluoride Induces a Volume Reduction in CA1 Hippocampal Slices Via MAP Kinase Pathway Through Volume Regulated Anion Channels. Exp. Neurobiol. 2016, 25, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Li, G.; Zhou, X.; Wang, C.; Qiao, Y.; Liao, D.; Shi, D. Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: Role of GSK-3Β/Β-catenin pathway. Chemosphere 2019, 214, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Chlubek, D. Fluoride and oxidative stress. Fluoride 2003, 36, 217–228. [Google Scholar]
- Basha, P.M.; Madhusudhan, N. Pre and post natal exposure of fluoride induced oxidative macromolecular alterations in developing central nervous system of rat and amelioration by antioxidants. Neurochem. Res. 2010, 35, 1017–1028. [Google Scholar] [CrossRef]
- Chioca, L.R.; Raupp, I.M.; Da Cunha, C.; Losso, E.M.; Andreatini, R. Subchronic fluoride intake induces impairment in habituation and active avoidance tasks in rats. Eur. J. Pharmacol. 2008, 579, 196–201. [Google Scholar] [CrossRef]
- Yazdi, S.M.; Sharifian, A.; Dehghani-Beshne, M.; Momeni, V.R.; Aminian, O. Effects of fluoride on psychomotor performance and memory of aluminum potroom workers. Fluoride 2011, 44, 158–162. [Google Scholar]
- Shivarajashankara, Y.M.; Shivashankara, A.R.; Gopalakrishna Bhat, P.; Muddanna Rao, S.; Hanumanth Rao, S. Histological changes in the brain of young fluoride-intoxicated rats. Fluoride 2002, 35, 12–21. [Google Scholar]
- Amunts, K.; Kedo, O.; Kindler, M.; Pieperhoff, P.; Mohlberg, H.; Shah, N.J.; Habel, U.; Schneider, F.; Zilles, K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat. Embryol. 2005, 210, 343–352. [Google Scholar] [CrossRef]
- Corbett, A.D.; Henderson, G.; McKnight, A.T.; Paterson, S.J. 75 Years of opioid research: The exciting but vain quest for the Holy Grail. Br. J. Pharmacol. 2006, 147 (Suppl. 1), 153–162. [Google Scholar] [CrossRef] [Green Version]
- Koob, G.F.; Stinus, L.; Moal, M.L.; Bloom, F.E. Opponent process theory of motivation: Neurobiological evidence from studies of opiate dependence. Neurosci. Biobehav. Rev. 1989, 13, 135–140. [Google Scholar] [CrossRef]
- Morales-González, J.A.; Gutiérrez-Salinas, J.; García-Ortiz, L.; Chima-Galán, M.d.C.; Madrigal-Santillán, E.; Esquivel-Soto, J.; Esquivel-Chirino, C.; González-Rubio, M. Effect of sodium fluoride ingestion on malondialdehyde concentration and the activity of antioxidant enzymes in rat erythrocytes. Int. J. Mol. Sci. 2010, 11, 2443–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, C.A.N.; Leite, A.d.L.; Silva, T.L.d.; Santos, L.D.d.; Nogueira, F.C.S.; Santos, K.S.; Oliveira, R.C.d.; Palma, M.S.; Domont, G.B.; Buzalaf, M.A.R. Proteomic analysis of urine in rats chronically exposed to fluoride. J. Biochem. Mol. Toxicol. 2011, 25, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Centeno, V.A.; Fontanetti, P.A.; Interlandi, V.; Ponce, R.H.; Gallará, R.V. Fluoride alters connexin expression in rat incisor pulp. Arch. Oral Biol. 2014, 60, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, S.; Sun, Z.; Niu, R.; Wang, J. In Vivo influence of sodium fluoride on sperm chemotaxis in male mice. Arch. Toxicol. 2014, 88, 533–539. [Google Scholar] [CrossRef]
- Lu, L.; Ying, Z.; He-feng, G.U.; Kai-qiang, Z.; Lin, M.A.; Rui-bo, C.; Si-yu, Z. The effect of fluoride on the metabolism of teeth and bone in rats. Shanghai J. Stomatol. 2014, 23, 129–132. [Google Scholar]
- Błaszczyk, I.; Ratajczak-Kubiak, E.; Birkner, E. Advantageous and harmfully effect of fluoride. Farm. Pol. 2009, 65, 623–626. [Google Scholar]
- Zohoori, F.V.; Innerd, A.; Azevedo, L.B.; Whitford, G.M.; Maguire, A. Effect of exercise on fluoride metabolism in adult humans: A pilot study. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Kolasa-Wołosiuk, A.; Tarnowski, M.; Baranowska-Bosiacka, I.; Gutowska, I. Long-term exposure to fluoride as a factor promoting changes in the expression and activity of cyclooxygenases (COX1 and COX2) in various rat brain structures. Neurotoxicology 2019, 74, 81–90. [Google Scholar] [CrossRef]
- Reddy, Y.P.; Tiwari, S.K.; Shaik, A.P.; Alsaeed, A.; Sultana, A.; Reddy, P.K. Effect of sodium fluoride on neuroimmunological parameters, oxidative stress and antioxidative defenses. Toxicol. Mech. Methods 2014, 24, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Chirumari, K.; Reddy, P.K. Dose-dependent effects of fluoride on neurochemical milieu in the hippocampus and neocortex of rat brain. Fluoride 2007, 40, 101–110. [Google Scholar]
- Pereira, M.; Dombrowski, P.A.; Losso, E.M.; Chioca, L.R.; Da Cunha, C.; Andreatini, R. Memory impairment induced by sodium fluoride is associated with changes in brain monoamine levels. Neurotox. Res. 2011, 19, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Aizawa, Y.; Nakano, K.; Liu, Y.; Horiuchi, T.; Itai, K.; Tsunoda, H. Changes in fluoride levels in the liver, kidney, and brain and in neurotransmitters of mice after subacute administration of fluoride. Fluoride 2005, 38, 284–292. [Google Scholar]
- Di Chiara, G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 2000, 30, 295–314. [Google Scholar] [CrossRef]
- Listos, J.; Baranowska-Bosiacka, I.; Wąsik, A.; Talarek, S.; Tarnowski, M.; Listos, P.; Łupina, M.; Antkiewicz-Michaluk, L.; Gutowska, I.; Tkacz, M.; et al. The adenosinergic system is involved in sensitization to morphine withdrawal signs in rats-neurochemical and molecular basis in dopaminergic system. Psychopharmacology 2016, 233, 2383–2397. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.L.; Kemmling, A.K.; Balerio, G.N. Baclofnen reestablishes striatal and cortical dopamine concentrations during naloxone-precipitated withdrawal. Neurochem. Int. 2003, 42, 293–298. [Google Scholar] [CrossRef]
- Devoto, P.; Flore, G.; Pira, L.; Diana, M.; Gessa, G. Co-release of noradrenaline and dopamine in the prefrontal cortex after acute morphine and during morphine withdrawal. Psychopharmacology 2002, 160, 220–224. [Google Scholar] [CrossRef]
- Sotnikova, T.D.; Beaulieu, J.M.; Espinoza, S.; Masri, B.; Zhang, X.; Salahpour, A.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. The dopamine metabolite 3-methoxytyramine is a neuromodulator. PLoS ONE 2010, 5, e13452. [Google Scholar] [CrossRef]
- Miller, J.W.; Selhub, J.; Joseph, J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: Protective effects of O-methylation and melatonin. Free Radic. Biol. Med. 1996, 21, 241–249. [Google Scholar] [CrossRef]
- Reddy, P.Y.; Reddy, K.P.; Kumar, K.P. Neurodegenerative changes in different regions of brain, spinal cord and sciatic nerve of rats treated with sodium fluoride. J. Med. 2011, 1, 30–35. [Google Scholar]
- Anuradha, C.D.; Kanno, S.; Hirano, S. Oxidative damage to mitochondria is a preliminary step to caspase-3 activation in fluoride-induced apoptosis in HL-60 cells. Free Radic. Biol. Med. 2001, 31, 367–373. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Wang, P.; Birukova, A.A.; Verin, A.D.; Garcia, J.G.N. Mechanism of fluoride-induced MAP kinase activation in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1139–L1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, Y.; Zhang, K.; Li, J.; Wang, J. Effects of NaF on the expression of intracellular Ca2+ fluxes and apoptosis and the antagonism of taurine in murine neuron. Toxicol. Mech. Methods 2012, 22, 305–308. [Google Scholar]
- Song, J.S.; Lee, H.Y.; Lee, E.; Hwang, H.J.; Kim, J.H. Cytotoxicity and apoptosis induction of sodium fluoride in human promyelocytic leukemia (HL-60) cells. Environ. Toxicol. Pharmacol. 2002, 11, 85–91. [Google Scholar] [CrossRef]
- Song, J.S.; Park, Y.D.; Hyun, J.W.; Kim, J.H. Induction of apoptosis by sodium fluorosilicate treatment in human osteogenic sarcoma (HOS) cells. Anticancer Res. 2005, 25, 391–395. [Google Scholar]
- Tsunoda, M.; Kido, T.; Hosokawa, M.; Sugaya, C.; Tusji, M.; Zhang, Y. Effects of fluoride onneurotransmitters in brain regions of rats exposed to fluoride in drinking water for twomonths. Fluoride 2012, 45, 208–209. [Google Scholar]
- Hastings, T.G.; Lewis, D.A.; Zigmond, M.J. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. USA 1996, 93, 1956–1961. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, D.C.; Vázquez, I.E.; Brizuela, N.O.; Alvarez, R.G.; Mejía, G.B.; García, E.H.; Santamaría, D.; De Apreza, M.L.R.; Olguín, H.J. Assessment of oxidative damage induced by acute doses of morphine sulfate in postnatal and adult rat brain. Neurochem. Res. 2006, 31, 549–554. [Google Scholar] [CrossRef]
- Özmen, I.; Naziroǧlu, M.; Alici, H.A.; Şahin, F.; Cengiz, M.; Eren, I. Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem. Res. 2007, 32, 19–25. [Google Scholar] [CrossRef]






| Prefrontal Cortex | Striatum | Hippocampus | Cerebellum | |||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D1 | D2 | D1 | D2 | D1 | D2 | |
| Control | ++ | + | + | ++ | + | + | + | + |
| Fluoride | +++ | + | ++ | +++ | + | ++ | ++ | +++ |
| Morphine | + | + | −/+ | + | + | + | ++ | ++ |
| Morphine + Fluoride | + | + | + | ++ | + | + | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupnicka, P.; Listos, J.; Tarnowski, M.; Kolasa-Wołosiuk, A.; Wąsik, A.; Łukomska, A.; Barczak, K.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats. Int. J. Mol. Sci. 2020, 21, 2361. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072361
Kupnicka P, Listos J, Tarnowski M, Kolasa-Wołosiuk A, Wąsik A, Łukomska A, Barczak K, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats. International Journal of Molecular Sciences. 2020; 21(7):2361. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072361
Chicago/Turabian StyleKupnicka, Patrycja, Joanna Listos, Maciej Tarnowski, Agnieszka Kolasa-Wołosiuk, Agnieszka Wąsik, Agnieszka Łukomska, Katarzyna Barczak, Izabela Gutowska, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2020. "Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats" International Journal of Molecular Sciences 21, no. 7: 2361. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072361