Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
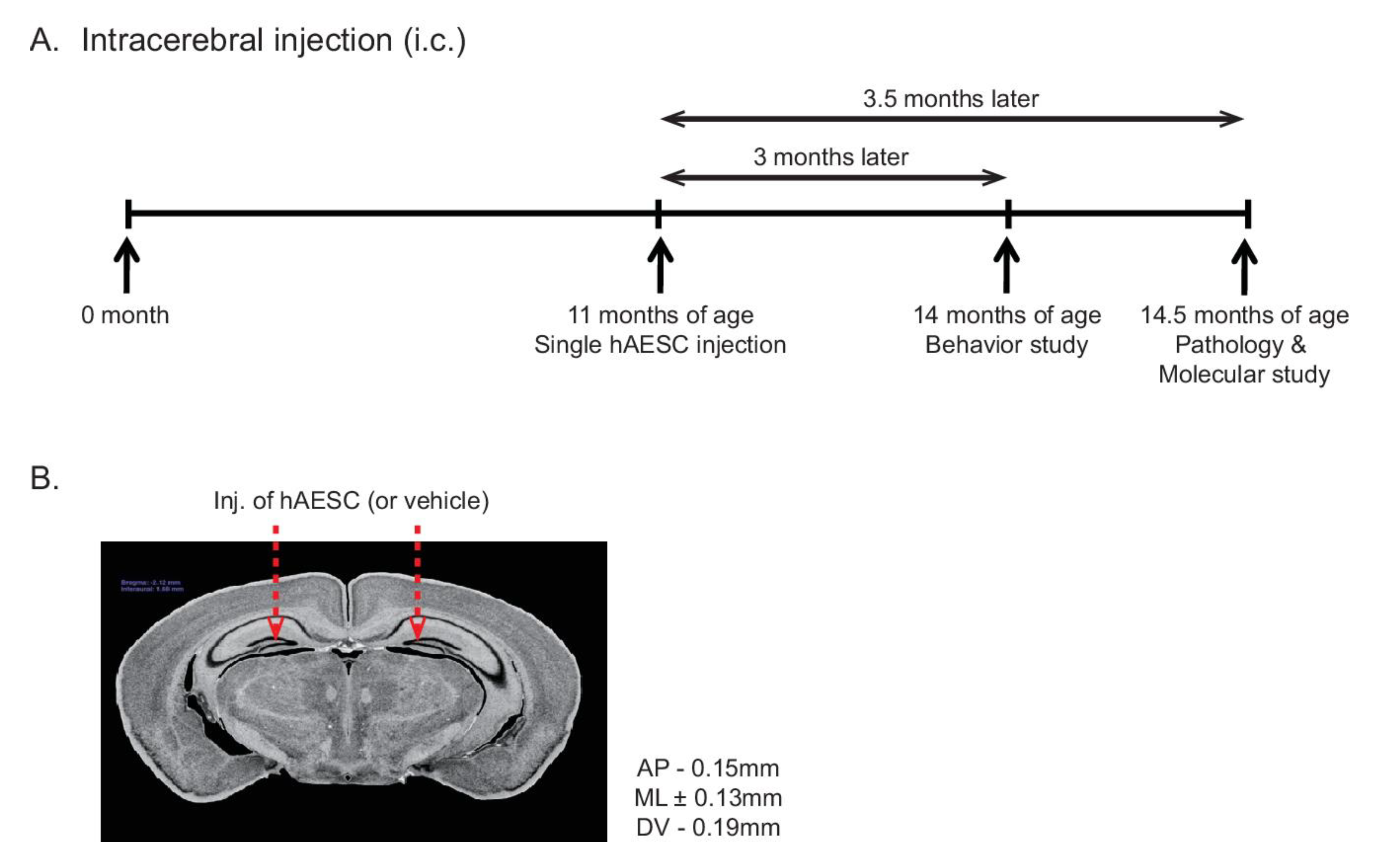
2.1. Intracerebral Transplantation of hAESCs into Tg2576 Transgenic Mice
2.2. Transplantation of hAESCs Alleviates Cognitive Deficits in Tg2576 Alzheimer’s Disease Transgenic Mice
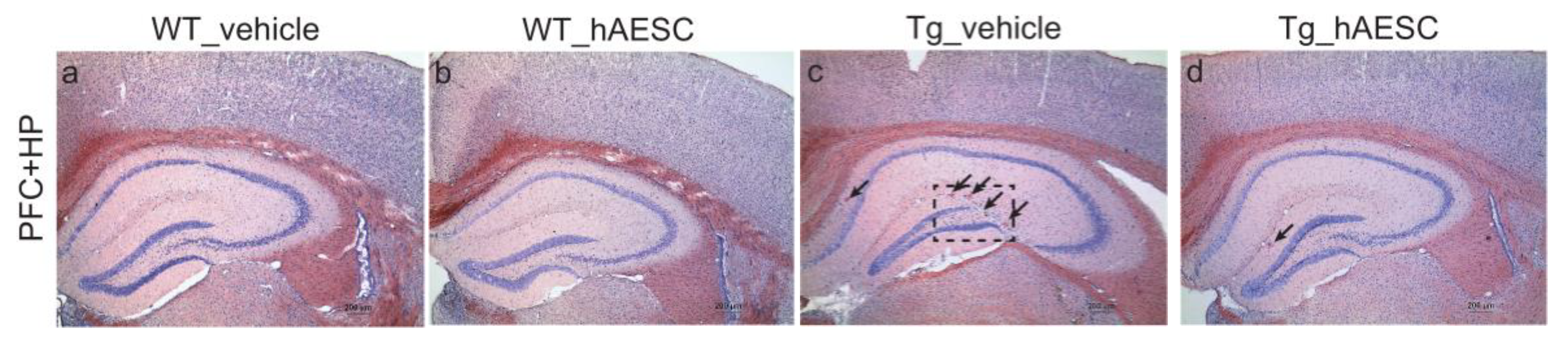
2.3. Transplantation of hAESCs Reduces Amyloid Burden in Tg2576 Alzheimer’s Disease Transgenic Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of hAESCs
4.3. Behavioral Tests
4.4. Collection of Brain Tissue
4.5. Congo Red Staining
4.6. BACE Activity
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | amyloid beta |
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| BACE | Beta-secretase |
| hAESCs | Human amniotic epithelial stem cells |
| PET | Positron Emission Tomography |
| Tg | Transgenic |
| WT | Wild type |
References
- Huang, L.K.; Chao, S.P.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- CDC. Alzheimer’s disease. Centers for Disease control and Prevention 2019. Available online: https://www.cdc.gov/dotw/alzheimers/index.html (accessed on 12 March 2020).
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glynn-Servedio, B.E.; Ranola, T.S. AChE Inhibitors and NMDA Receptor Antagonists in Advanced Alzheimer’s Disease. Consult. Pharm. 2017, 32, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Sajadimajd, S.; Jafari, S.; Izadi, Z.; Sarvari, S.; Sharifi, M.; Falahati, M.; Moakedi, F.; Muganda, W.C.A.; Muller, M.; et al. Novel therapeutic strategies for Alzheimer’s disease: Implications from cell-based therapy and nanotherapy. Nanomedicine 2020, 24, 102149. [Google Scholar] [CrossRef] [PubMed]
- Formicola, B.; Cox, A.; Dal Magro, R.; Masserini, M.; Re, F. Nanomedicine for the Treatment of Alzheimer’s Disease. J. Biomed. Nanotechnol. 2019, 15, 1997–2024. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Ganesan, P.; Park, S.Y.; Kim, J.S.; Choi, D.K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Miki, T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am. J. Reprod. Immunol. 2018, 80, e13003. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Tahan, A.C.; Tahan, V. Placental amniotic epithelial cells and their therapeutic potential in liver diseases. Front. Med. (Lausanne) 2014, 1, 48. [Google Scholar] [CrossRef] [Green Version]
- Bali, P.; Lahiri, D.K.; Banik, A.; Nehru, B.; Anand, A. Potential for Stem Cells Therapy in Alzheimer’s Disease: Do Neurotrophic Factors Play Critical Role? Curr. Alzheimer. Res. 2017, 14, 208–220. [Google Scholar] [CrossRef]
- Tang, J. How close is the stem cell cure to the Alzheimer’s disease: Future and beyond? Neural Regen Res. 2012, 7, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, K.; Vaidya, M. Stem Cell Therapies for Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2018, 1056, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Muttini, A.; Barboni, B.; Valbonetti, L.; Russo, V.; Maffulli, N. Amniotic Epithelial Stem Cells: Salient Features and Possible Therapeutic Role. Sports Med. Arthrosc. Rev. 2018, 26, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, F.; Bellofatto, K.; Wassmer, C.H.; Perez, L.; Lavallard, V.; Parnaud, G.; Cottet-Dumoulin, D.; Kerr-Conte, J.; Pattou, F.; Bosco, D.; et al. Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Maymo, J.L.; Riedel, R.; Perez-Perez, A.; Magatti, M.; Maskin, B.; Duenas, J.L.; Parolini, O.; Sanchez-Margalet, V.; Varone, C.L. Proliferation and survival of human amniotic epithelial cells during their hepatic differentiation. PLoS ONE 2018, 13, e0191489. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.; Grubbs, B. Therapeutic potential of placenta-derived stem cells for liver diseases: Current status and perspectives. J. Obstet. Gynaecol. Res. 2014, 40, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, J.; Tsang, K.S.; Yang, H.; Gao, W.Q. Therapeutic Potential of Human Amniotic Epithelial Cells on Injuries and Disorders in the Central Nervous System. Stem Cells Int. 2019, 2019, 5432301. [Google Scholar] [CrossRef] [Green Version]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef]
- Andrewartha, N.; Yeoh, G. Human Amnion Epithelial Cell Therapy for Chronic Liver Disease. Stem Cells Int. 2019, 2019, 8106482. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.T.; Li, C.; Dong, Z.Y.; Liu, J.M.; Li, W.; Liu, Y.; Xue, H.; Chen, D. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol. Int. 2008, 32, 1546–1558. [Google Scholar] [CrossRef]
- Studart, A.N.; Nitrini, R. Subjective cognitive decline: The first clinical manifestation of Alzheimer’s disease? Dement. Neuropsychol. 2016, 10, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Schwartz, C.E. Intellectual disability and autism spectrum disorders: Causal genes and molecular mechanisms. Neurosci. Biobehav. Rev. 2014, 46(Pt. 2), 161–174. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.; Chen, C.; Dong, W.; Hui, G.; Liu, T.; Guo, L. Therapeutic effects of human amniotic epithelial cell transplantation on double-transgenic mice co-expressing APPswe and PS1DeltaE9-deleted genes. Sci. China Life Sci. 2012, 55, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Sankar, V.; Muthusamy, R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 2003, 118, 11–17. [Google Scholar] [CrossRef]
- Jiao, H.; Shi, K.; Zhang, W.; Yang, L.; Yang, L.; Guan, F.; Yang, B. Therapeutic potential of human amniotic membrane-derived mesenchymal stem cells in APP transgenic mice. Oncol. Lett. 2016, 12, 1877–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H. Amyloid-beta and cognition in aging and Alzheimer’s disease: Molecular and neurophysiological mechanisms. J. Alzheimers Dis. 2013, 33 (Suppl. 1), S79–S86. [Google Scholar] [CrossRef]
- Rowe, C.C.; Villemagne, V.L. Amyloid imaging with PET in early Alzheimer disease diagnosis. Med. Clin. North. Am. 2013, 97, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.Q.; Hampel, H. Neurodegenerative disease biomarkers: Guideposts for disease prevention through early diagnosis and intervention. Prog. Neurobiol. 2011, 95, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Resnick, S.M.; Sojkova, J. Amyloid imaging and memory change for prediction of cognitive impairment. Alzheimers Res. Ther. 2011, 3, 3. [Google Scholar] [CrossRef]
- Tolboom, N.; Yaqub, M.; van der Flier, W.M.; Boellaard, R.; Luurtsema, G.; Windhorst, A.D.; Barkhof, F.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. Detection of Alzheimer pathology in vivo using both 11C-PIB and 18F-FDDNP PET. J. Nucl. Med. 2009, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Rowe, C.C.; Ng, S.; Ackermann, U.; Gong, S.J.; Pike, K.; Savage, G.; Cowie, T.F.; Dickinson, K.L.; Maruff, P.; Darby, D.; et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007, 68, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.E.; Savage, G.; Villemagne, V.L.; Ng, S.; Moss, S.A.; Maruff, P.; Mathis, C.A.; Klunk, W.E.; Masters, C.L.; Rowe, C.C. Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer’s disease. Brain 2007, 130, 2837–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolboom, N.; van der Flier, W.M.; Yaqub, M.; Koene, T.; Boellaard, R.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. Differential association of [11C]PIB and [18F]FDDNP binding with cognitive impairment. Neurology 2009, 73, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Westerman, M.A.; Cooper-Blacketer, D.; Mariash, A.; Kotilinek, L.; Kawarabayashi, T.; Younkin, L.H.; Carlson, G.A.; Younkin, S.G.; Ashe, K.H. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 2002, 22, 1858–1867. [Google Scholar] [CrossRef] [Green Version]
- Kawarabayashi, T.; Younkin, L.H.; Saido, T.C.; Shoji, M.; Ashe, K.H.; Younkin, S.G. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2001, 21, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Peters, F.; Salihoglu, H.; Pratsch, K.; Herzog, E.; Pigoni, M.; Sgobio, C.; Lichtenthaler, S.F.; Neumann, U.; Herms, J. Tau deletion reduces plaque-associated BACE1 accumulation and decelerates plaque formation in a mouse model of Alzheimer’s disease. EMBO J. 2019, 38, e102345. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. Role of BACE1 in Alzheimer’s synaptic function. Transl. Neurodegener. 2017, 6, 23. [Google Scholar] [CrossRef]
- Fang, C.H.; Jin, J.; Joe, J.H.; Song, Y.S.; So, B.I.; Lim, S.M.; Cheon, G.J.; Woo, S.K.; Ra, J.C.; Lee, Y.Y.; et al. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: Comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transpl. 2012, 21, 1687–1696. [Google Scholar] [CrossRef]
- Kim, S.; Chang, K.A.; Kim, J.; Park, H.G.; Ra, J.C.; Kim, H.S.; Suh, Y.H. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS ONE 2012, 7, e45757. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.Y.; Suh, Y.-H.; Chang, K.-A. Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2658. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072658
Kim KY, Suh Y-H, Chang K-A. Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2020; 21(7):2658. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072658
Chicago/Turabian StyleKim, Ka Young, Yoo-Hun Suh, and Keun-A Chang. 2020. "Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 21, no. 7: 2658. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072658





