Peptidylarginine Deiminase of Porphyromonas gingivalis Modulates the Interactions between Candida albicans Biofilm and Human Plasminogen and High-Molecular-Mass Kininogen
Abstract
:1. Introduction
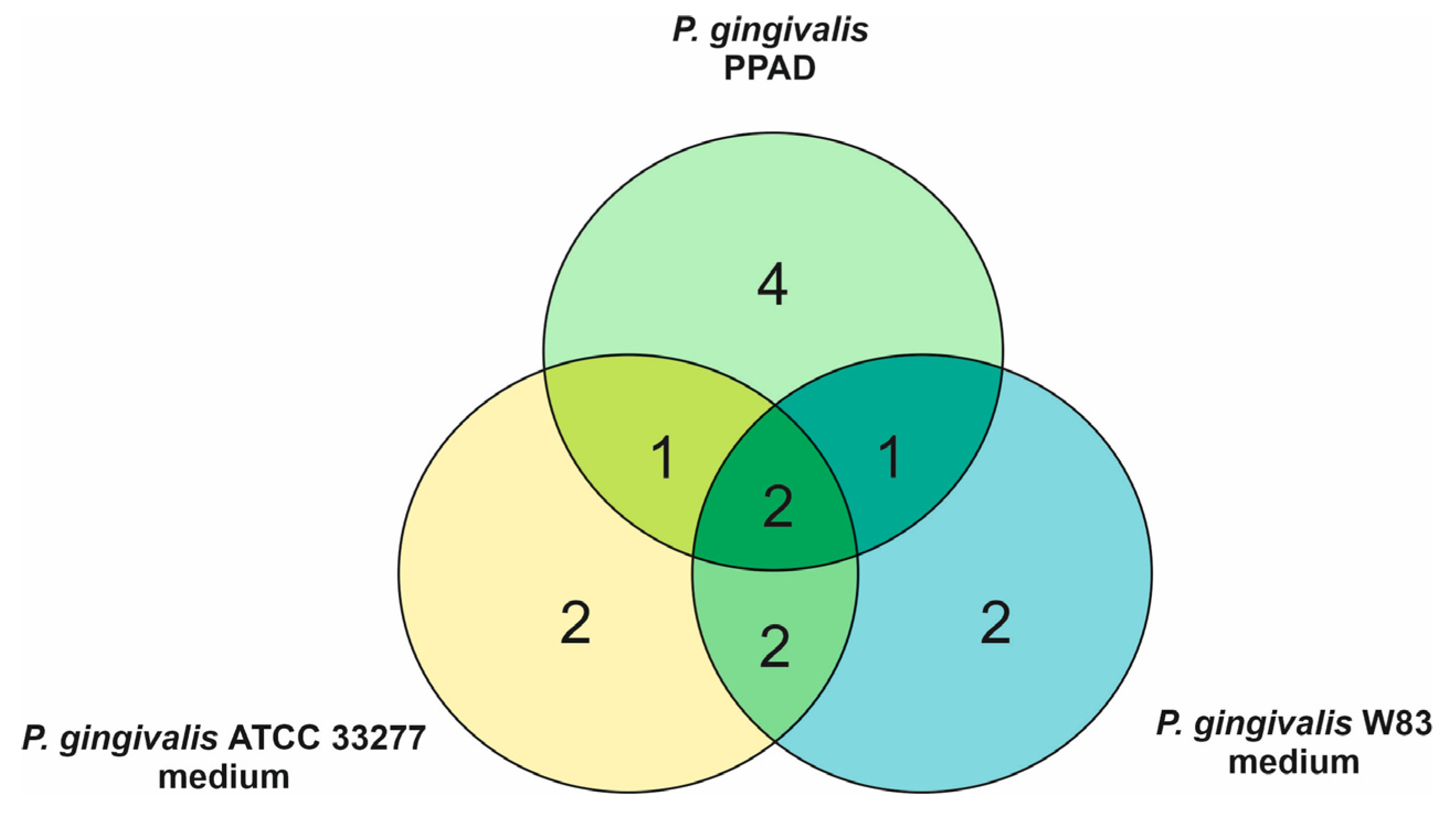
2. Results
2.1. Effects of Citrullination of Fungal Surface-Exposed Proteins on the Binding of Human Plasma Proteins to C. albicans Hyphae
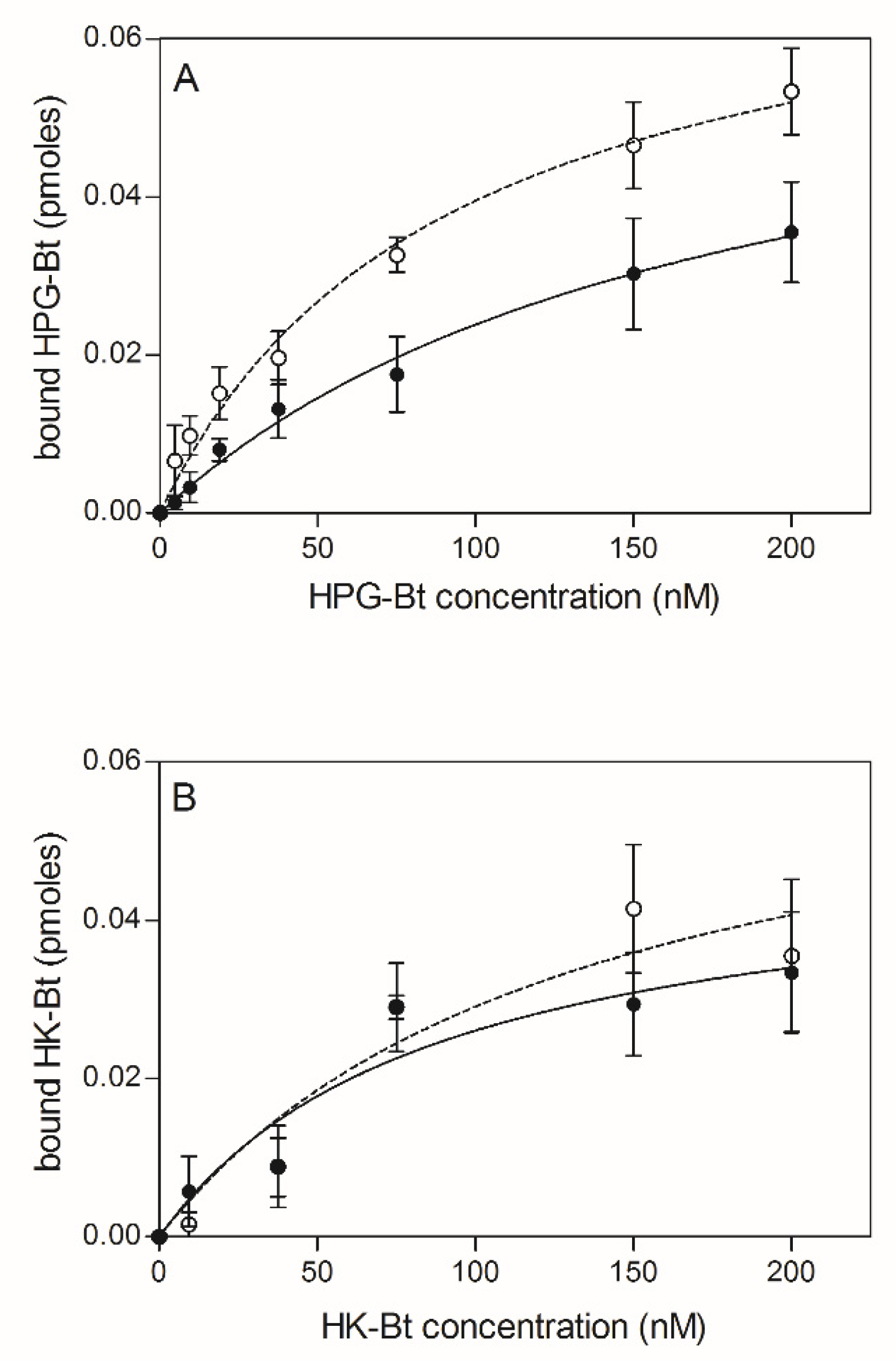
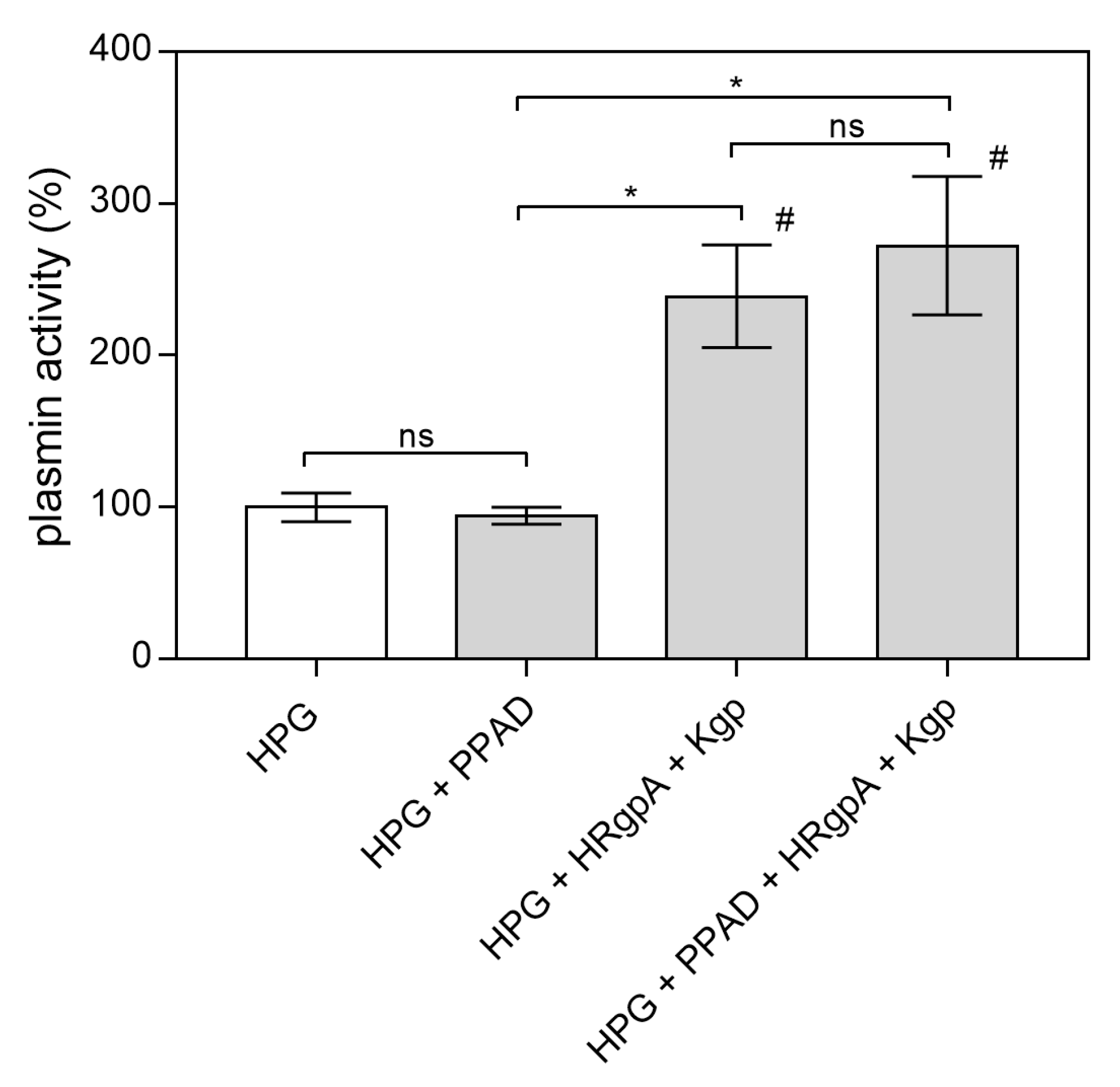
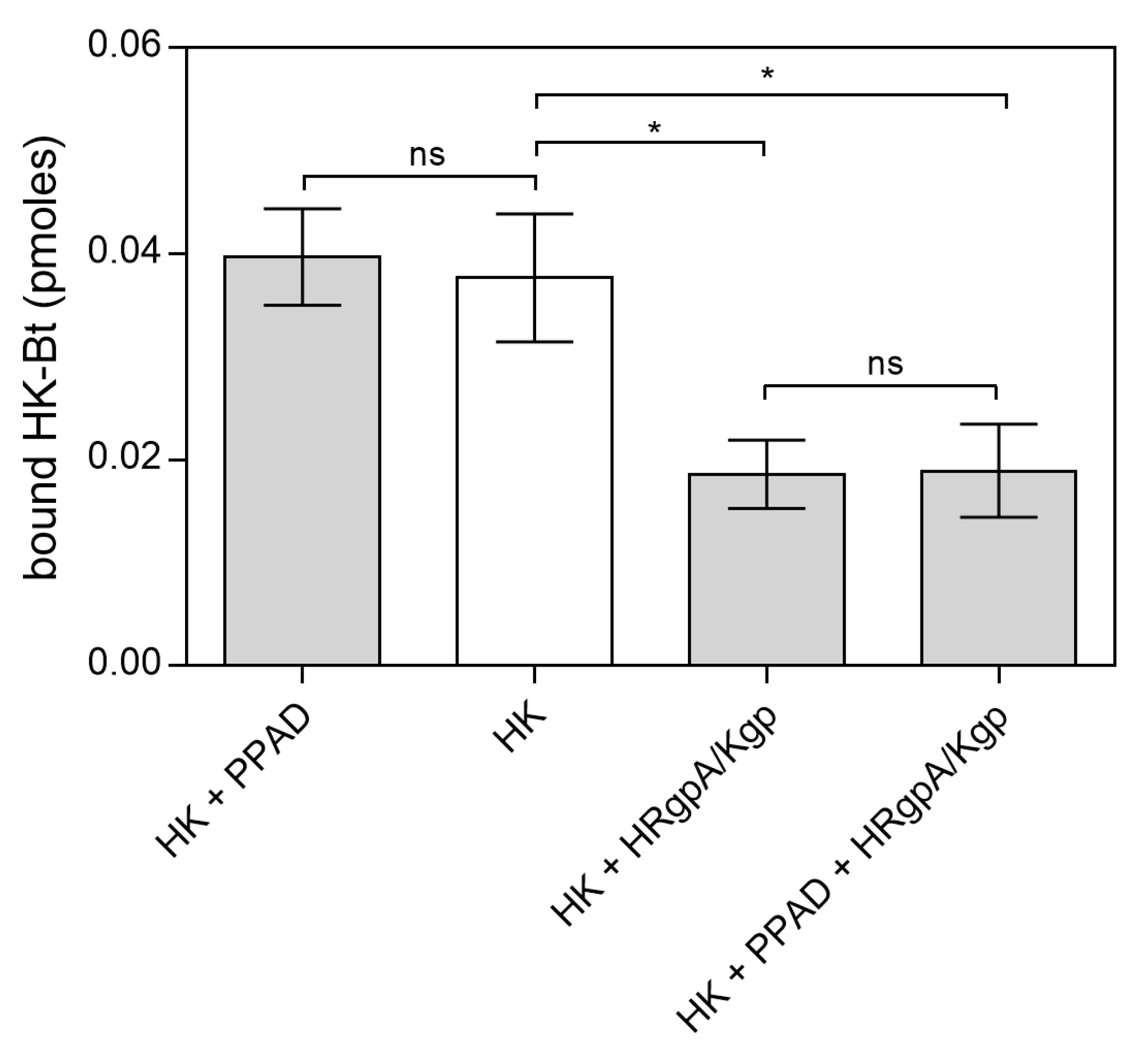
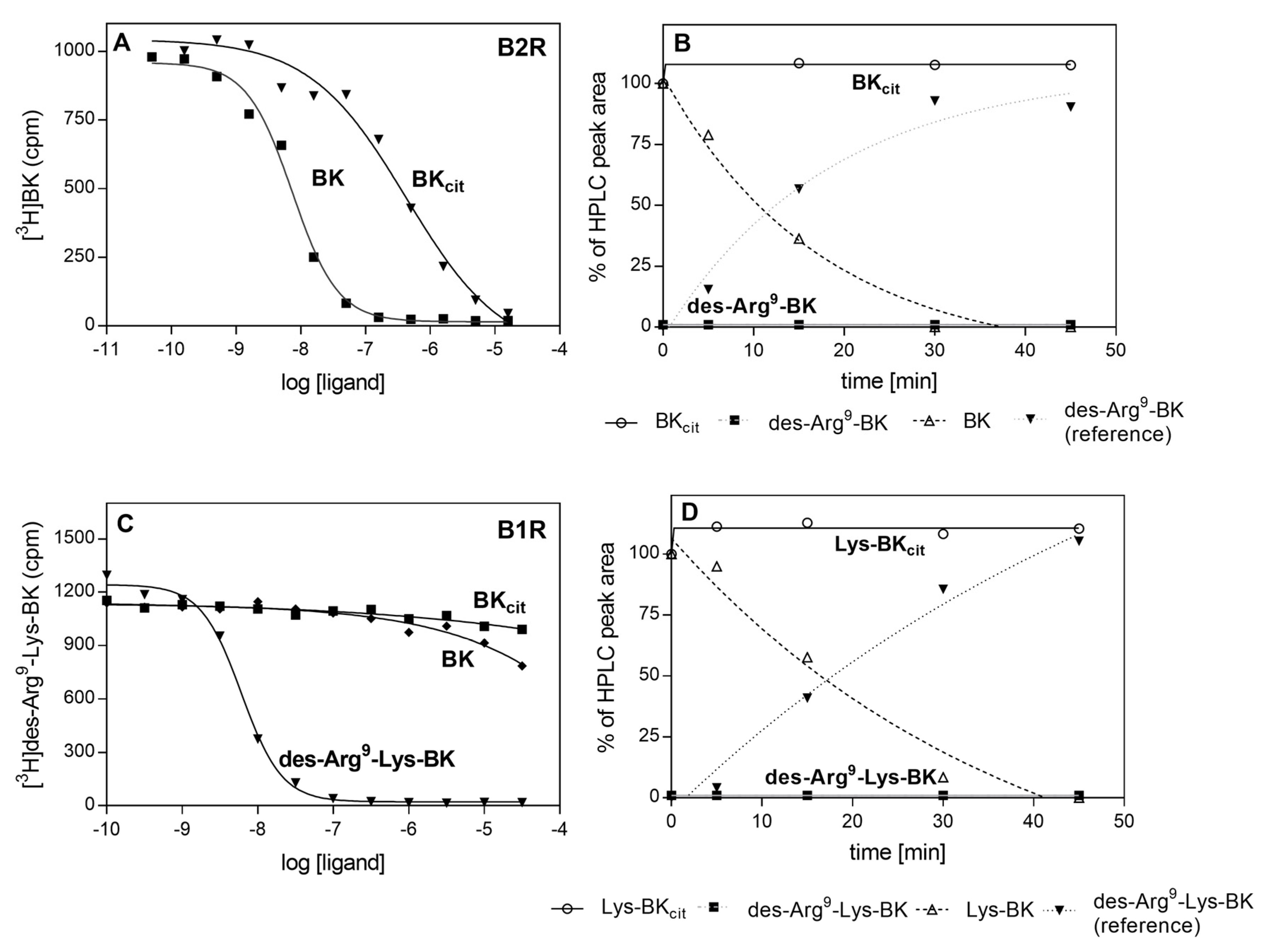
2.2. Effects of HPG and HK Citrullination on Their Binding to C. albicans Hyphae
3. Discussion
4. Materials and Methods
4.1. Growth Conditions for Bacterial and Fungal Cultures
4.2. Biotinylation of Human Proteins
4.3. Modification of C. albicans Surface Proteins by Bacterial Enzymes
4.3.1. Identification of Proteins Secreted by P. gingivalis into the Culture Medium
4.3.2. Growth of C. albicans Cells in Media Collected after Culture of P. gingivalis
4.3.3. Incubation of C. albicans Cells with PPAD from P. gingivalis
4.4. Cell Surface Shaving with Trypsin and Identification of C. albicans Proteins Modified by PPAD
4.5. Analysis of the Modifications of Human Proteins by Bacterial PPAD
4.6. Binding of HPG-Bt and HK-Bt to C. albicans Cells Pre-Treated with Bacterial Enzymes
4.7. Binding of Citrullinated HPG and HK to C. albicans Cells
4.8. Plasmin Activity Assay
4.9. Competitive Radioreceptor Assay for Kinins
4.10. Kinin Degradation by Carboxypeptidase M
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BK | bradykinin |
| B1R | kinin receptor B1 |
| B2R | kinin receptor B2 |
| CPM | carboxypeptidase M |
| HK | high-molecular-mass kininogen |
| HK-Bt | biotinylated high-molecular-mass kininogen |
| HPG | plasminogen |
| HPG-Bt | biotinylated plasminogen |
| HPLC | high performance liquid chromatography |
| HRgpA | gingipain R |
| Kgp | gingipain K |
| LC-MS/MS | liquid chromatography coupled-tandem mass spectrometry |
| PPAD | peptidylarginine deiminase |
| SA-HRP/TMB | streptavidin conjugated to horseradish peroxidase/ 3,3′,5,5′-tetramethylbenzidine |
| SAPs | secreted aspartyl proteases |
References
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arweiler, N.B.; Netuschil, L. The oral microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Jenkinson, H.F. Interkingdom networking within the oral microbiome. Microbes Infect. 2015, 17, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008, 23, 196–205. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Lopez-Ribot, J.L. Candida interactions with the oral bacterial microbiota. J. Fungi 2018, 4, 122. [Google Scholar] [CrossRef] [Green Version]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72–122. [Google Scholar] [CrossRef]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.J.; France, J.; Crean, S.; Singhrao, S.K. The Porphyromonas gingivalis/Host Interactome Shows Enrichment in GWASdb Genes Related to Alzheimer’s Disease, Diabetes and Cardiovascular Diseases. Front. Aging Neurosci. 2017, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- de Molon, R.S.; Rossa, C.J.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef] [Green Version]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.P., Jr.; Keeney, P.M.; Brohawn, D.G. RNA Sequencing Reveals Small and Variable Contributions of Infectious Agents to Transcriptomes of Postmortem Nervous Tissues From Amyotrophic Lateral Sclerosis, Alzheimer’s Disease and Parkinson’s Disease Subjects, and Increased Expression of Genes From Disease-Activated Microglia. Front. Neurosci. 2019, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Tamai, R.; Sugamata, M.; Kiyoura, Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb. Pathog. 2011, 51, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Karkowska-Kuleta, J.; Bartnicka, D.; Zawrotniak, M.; Zielinska, G.; Kieronska, A.; Bochenska, O.; Ciaston, I.; Koziel, J.; Potempa, J.; Baster, Z.; et al. The activity of bacterial peptidylarginine deiminase is important during formation of dual-species biofilm by periodontal pathogen Porphyromonas gingivalis and opportunistic fungus Candida albicans. Pathog. Dis. 2018, 76, fty033. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als 3. MBio 2018, 9, e00202-18. [Google Scholar] [CrossRef] [Green Version]
- Haverman, T.M.; Laheij, A.M.G.A.; de Soet, J.J.; de Lange, J.; Rozema, F.R. Candida and Porphyromonas gingivalis: The effect on wound closure in vitro. J. Oral Microbiol. 2017, 9, 1328266. [Google Scholar] [CrossRef] [Green Version]
- McGraw, W.T.; Potempa, J.; Farley, D.; Travis, J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect. Immun. 1999, 67, 3248–3256. [Google Scholar] [CrossRef] [Green Version]
- Gabarrini, G.; de Smit, M.; Westra, J.; Brouwer, E.; Vissink, A.; Zhou, K.; Rossen, J.W.; Stobernack, T.; van Dijl, J.M.; van Winkelhoff, A.J. The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis. Sci. Rep. 2015, 5, 13936. [Google Scholar] [CrossRef]
- Chen, Z.; Potempa, J.; Polanowski, A.; Wikstrom, M.; Travis, J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J. Biol. Chem. 1992, 267, 18896–18901. [Google Scholar]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K.A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Mizgalska, D.; Garcia-Ferrer, I.; Kantyka, T.; Guevara, T.; Szmigielski, B.; Sroka, A.; Millán, C.; Usón, I.; Veillard, F.; et al. Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci. Rep. 2015, 5, 11969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereta, G.; Goulas, T.; Madej, M.; Bielecka, E.; Solà, M.; Potempa, J.; Xavier Gomis-Rüth, F. Structure, function, and inhibition of a genomic/clinical variant of Porphyromonas gingivalis peptidylarginine deiminase. Protein Sci. 2019, 28, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Stobernack, T.; du Teil Espina, M.; Mulder, L.M.; Palma Medina, L.M.; Piebenga, D.R.; Gabarrini, G.; Zhao, X.; Janssen, K.M.J.; Hulzebos, J.; Brouwer, E.; et al. Secreted Bacterial Peptidylarginine Deiminase Can Neutralize Human Innate Immune Defenses. MBio 2018, 9, e01704-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karkowska-Kuleta, J.; Rapala-Kozik, M.; Kozik, A. Fungi pathogenic to humans: Molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim. Pol. 2009, 56, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, D.; Karkowska-Kuleta, J.; Zawrotniak, M.; Satała, D.; Michalik, K.; Zielinska, G.; Bochenska, O.; Kozik, A.; Ciaston, I.; Koziel, J.; et al. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci. Rep. 2019, 9, 4376. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, M.; Dongari-Bagtzoglou, A. The Relationship of Candida albicans with the Oral Bacterial Microbiome in Health and Disease. Adv. Exp. Med. Biol. 2019, 1197, 69–78. [Google Scholar] [CrossRef]
- Imamura, T.; Pike, R.N.; Potempa, J.; Travis, J. Pathogenesis of periodontitis: A major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J. Clin. Investig. 1994, 94, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Bunn, C.L.; Bartold, P.M. Effect of lipopolysaccharide from periodontal pathogens on the production of tissue plasminogen activator and plasminogen activator inhibitor 2 by human gingival fibroblasts. J. Periodontal Res. 2001, 36, 25–31. [Google Scholar] [CrossRef]
- Yang, S.F.; Hsieh, Y.S.; Huang, F.M.; Yang, L.C.; Chang, Y.C. Effect of black-pigmented bacteria on the plasminogen-plasmin system in human pulp and osteoblastic cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Klarström Engström, K.; Khalaf, H.; Kälvegren, H.; Bengtsson, T. The role of Porphyromonas gingivalis gingipains in platelet activation and innate immune modulation. Mol. Oral Microbiol. 2015, 30, 62–73. [Google Scholar] [CrossRef]
- Kozik, A.; Gogol, M.; Bochenska, O.; Karkowska-Kuleta, J.; Wolak, N.; Kamysz, W.; Aoki, W.; Ueda, M.; Faussner, A.; Rapala-Kozik, M. Kinin release from human kininogen by 10 aspartic proteases produced by pathogenic yeast Candida albicans. BMC Microbiol. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, Y.; Sato, H.; Seiki, M.; Kido, H. Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin. A possible cell surface activator. FEBS Lett. 1997, 402, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Wehner, C.; Janjić, K.; Agis, H. Relevance of the plasminogen system in physiology, pathology, and regeneration of oral tissues—From the perspective of dental specialties. Arch. Oral Biol. 2017, 74, 136–145. [Google Scholar] [CrossRef]
- Joseph, K.; Kaplan, A.P. Formation of bradykinin: A major contributor to the innate inflammatory response. Adv. Immunol. 2005, 86, 159–208. [Google Scholar]
- Fleetwood, A.J.; O’Brien-Simpson, N.M.; Veith, P.D.; Lam, R.S.; Achuthan, A.; Cook, A.D.; Singleton, W.; Lund, I.K.; Reynolds, E.C.; Hamilton, J.A. Porphyromonas gingivalis-derived RgpA-Kgp Complex Activates the Macrophage Urokinase Plasminogen Activator System: IMPLICATIONS FOR PERIODONTITIS. J. Biol. Chem. 2015, 290, 16031–16042. [Google Scholar] [CrossRef] [Green Version]
- Jong, A.Y.; Chen, S.H.; Stins, M.F.; Kim, K.S.; Tuan, T.L.; Huang, S.H. Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J. Med. Microbiol. 2003, 52 Pt 8, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Karkowska-Kuleta, J.; Kozik, A.; Rapala-Kozik, M. Binding and activation of the human plasma kinin-forming system on the cell walls of Candida albicans and Candida tropicalis. Biol Chem. 2010, 391, 97–103. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Karkowska-Kuleta, J.; Ryzanowska, A.; Golda, A.; Barbasz, A.; Faussner, A.; Kozik, A. Degradation of human kininogens with the release of kinin peptides by extracellular proteinases of Candida spp. Biol. Chem. 2010, 391, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019.
- Wun, T.C. Plasminogen activation: Biochemistry, physiology, and therapeutics. Crit. Rev. Biotechnol. 1988, 8, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. Plasma contact activation: A revised hypothesis. Biol. Res. 1998, 31, 251–262. [Google Scholar] [PubMed]
- Imamura, T.; Potempa, J.; Travis, J. Activation of the kallikrein-kinin system and release of new kinins through alternative cleavage of kininogens by microbial and human cell proteinases. Biol. Chem. 2004, 385, 989–996. [Google Scholar] [CrossRef]
- Bras, G.; Bochenska, O.; Rapala-Kozik, M.; Guevara-Lora, I.; Faussner, A.; Kozik, A. Extracellular aspartic protease SAP2 of Candida albicans yeast cleaves human kininogens and releases proinflammatory peptides, Met-Lys-bradykinin and des-Arg(9)-Met-Lys-bradykinin. Biol. Chem. 2012, 393, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bras, G.; Bochenska, O.; Rapala-Kozik, M.; Guevara-Lora, I.; Faussner, A.; Kamysz, W.; Kozik, A. Release of biologically active kinin peptides, Met-Lys-bradykinin and Leu-Met-Lys-bradykinin from human kininogens by two major secreted aspartic proteases of Candida parapsilosis. Peptides 2013, 48, 114–123. [Google Scholar] [CrossRef]
- Leeb-Lundberg, L.M.F.; Marceau, F.; Müller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman, R.W.; Schmaier, A.H. Contact system: A vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 1997, 90, 3819–3843. [Google Scholar] [CrossRef] [Green Version]
- Nickel, K.F.; Renné, T. Crosstalk of the plasma contact system with bacteria. Thromb. Res. 2012, 130 (Suppl. 1), S78–S83. [Google Scholar] [CrossRef]
- Fraga, T.R.; Courrol Ddos, S.; Castiblanco-Valencia, M.M.; Hirata, I.Y.; Vasconcellos, S.A.; Juliano, L.; Barbosa, A.S.; Isaac, L. Immune evasion by pathogenic Leptospira strains: The secretion of proteases that directly cleave complement proteins. J. Infect. Dis. 2014, 209, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Peetermans, M.; Vanassche, T.; Liesenborghs, L.; Lijnen, R.H.; Verhamme, P. Bacterial pathogens activate plasminogen to breach tissue barriers and escape from innate immunity. Crit. Rev. Microbiol. 2016, 42, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Shende, R.; Wong, S.S.W.; Rapole, S.; Beau, R.; Ibrahim-Granet, O.; Monod, M.; Gührs, K.H.; Pal, J.K.; Latgé, J.P.; Madan, T.; et al. Aspergillus fumigatus conidial metalloprotease Mep1p cleaves host complement proteins. J. Biol. Chem. 2018, 293, 15538–15555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayón-Núñez, D.A.; Fragoso, G.; Bobes, R.J.; Laclette, J.P. Plasminogen-binding proteins as an evasion mechanism of the host’s innate immunity in infectious diseases. Biosci. Rep. 2018, 38, BSR20180705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehmcke-Hecht, S.; Köhler, J. Interaction of the Human Contact System with Pathogens-An Update. Front. Immunol. 2018, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Joosten, L.A.; Kullberg, B.J.; Netea, M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012, 80, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans—Biology, molecular characterization, pathogenicity, and advances in diagnosis and control—An update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Crowe, J.D.; Sievwright, I.K.; Auld, G.C.; Moore, N.R.; Gow, N.A.; Booth, N.A. Candida albicans binds human plasminogen: Identification of eight plasminogen-binding proteins. Mol. Microbiol. 2003, 47, 1637–1651. [Google Scholar] [CrossRef]
- Funk, J.; Schaarschmidt, B.; Slesiona, S.; Hallström, T.; Horn, U.; Brock, M. The glycolytic enzyme enolase represents a plasminogen-binding protein on the surface of a wide variety of medically important fungal species. Int. J. Med. Microbiol. 2016, 306, 59–68. [Google Scholar] [CrossRef]
- Karkowska-Kuleta, J.; Kedracka-Krok, S.; Rapala-Kozik, M.; Kamysz, W.; Bielinska, S.; Karafova, A.; Kozik, A. Molecular determinants of the interaction between human high molecular weight kininogen and Candida albicans cell wall: Identification of kininogen-binding proteins on fungal cell wall and mapping the cell wall-binding regions on kininogen molecule. Peptides 2011, 32, 2488–2496. [Google Scholar] [CrossRef]
- Seweryn, K.; Karkowska-Kuleta, J.; Wolak, N.; Bochenska, O.; Kedracka-Krok, S.; Kozik, A.; Rapala-Kozik, M. Kinetic and thermodynamic characterization of the interactions between the components of human plasma kinin-forming system and isolated and purified cell wall proteins of Candida albicans. Acta Biochim. Pol. 2015, 62, 825–835. [Google Scholar] [CrossRef]
- Gera, L.; Roy, C.; Bawolak, M.T.; Bouthillier, J.; Adam, A.; Marceau, F. Met-Lys-bradykinin-Ser-Ser, a peptide produced by the neutrophil from kininogen, is metabolically activated by angiotensin converting enzyme in vascular tissue. Pharmacol. Res. 2011, 64, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Frick, I.M.; Björck, L.; Herwald, H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007, 98, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urzúa, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Canabarro, A.; Valle, C.; Farias, M.R.; Santos, F.B.; Lazera, M.; Wanke, B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J. Periodontal Res. 2013, 48, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Percudani, D.; Lodi, G.; Bertani, P.; Manfredi, M. Prevalence of Candida Species in Endodontic Infections: Systematic Review and Meta-analysis. J. Endod. 2018, 44, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Stobernack, T.; Glasner, C.; Junker, S.; Gabarrini, G.; de Smit, M.; de Jong, A.; Otto, A.; Becher, D.; van Winkelhoff, A.J.; van Dijl, J.M. Extracellular Proteome and Citrullinome of the Oral Pathogen Porphyromonas gingivalis. J. Proteome Res. 2016, 15, 4532–4543. [Google Scholar] [CrossRef]
- Larsen, D.N.; Mikkelsen, C.E.; Kierkegaard, M.; Bereta, G.P.; Nowakowska, Z.M.; Kaczmarek, J.Z.; Potempa, J.; Højrup, P. Citrullinome of Porphyromonas gingivalis Outer Membrane Vesicles: Confident Identification of Citrullinated Peptides. Mol. Cell Proteomics. 2020, 19, 167–180. [Google Scholar] [CrossRef]
- Vermilyea, D.M.; Ottenberg, G.K.; Davey, M.E. Citrullination mediated by PPAD constrains biofilm formation in P. gingivalis strain 381. NPJ Biofilms Microbiomes 2019, 5, 7. [Google Scholar] [CrossRef]
- Miles, L.A.; Dahlberg, C.M.; Plescia, J.; Felez, J.; Kato, K.; Plow, E.F. Role of cell-surface lysines in plasminogen binding to cells: Identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 1991, 30, 1682–1691. [Google Scholar] [CrossRef]
- Grenier, D. Degradation of host protease inhibitors and activation of plasminogen by proteolytic enzymes from Porphyromonas gingivalis and Treponema denticola. Microbiology 1996, 142 Pt 4, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Calixto, J.B.; Cabrini, D.A.; Ferreira, J.; Campos, M.M. Kinins in pain and inflammation. Pain 2000, 87, 1–5. [Google Scholar] [CrossRef]
- Hu, S.W.; Huang, C.H.; Huang, H.C.; Lai, Y.Y.; Lin, Y.Y. Transvascular dissemination of Porphyromonas gingivalis from a sequestered site is dependent upon activation of the kallikrein/kinin pathway. J. Periodontal Res. 2006, 41, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Rapala-Kozik, M.; Bras, G.; Chruscicka, B.; Karkowska-Kuleta, J.; Sroka, A.; Herwald, H.; Nguyen, K.A.; Eick, S.; Potempa, J.; Kozik, A. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infect. Immun. 2011, 79, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchi, P.; Meini, S.; Quartara, L.; Giolitti, A.; Zappitelli, S.; Rotondaro, L.; Maggi, C.A. Interaction of linear and cyclic peptide antagonists at the human B(2) kinin receptor. Peptides 2002, 23, 1457–1463. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Davis, R.M.; Tan, F. Human carboxypeptidase M. Purification and characterization of a membrane-bound carboxypeptidase that cleaves peptide hormones. J. Biol. Chem. 1989, 264, 2236–2241. [Google Scholar]
- Reverter, D.; Maskos, K.; Tan, F.; Skidgel, R.A.; Bode, W. Crystal structure of human carboxypeptidase M, a membrane-bound enzyme that regulates peptide hormone activity. J. Mol. Biol. 2004, 338, 257–269. [Google Scholar] [CrossRef]
- Pyrc, K.; Milewska, A.; Kantyka, T.; Sroka, A.; Maresz, K.; Kozieł, J.; Nguyen, K.A.; Enghild, J.J.; Knudsen, A.D.; Potempa, J. Inactivation of epidermal growth factor by Porphyromonas gingivalis as a potential mechanism for periodontal tissue damage. Infect. Immun. 2013, 81, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Bielecka, E.; Scavenius, C.; Kantyka, T.; Jusko, M.; Mizgalska, D.; Szmigielski, B.; Potempa, B.; Enghild, J.J.; Prossnitz, E.R.; Blom, A.M.; et al. Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J. Biol. Chem. 2014, 289, 32481–32487. [Google Scholar] [CrossRef] [Green Version]
- Gawron, K.; Bereta, G.; Nowakowska, Z.; Lazarz-Bartyzel, K.; Lazarz, M.; Szmigielski, B.; Mizgalska, D.; Buda, A.; Koziel, J.; Oruba, Z.; et al. Peptidylarginine deiminase from Porphyromonas gingivalis contributes to infection of gingival fibroblasts and induction of prostaglandin E2 -signaling pathway. Mol. Oral Microbiol. 2014, 29, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Karkowska-Kuleta, J.; Zajac, D.; Bras, G.; Bochenska, O.; Rapala-Kozik, M.; Kozik, A. Binding of human plasminogen and high-molecular-mass kininogen by cell surface-exposed proteins of Candida parapsilosis. Acta Biochim. Pol. 2017, 64, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Nguyen, K.A. Purification and Characterization of Gingipains. Curr. Protoc. Protein Sci. 2007, 49, 21.20.1–21.20.27. [Google Scholar] [CrossRef] [PubMed]
- Karkowska-Kuleta, J.; Zajac, D.; Bochenska, O.; Kozik, A. Surfaceome of pathogenic yeasts, Candida parapsilosis and Candida tropicalis, revealed with the use of cell surface shaving method and shotgun proteomic approach. Acta Biochim. Pol. 2015, 62, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, R.; van Beers, J.J.; El-Azzouny, M.; Visser, N.F.; Božič, B.; Pruijn, G.J.; Heck, A.J. Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res. Ther. 2012, 14, R114. [Google Scholar] [CrossRef] [Green Version]
- Hensen, S.M.; Pruijn, G.J. Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination. Mol. Cell Proteomics. 2014, 13, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Kadowaki, T. Suppression of Pathogenicity of Porphyromonas gingivalis by Newly Developed Gingipain Inhibitors. Mol. Pharmacol. 2004, 66, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Zubakova, R.; Gille, A.; Faussner, A.; Hilgenfeldt, U. Ca2+ signalling of kinins in cells expressing rat, mouse and human B1/B2-receptor. Int. Immunopharmacol. 2008, 8, 276–281. [Google Scholar] [CrossRef]
- Nägler, D.K.; Kraus, S.; Feierler, J.; Mentele, R.; Lottspeich, F.; Jochum, M.; Faussner, A. A cysteine-type carboxypeptidase, cathepsin X, generates peptide receptor agonists. Int. Immunopharmacol. 2010, 10, 134–139. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkowska-Kuleta, J.; Surowiec, M.; Gogol, M.; Koziel, J.; Potempa, B.; Potempa, J.; Kozik, A.; Rapala-Kozik, M. Peptidylarginine Deiminase of Porphyromonas gingivalis Modulates the Interactions between Candida albicans Biofilm and Human Plasminogen and High-Molecular-Mass Kininogen. Int. J. Mol. Sci. 2020, 21, 2495. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072495
Karkowska-Kuleta J, Surowiec M, Gogol M, Koziel J, Potempa B, Potempa J, Kozik A, Rapala-Kozik M. Peptidylarginine Deiminase of Porphyromonas gingivalis Modulates the Interactions between Candida albicans Biofilm and Human Plasminogen and High-Molecular-Mass Kininogen. International Journal of Molecular Sciences. 2020; 21(7):2495. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072495
Chicago/Turabian StyleKarkowska-Kuleta, Justyna, Magdalena Surowiec, Mariusz Gogol, Joanna Koziel, Barbara Potempa, Jan Potempa, Andrzej Kozik, and Maria Rapala-Kozik. 2020. "Peptidylarginine Deiminase of Porphyromonas gingivalis Modulates the Interactions between Candida albicans Biofilm and Human Plasminogen and High-Molecular-Mass Kininogen" International Journal of Molecular Sciences 21, no. 7: 2495. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072495




