A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models
Abstract
:1. Introduction
2. Results
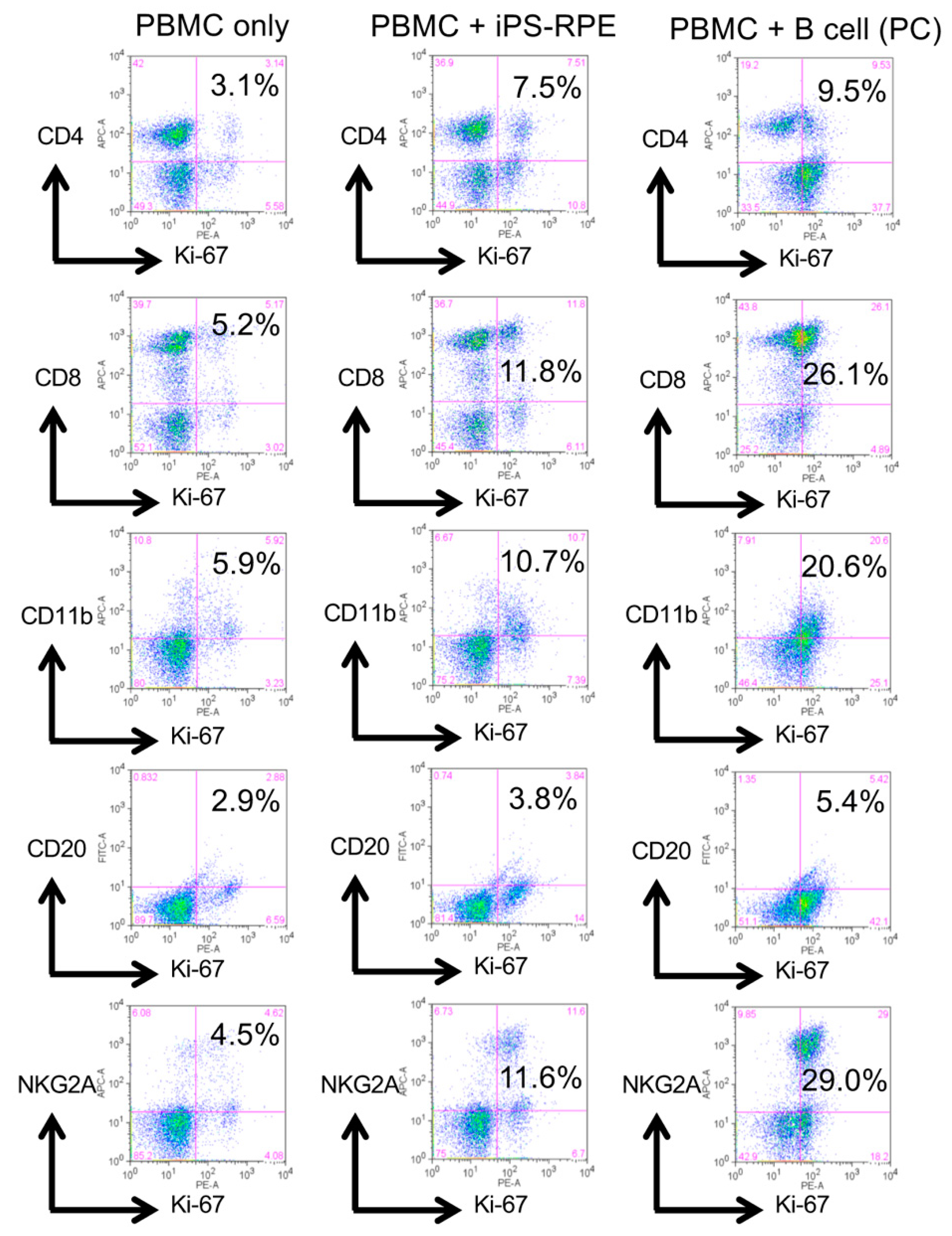
2.1. Results of LGIR In Vitro Tests in Monkeys
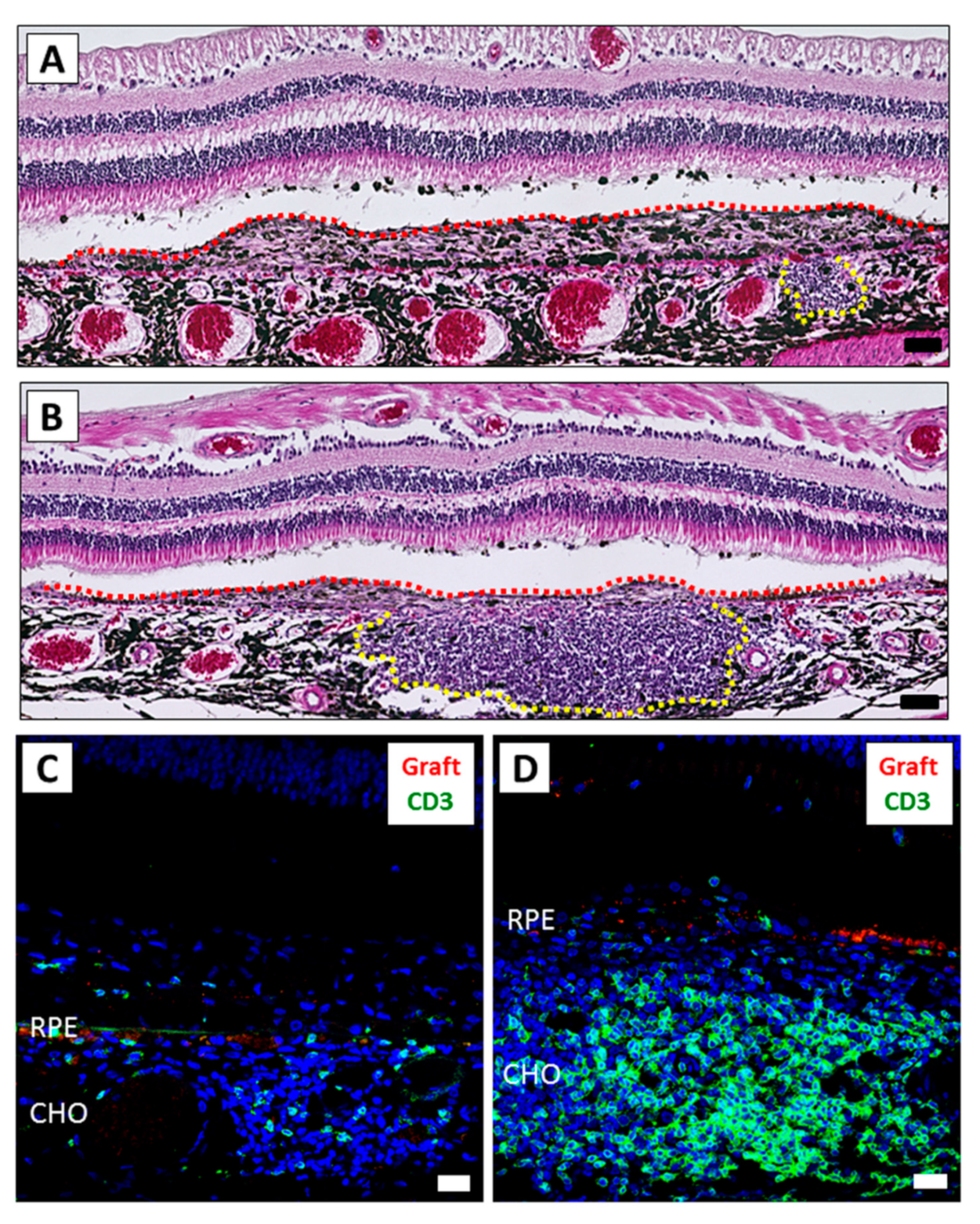
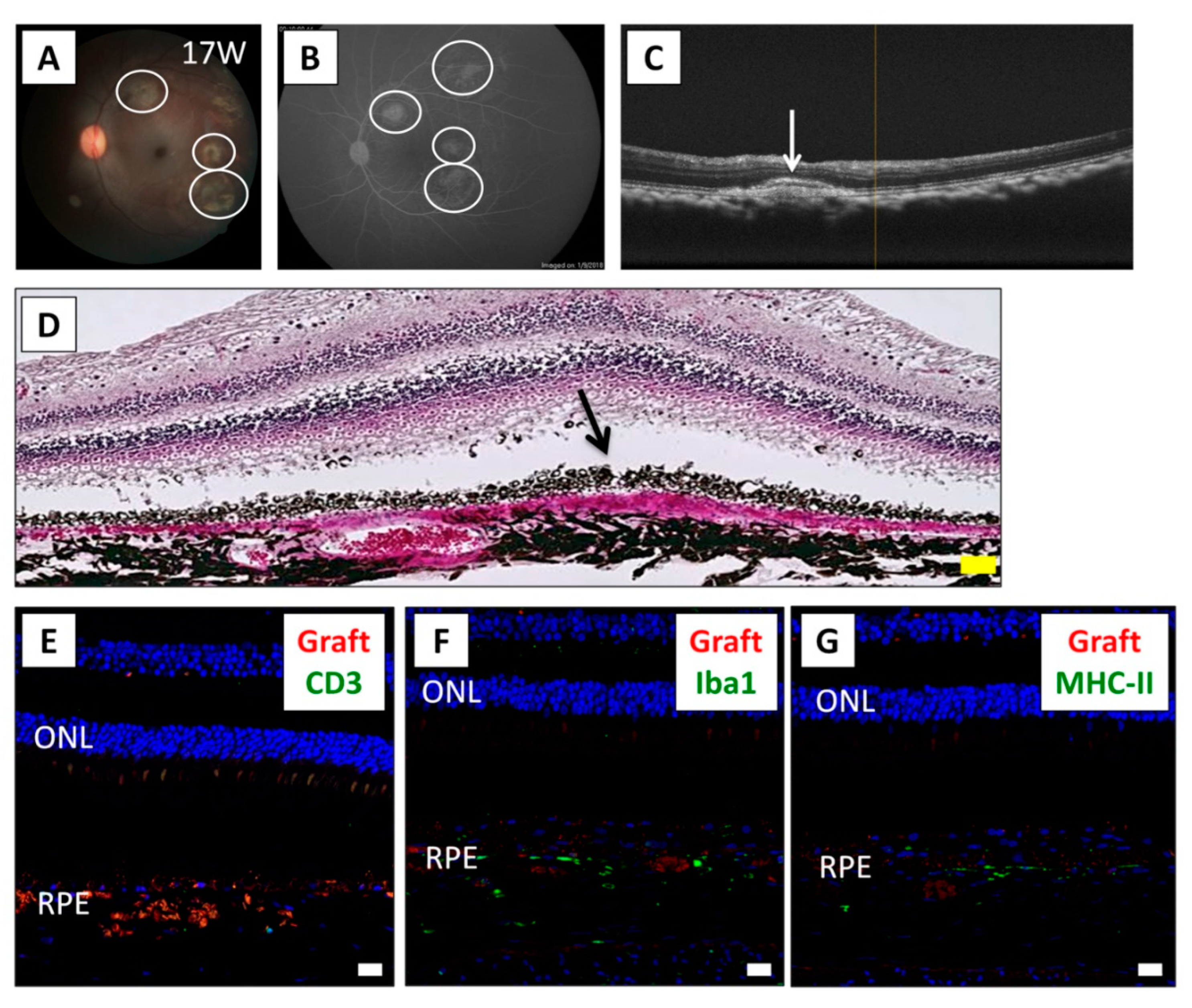
2.2. Results of Human iPSC-RPE Xenotransplantation
2.3. Results of In Vitro Drug-LGIR Tests
2.4. Effects of Local Steroid Administration in RPE Cell Transplantation
2.5. Effects of Systemic Cyclosporine A Administration in RPE Cell Transplantation
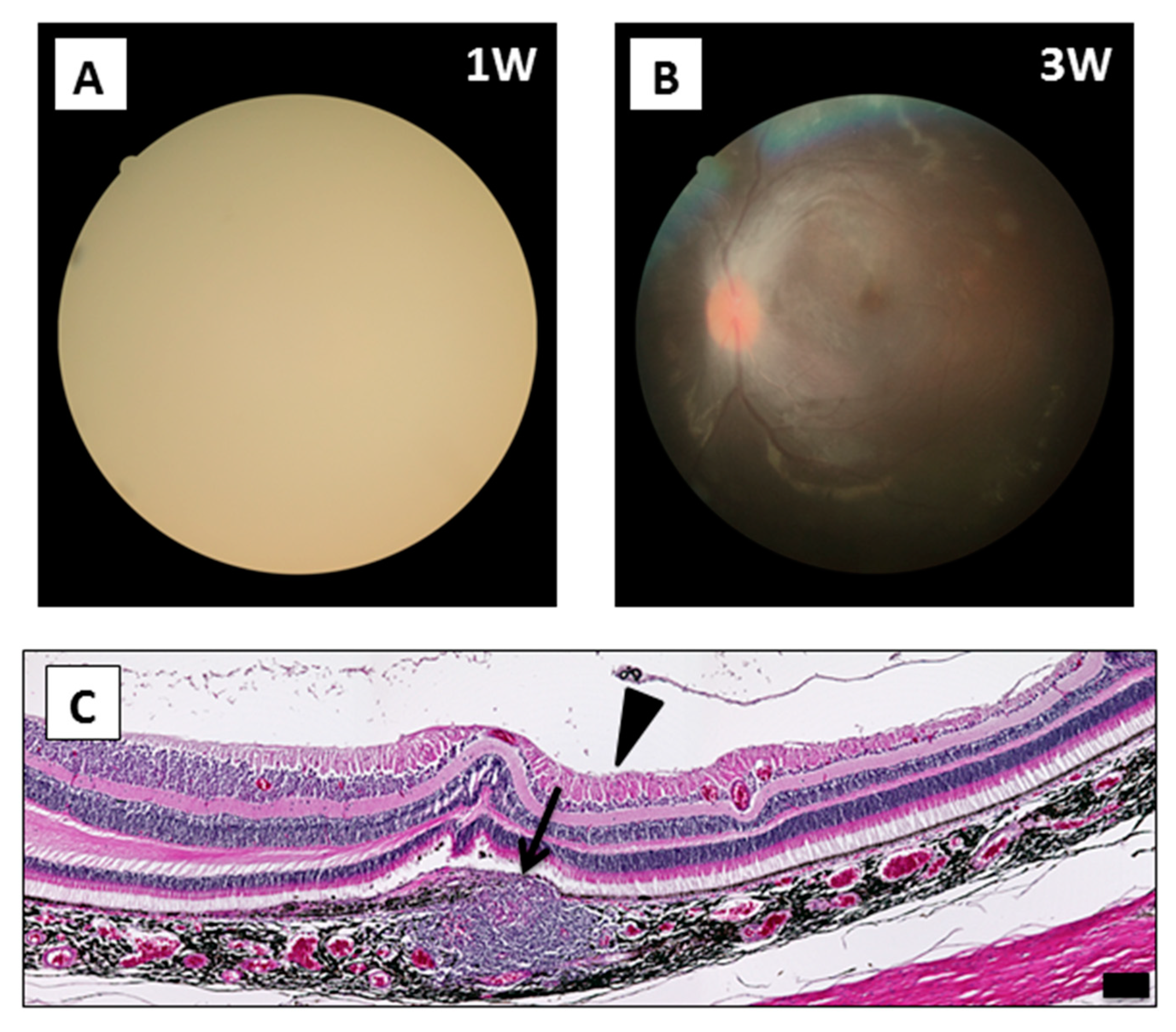
2.6. Surgical Complications in RPE Cell Xenotransplantation
3. Discussion
4. Material and Methods
4.1. RPE Cell Transplantation in Monkeys
4.2. Local Steroid and Oral Cyclosporine A Administration
4.3. Clinical Evaluation
4.4. Histological Assessment
4.5. Drug-LGIR Tests and FACS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| ERM | epiretinal membrane |
| ESC | embryonic stem cells |
| FA | fluorescein angiography |
| FACS | fluorescence-activated cell sorting |
| H&E | hematoxylin and eosin |
| MHC | major histocompatibility complex |
| NK | natural killer |
| IHC | immunohistochemistry |
| iPSC | induced pluripotent stem cells |
| IVTA | intravitreal triamcinolone acetonide |
| LGIR | lymphocytes–grafts immune reaction |
| OCT | optical coherence tomography |
| PBMC | peripheral blood mononuclear cells |
| RPE | retinal pigment epithelial cells |
| STTA | sub-Tenon conjunctival injection of triamcinolone acetonide |
References
- Okada, A.A.; Wakabayashi, T.; Morimura, Y.; Kawahara, S.; Kojima, E.; Asano, Y.; Hida, T. Trans-Tenon’s retrobulbar triamcinolone infusion for the treatment of uveitis. Br. J. Ophthalmol. 2003, 87, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M. Immunotherapy for Behcet’s disease. Int. Rev. Immunol. 1997, 14, 49–66. [Google Scholar] [CrossRef]
- Ohno, S.; Nakamura, S.; Hori, S.; Shimakawa, M.; Kawashima, H.; Mochizuki, M.; Sugita, S.; Ueno, S.; Yoshizaki, K.; Inaba, G. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet’s disease with refractory uveoretinitis. J. Rheumatol. 2004, 31, 1362–1368. [Google Scholar] [PubMed]
- Diaz-Llopis, M.; Salom, D.; Garcia-de-Vicuna, C.; Cordero-Coma, M.; Ortega, G.; Ortego, N.; Suarez-de-Figueroa, M.; Rio-Pardo, M.J.; Fernandez-Cid, C.; Fonollosa, A.; et al. Treatment of refractory uveitis with adalimumab: A prospective multicenter study of 131 patients. Ophthalmology 2012, 119, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. New Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Jin, Z.B.; Gao, M.L.; Deng, W.L.; Wu, K.C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2019, 69, 38–56. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Algvere, P.V.; Berglin, L.; Gouras, P.; Sheng, Y.; Kopp, E.D. Transplantation of RPE in age-related macular degeneration: Observations in disciform lesions and dry RPE atrophy. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 149–158. [Google Scholar] [CrossRef]
- McGill, T.J.; Stoddard, J.; Renner, L.M.; Messaoudi, I.; Bharti, K.; Mitalipov, S.; Lauer, A.; Wilson, D.J.; Neuringer, M. Allogeneic iPSC-Derived RPE Cell Graft Failure Following Transplantation Into the Subretinal Space in Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Kennelly, K.P.; Holmes, T.M.; Wallace, D.M.; O’Farrelly, C.; Keegan, D.J. Early Subretinal Allograft Rejection Is Characterized by Innate Immune Activity. Cell Transpl. 2017, 26, 983–1000. [Google Scholar] [CrossRef] [Green Version]
- Rezai, K.A.; Farrokh-Siar, L.; Godowski, K.; Patel, S.C.; Ernest, J.T. A model for xenogenic immune response. Graefes. Arch. Clin. Exp. Ophthalmol. 2000, 238, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Gouras, P.; Cao, H.; Berglin, L.; Kjeldbye, H.; Lopez, R.; Rosskothen, H. Patch transplants of human fetal retinal pigment epithelium in rabbit and monkey retina. Investig. Ophthalmol. Vis. Sci. 1995, 36, 381–390. [Google Scholar]
- Ilmarinen, T.; Hiidenmaa, H.; Kööbi, P.; Nymark, S.; Sorkio, A.; Wang, J.H.; Stanzel, B.V.; Thieltges, F.; Alajuuma, P.; Oksala, O.; et al. Ultrathin Polyimide Membrane as Cell Carrier for Subretinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigment Epithelium. PLoS ONE 2015, 10, e0143669. [Google Scholar] [CrossRef] [PubMed]
- Petrus-Reurer, S.; Bartuma, H.; Aronsson, M.; Westman, S.; Lanner, F.; Andre, H.; Kvanta, A. Integration of Subretinal Suspension Transplants of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in a Large-Eyed Model of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Petrus-Reurer, S.; Winblad, N.; Kumar, P.; Gorchs, L.; Chrobok, M.; Wagner, A.K.; Bartuma, H.; Lardner, E.; Aronsson, M.; Plaza Reyes, A.; et al. Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II. Stem Cell Rep. 2020, 14, 648–662. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Lu, X.; Zhang, C.; Yu, H.; Zhao, T. Cells derived from iPSC can be immunogenic-yes or no? Protein Cell 2014, 5, 1–3. [Google Scholar] [CrossRef]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kimura, T.; Futagami, T.; Suegami, S.; Takahashi, M. Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Rep. 2016, 7, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Sugita, S.; Makabe, K.; Fujii, S.; Iwasaki, Y.; Kamao, H.; Shiina, T.; Ogasawara, K.; Takahashi, M. Detection of Retinal Pigment Epithelium-Specific Antibody in iPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem Cell Rep. 2017, 9, 1501–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makabe, K.; Sugita, S.; Hono, A.; Kamao, H.; Takahashi, M. Mycoplasma Ocular Infection in Subretinal Graft Transplantation of iPS Cells-Derived Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1298–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Makabe, K.; Iwasaki, Y.; Fujii, S.; Takahashi, M. Natural Killer Cell Inhibition by HLA-E Molecules on Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Huang, B. The immune response of stem cells in subretinal transplantation. Stem Cell Res. Ther. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Koss, M.J.; Falabella, P.; Stefanini, F.R.; Pfister, M.; Thomas, B.B.; Kashani, A.H.; Brant, R.; Zhu, D.; Clegg, D.O.; Hinton, D.R.; et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: A feasibility and safety study in Yucatán minipigs. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1553–1565. [Google Scholar] [CrossRef]
- Plaza Reyes, A.; Petrus-Reurer, S.; Antonsson, L.; Stenfelt, S.; Bartuma, H.; Panula, S.; Mader, T.; Douagi, I.; André, H.; Hovatta, O.; et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem Cell Rep. 2016, 6, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.C.; Gouras, P.; Doi, K.; Tsang, S.H.; Goff, S.P.; Ashton, P. Local immunosuppression prolongs survival of RPE xenografts labeled by retroviral gene transfer. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3134–3141. [Google Scholar]
- Xing, K.; Gu, B.; Zhang, P.; Wu, X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: An insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015, 16, 39. [Google Scholar] [CrossRef] [Green Version]
- Ganter, S.; Northoff, H.; Männel, D.; Gebicke-Härter, P.J. Growth control of cultured microglia. J. Neurosci. Res. 1992, 33, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Stanzel, B.V.; Liu, Z.; Somboonthanakij, S.; Wongsawad, W.; Brinken, R.; Eter, N.; Corneo, B.; Holz, F.G.; Temple, S.; Stern, J.H.; et al. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Rep. 2014, 2, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhry, S.; Ghosh, S. Intravitreal and posterior subtenon triamcinolone acetonide in idiopathic bilateral uveitic macular oedema. Clin. Exp. Ophthalmol. 2007, 35, 713–718. [Google Scholar] [CrossRef] [PubMed]
- VanderBeek, B.L.; Bonaffini, S.G.; Ma, L. The Association between Intravitreal Steroids and Post-Injection Endophthalmitis Rates. Ophthalmology 2015, 122, 2311–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charalampidou, S.; Nolan, J.; Ormonde, G.O.; Beatty, S. Visual perceptions induced by intravitreous injections of therapeutic agents. Eye (Lond) 2011, 25, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, H.; Kawano, H.; Sonoda, S.; Nakamura, M.; Sakamoto, T. Particle-induced endophthalmitis: Possible mechanisms of sterile endophthalmitis after intravitreal triamcinolone. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.A.; Potter, M.J.; Cui, J.Z.; Chang, T.S.; Ma, P.; Maberley, A.L.; Ross, W.H.; White, V.A.; Samad, A.; Jia, W.; et al. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Can. J. Ophthalmol. 2002, 37, 211–220. [Google Scholar] [CrossRef]
- Sugita, S.; Kamao, H.; Iwasaki, Y.; Okamoto, S.; Hashiguchi, T.; Iseki, K.; Hayashi, N.; Mandai, M.; Takahashi, M. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1051–1062. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, S.; Sugita, S.; Futatsugi, Y.; Ishida, M.; Edo, A.; Makabe, K.; Kamao, H.; Iwasaki, Y.; Sakaguchi, H.; Hirami, Y.; et al. A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models. Int. J. Mol. Sci. 2020, 21, 3077. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21093077
Fujii S, Sugita S, Futatsugi Y, Ishida M, Edo A, Makabe K, Kamao H, Iwasaki Y, Sakaguchi H, Hirami Y, et al. A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models. International Journal of Molecular Sciences. 2020; 21(9):3077. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21093077
Chicago/Turabian StyleFujii, Shota, Sunao Sugita, Yoko Futatsugi, Masaaki Ishida, Ayaka Edo, Kenichi Makabe, Hiroyuki Kamao, Yuko Iwasaki, Hirokazu Sakaguchi, Yasuhiko Hirami, and et al. 2020. "A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models" International Journal of Molecular Sciences 21, no. 9: 3077. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21093077



