The In Vitro Non-Tetramerizing ZapAI83E Mutant Is Unable to Recruit ZapB to the Division Plane In Vivo in Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. ZapAI83E Does Not Complement the ∆zapA Phenotype
2.2. ZapAI83E Localizes Diffusely Throughout the Cell
2.3. ZapAI83E Interacts with FtsZ In Vivo
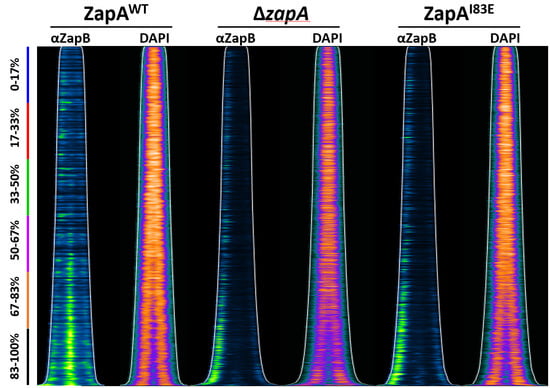
2.4. ZapB Delocalizes Unipolarly in Cells with ZapAI83E or Without ZapA
2.5. Unipolar ZapB Signal Anticorrelates with Signal for the Chromosome
2.6. Min Oscillation Is Not Affected by Polar ZapB, Polar ZapB Is Likely Folded and Functional
2.7. ZapA and MatP Effects on ZapB Localization
3. Discussion and Conclusions
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Site-directed Mutagenesis and Plasmid Construction
4.3. Immunolabeling
4.4. Microscopy and Image Analysis
4.5. FRET Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gueiros-Filho, F.J.; Losick, R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 2002, 16, 2544–2556. [Google Scholar] [CrossRef] [Green Version]
- Small, E.; Marrington, R.; Rodger, A.; Scott, D.J.; Sloan, K.; Roper, D.; Dafforn, T.R.; Addinall, S.G. FtsZ Polymer-bundling by the Escherichia coli ZapA Orthologue, YgfE, Involves a Conformational Change in Bound GTP. J. Mol. Biol. 2007, 369, 210–221. [Google Scholar] [CrossRef]
- Mohammadi, T.; Ploeger, G.E.J.; Verheul, J.; Comvalius, A.D.; Martos, A.; Alfonso, C.; Van Marle, J.; Rivas, G.; Den Blaauwen, T. The GTPase activity of Escherichia coli FtsZ determines the magnitude of the FtsZ polymer bundling by ZapA in vitro. Biochemistry 2009, 48, 11056–11066. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.E.; Gueiros-Filho, F.J.; Erickson, H.P. Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J. Bacteriol. 2004, 186, 5775–5781. [Google Scholar] [CrossRef] [Green Version]
- Caldas, P.; López-Pelegrín, M.; Pearce, D.J.G.; Budanur, N.B.; Brugués, J.; Loose, M. Cooperative ordering of treadmilling filaments in cytoskeletal networks of FtsZ and its crosslinker ZapA. Nat. Commun. 2019, 10, 5744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Gómez, R.; Cheng, X.; Hicks, M.R.; Smith, C.J.I.; Roper, D.I.; Addinall, S.; Rodger, A.; Dafforn, T.R. Tetramerization of ZapA is required for FtsZ bundling. Biochem. J. 2013, 449, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseboom, W.; Nazir, M.; Meiresonne, N.; Mohammadi, T.; Verheul, J.; Buncherd, H.; Bonvin, A.; De Koning, L.; De Koster, C.; De Jong, L.; et al. Mapping the Contact Sites of the Escherichia coli Division-Initiating Proteins FtsZ and ZapA by BAMG Cross-Linking and Site-Directed Mutagenesis. Int. J. Mol. Sci. 2018, 19, 2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, H.H.; Moncrieffe, M.C.; Löwe, J. The Crystal Structure of ZapA and its Modulation of FtsZ Polymerisation. J. Mol. Biol. 2004, 341, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Roach, E.J.; Kimber, M.S.; Khursigara, C.M. Crystal Structure and Site-directed Mutational Analysis Reveals Key Residues Involved in Escherichia coli ZapA Function. J. Biol. Chem. 2014, 289, 23276–23286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espéli, O.; Borne, R.; Dupaigne, P.; Thiel, A.; Gigant, E.; Mercier, R.; Boccard, F. A MatP-divisome interaction coordinates chromosome segregation with cell division in E. coli. EMBO J. 2012, 31, 3198–3211. [Google Scholar] [CrossRef] [Green Version]
- Mercier, R.; Petit, M.A.; Schbath, S.; Robin, S.; El Karoui, M.; Boccard, F.; Espéli, O. The MatP/matS Site-Specific System Organizes the Terminus Region of the E. coli Chromosome into a Macrodomain. Cell 2008, 135, 475–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, E.; Gerdes, K. FtsZ-ZapA-ZapB Interactome of Escherichia coli. J. Bacteriol. 2012, 194, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebersbach, G.; Galli, E.; Møller-Jensen, J.; Löwe, J.; Gerdes, K. Novel coiled-coil cell division factor ZapB stimulates Z ring assembly and cell division. Mol. Microbiol. 2008, 68, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Gerdes, K. Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol. Microbiol. 2010, 76, 1514–1526. [Google Scholar] [CrossRef]
- Castillo, D.E.; Yang, D.; Siopsis, G.; Männik, J. The role of MatP, ZapA and ZapB in chromosomal organization and dynamics in Escherichia coli. Nucleic Acids Res. 2015, 44, 1216–1226. [Google Scholar]
- Buss, J.A.; Peters, N.T.; Xiao, J.; Bernhardt, T.G. ZapA and ZapB form an FtsZ-independent structure at midcell. Mol. Microbiol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Vischer, N.O.E.; Verheul, J.; Postma, M.; Van den Berg van Saparoea, B.; Galli, E.; Natale, P.; Gerdes, K.; Luirink, J.; Vollmer, W.; Vicente, M.; et al. Cell age dependent concentration of Escherichia coli divisome proteins analyzed with ImageJ and ObjectJ. Front. Microbiol. 2015, 6, 586. [Google Scholar] [CrossRef] [Green Version]
- Hale, C.A.; De Boer, P.A. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 1997, 88, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Alexeeva, S.; Gadella, T.W.J.; Verheul, J.; Verhoeven, G.S.; Den Blaauwen, T. Direct interactions of early and late assembling division proteins in Escherichia coli cells resolved by FRET. Mol. Microbiol. 2010, 77, 384–398. [Google Scholar] [CrossRef]
- Meiresonne, N.; Alexeeva, S.; Van der Ploeg, R.; Den Blaauwen, T. Detection of Protein Interactions in the Cytoplasm and Periplasm of Escherichia coli by Förster Resonance Energy Transfer. Bio-Protocol 2018, 7. [Google Scholar] [CrossRef]
- Bisicchia, P.; Arumugam, S.; Schwille, P.; Sherratt, D. MinC, MinD, and MinE drive counter-oscillation of early-cell-division proteins prior to Escherichia coli septum formation. MBio 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lioy, V.S.; Cournac, A.; Marbouty, M.; Duigou, S.; Mozziconacci, J.; Espéli, O.; Boccard, F.; Koszul, R. Multiscale Structuring of the E. coli Chromosome by Nucleoid-Associated and Condensin Proteins. Cell 2018, 172, 771–783.e18. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-W.; Burkhardt, D.; Gross, C.; Weissman, J.S. Quantifying Absolute Protein Synthesis Rates Reveals Principles Underlying Allocation of Cellular Resources. Cell 2014, 157, 624–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2. [Google Scholar] [CrossRef] [Green Version]
- Bethesda Research Laboratories E. coli DH5 alpha competent cells. Focus Bethesda Res. Lab. 1986, 8, 9. [Google Scholar]
- Bernhardt, T.G.; De Boer, P.A.J. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 2003, 48, 1171–1182. [Google Scholar] [CrossRef] [Green Version]
- Den Blaauwen, T.; Aarsman, M.E.G.; Vischer, N.O.E.; Nanninga, N. Penicillin-binding protein PBP2 of Escherichia coli localizes preferentially in the lateral wall and at mid-cell in comparison with the old cell pole. Mol. Microbiol. 2003, 47, 539–547. [Google Scholar] [CrossRef]
- Van der Ploeg, R.; Verheul, J.; Vischer, N.O.E.; Alexeeva, S.; Hoogendoorn, E.; Postma, M.; Banzhaf, M.; Vollmer, W.; Den Blaauwen, T. Colocalization and interaction between elongasome and divisome during a preparative cell division phase in Escherichia coli. Mol. Microbiol. 2013, 87, 1074–1087. [Google Scholar] [CrossRef]
- Buddelmeijer, N.; Aarsman, M.; Den Blaauwen, T. Immunolabeling of Proteins in situ in Escherichia coli K12 Strains. BIO-PROTOCOL 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Koppelman, C.-M.; Aarsman, M.E.G.; Postmus, J.; Pas, E.; Muijsers, A.O.; Scheffers, D.-J.; Nanninga, N.; Den Blaauwen, T. R174 of Escherichia coli FtsZ is involved in membrane interaction and protofilament bundling, and is essential for cell division. Mol. Microbiol. 2004, 51, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, T. Statistics to Use: Kolmogorov-Smirnov Test. Available online: http://www.physics.csbsju.edu/stats/ (accessed on 21 April 2020).
- Clark, D.J.; Maaløe, O. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 1967, 23, 99–112. [Google Scholar] [CrossRef]






| Name | Full Name | Reference |
|---|---|---|
| DH5α | F-, supE44, hsdR17, recA1, endA1, gyrA96, thi1, relA1 | [25] |
| TB28 | rph1 ilvG rfb-50 ΔlacIYZA::FRT | [26] |
| TB28 ∆zapA | TB28 ∆zapA | [14] |
| sNM02 | TB28 ∆matP | This work |
| sNM03 | TB28 ∆zapA ∆matP | This work |
| Name | Full Name/Characteristics | Reference |
|---|---|---|
| pTHV037 | ptrc99Adown, AmpR, ColE1 ori | [27] |
| pSAV057 | ptrc99Adown, CamR, P15 ori | [19] |
| pGP021 | pTHV–ZapA | [3] |
| pGP016 | pET302His6ZapA, ptrc99A, AmpR, ColE1 ori | [3] |
| pET302His6ZapAI83E | pET302His6ZapAI83E | This work |
| pRP071 | pTHV–ZapAI83E | This work |
| pSAV050 | pTHV–mCh–mKO | [19] |
| pSAV072 | pSAV–mKO–FtsZ | [19] |
| pSAV077 | pTHV–mCh–ZapA | [19] |
| pNM137 | pTHV–mCh–ZapAI83E | This work |
| pBB008 | pTHV–mCh–PBP1b | [28] |
| pBB004 | pSAV–mKO–PBP1a | [28] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meiresonne, N.Y.; den Blaauwen, T. The In Vitro Non-Tetramerizing ZapAI83E Mutant Is Unable to Recruit ZapB to the Division Plane In Vivo in Escherichia coli. Int. J. Mol. Sci. 2020, 21, 3130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21093130
Meiresonne NY, den Blaauwen T. The In Vitro Non-Tetramerizing ZapAI83E Mutant Is Unable to Recruit ZapB to the Division Plane In Vivo in Escherichia coli. International Journal of Molecular Sciences. 2020; 21(9):3130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21093130
Chicago/Turabian StyleMeiresonne, Nils Y., and Tanneke den Blaauwen. 2020. "The In Vitro Non-Tetramerizing ZapAI83E Mutant Is Unable to Recruit ZapB to the Division Plane In Vivo in Escherichia coli" International Journal of Molecular Sciences 21, no. 9: 3130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21093130