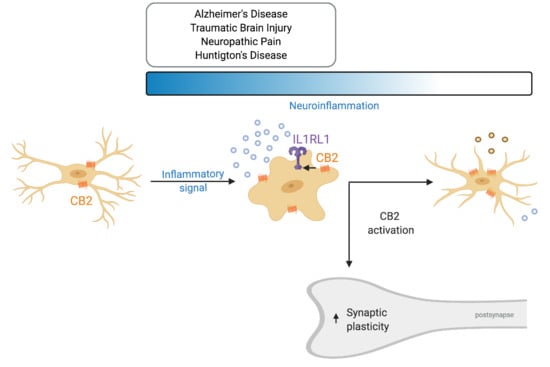
CB2 Receptor in Microglia: The Guardian of Self-Control
Abstract
:1. Introduction
1.1. Microglia and Neuroinflammation
1.2. The CB2 Receptor Is Expressed in the Brain not only under Pathological Conditions
2. The Role of CB2Rin Physiology and Neuroinflammation
2.1. CB2R and Microglia In Vitro
| Inflammatory Modulator | Model | CB2R Activity Modification | Outcome | Further Proof | Reference |
|---|---|---|---|---|---|
| Pharmacological modification of CB2R activity | |||||
| none | BV2 cells | ACPA | ↑ Migration | CB1R antagonist did not affect ACPA-induced migration. CB2R antagonists inhibited migration | [49] |
| 2-AG | ↑ Migration | cannabinol and cannabidiol blocks this effect | [16] | ||
| Murine primary microglial cells | JWH-015 | = NO, TNFα, CD40 | [52] | ||
| Rat primary microglial cells | HU-210; WIN 55,212-2; JWH-133 | = TNFα | [53] | ||
| Human/Rat microglial cells | AEA; 2-AG | ↑ Arg1 | [56] | ||
| LPS | Rat primary microglial cells | AEA; WIN 55,212-2 | ↓ iNOS, | ||
| AEA; 2-AG | ↑ Arg1, SOCS3 | ||||
| LPS/IFNγ | Murine N9 microglial cells | AM1241 (pretreatment) | ↓ iNOS, ↑ Arg1, IL10, BDNF, GDNF ↓ TNFα, IL1β, IL-6 | Effects reversed with CB2R antagonist AM630 or an inhibitor of protein kinase C | [55] |
| Murine primary microglial cells | AEA; JWH-133 | ↑ IL-10 | Effect reversed by CB2R antagonist SR144528 | [54] | |
| IFNγ | JWH-015 | ↓ CD40 | [52] | ||
| IFNγ/CD40L Aβ1–42/CD40L | JWH-015 | ↓ NO, TNFα ↑ phagocytosis (of Aβ) | |||
| Aβ | Rat primary microglial cells | HU-210; WIN 55,212-2; JWH-133 | ↓ TNFα ↓ microglia activation ↔ microglia morphology | [53] | |
| IL-4/IL-13 | none | ↑ 2-AG, Arg1 | Arg1 increase blocked by CB1R and CB2R antagonists | [56] | |
| TGFβ | none | ↑ AEA | |||
| Genetic deletion of CB2R | |||||
| none | Murine primary microglial cells | Cnr2tm1Dgen | ↓ phagocytosis ↓ Arg1 | [56] | |
| LPS/IFNγ | Cnr2tim1Zim | ↓ TNFα, ICAM, CD40, IL-6, CCL2 = phagocytosis | [51] | ||
| IL-4/IL-13 | Cnr2tm1Dgen | ↓ Arg1 ↔ microglia morphology ↓ phagocytosis | [56] | ||
2.2. CB2Rs and Microglia: In Vivo Studies
2.2.1. Alzheimer’s Disease
2.2.2. Huntington’s Disease (HD)
2.2.3. Neuropathic Pain
2.2.4. Traumatic Brain Injury (TBI)
| Disease Model | Model | CB2R Activity Modification | Molecular Effects | Behavioral Phenotype | Reference |
|---|---|---|---|---|---|
| AD | Pharmacological modification of CB2R activity | ||||
| APP/PS1 (APPswe/PS1dE9) | JWH-133 | ↓ Microglial activity ↓ IL-1β, IL-6, TNFα, IL-10 secretion ↓ Oxidative damage = Aβ plaque load | ↑ V-maze | [65] | |
| Cannabidiol | = Aβ plaque load | ↑ Novel object recognition ↑ Social recognition | [67] | ||
| JWH-133 | = Aβ plaque load ↓ Microglial activity ↓ IL6, TNFα, iNOS expression (region-dependent) | ↑ Novel object recognition ↔ Spatial memory impairment (MWM) | [70] | ||
| APP2576 | WIN 55,212-2 JWH-133 | ↓ Microglial density (JWH-133) ↓ COX-2 ↓ IL-6, TNFα ↓ Aβ1-40 cortical levels | ↑ Novel object recognition (JHW-133) | [69] | |
| AβPP23/PS45 | HU-210 | = APP processing and neuritic laque formation = Neurogenesis | = Spatial memory impairment (MWM) = Contextual fear conditioning | [66] | |
| Genetic deletion of CB2R | |||||
| J20 | Cnr2tm1Dgen/J | ↑ Plaque-associated microglia ↑ soluble Aβ42 ↑ Aβ plaque load | [72] | ||
| APP/PS1 (APPswe/PS1dE9) | Cnr2tim1Zim/J | ↑ soluble Aβ40 = Aβ plaque load = Microglial activity | = Two-object recognition test | [71] | |
| ↓ Microglial activity ↓ TNFα expression ↓ Aβ plaque load ↓ neuronal loss ↓ Infiltrating immune cells | ↓ Spatial memory impairment (MWM) | [51,73] | |||
| HD | Pharmacological modification of CB2R activity | ||||
| R6/2 | GW405833 | ↓ Microglial activity ↓ Expression of IL-6 | ↓ Severity of motor symptoms | [86] | |
| Genetic deletion of CB2R | |||||
| R6/2 | Cnr2tim1Zim/J | ↑ Microglial activity | ↑ Severity of motor symptoms | [87] | |
| BACHD | ↑ Expression of IL-6 | ↑ Onset and severity of motor symptoms | [86] | ||
| Neuropathic pain | Pharmacological modification of CB2R activity | ||||
| Post-operative pain | JWH-015 | ↓ Microglial activity | ↓ Paw incision–induced hypersen- sitivity | [93] | |
| Partial sciatic nerve ligation | β-caryophyllene JWH-133 | ↓ Microglial density (BCP) | ↓ Pain response thermal sensitivity ↓ Pain response mechanical sensitivity | [95] | |
| Formalin Test | β-caryophyllene | ↓ Pain response Formalin test | [95] | ||
| Partial sciatic nerve ligation | ↓ Pain response thermal sensitivity | [98] | |||
| TBI | Pharmacological modification of CB2R activity | ||||
| GP1a | ↓ Macrophage infiltration ↓ Oedema size ↑ Macrophage M2 polarization ↓ Expression of iNOS, TNFα, IL-6, IL-1β ↑ Expression of Arg1, IL-10 | ↓ Severity of motor symptoms | [103] | ||
| Raloxifene | ↓ M1/M2 microglia ratio ↓ Microglial density | ↓ Severity of visual deficits | [104] | ||
| SM-189 | ↓ M1/M2 microglia ratio ↓ Microglial density | ↓ Severity of visual deficits | [106] | ||
2.3. Intracellular Signalling Pathways
2.4. CB2 Knockout Mouse Lines
2.5. CB2 Receptors on Microglia Influence Behaviour
Neuroinflammation and Cognition
3. Open Questions and Controversies
CB2Rs in Neuron-Microglia Interaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawson, L.J.; Perry, V.H.; Gordon, S. Turnover of Resident Microglia in the Normal Adult Mouse Brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia Emerge from Erythromyeloid Precursors via Pu.1-and Irf8-Dependent Pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Klett, F.F.; Lin, G.; Sagar, S.; Datta, M.; Drougard, A.; Stempfl, T.; Fabregat, A.A.; et al. A New Fate Mapping System Reveals Context-Dependent Random or Clonal Expansion of Microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Neuroforum 2005, 11, 95–96. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Torres, C.P.; Frouin, A.; Wilton, D.K.; et al. A Complement-Microglial Axis Drives Synapse Loss during Virus-Induced Memory Impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Inoue, H.N.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 1–16. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Bittner, T.; Jung, C.K.E.; Burgold, S.; Page, R.M.; Mitteregger, G.; Haass, C.; LaFerla, F.M.; Kretzschmar, H.; Herms, J. Microglial Cx3cr1 Knockout Prevents Neuron Loss in a Mouse Model of Alzheimer’s Disease. Nat. Neurosci. 2010, 13, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; Khoury, J.E. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Pablo, J.; Vaccari, D.R.; Dietrich, W.D.; Phillip, G.; Keane, R.W.; Repair, S.C.; Medical, W. Pattern Recognition Receptors and the Central Nervous System Repair. Exp. Neurol. 2016, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Suárez, D.F. Alternatively Activated Microglia and Macrophages in the Central Nervous System. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef]
- Witting, A.; Walter, L.; Wacker, J.; Mo, T.; Stella, N. P2X7 Receptors Control 2AG Production by Microglial Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 3214–3219. [Google Scholar] [CrossRef] [Green Version]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ 9-Tetrahydrocannabinol, Cannabidiol and Δ 9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Devane, W.; Dysarz, F., 3rd; Johnson, M.; Melvin, L.; Howlet, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Munro, S.; Thomas, K.L.; Shaar, M.A. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Neuroscience: Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Özdoğan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A.; et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumpf, A.; Parthier, D.; Sammons, R.P.; Stempel, A.V.; Breustedt, J.; Rost, B.R.; Schmitz, D. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Self-Inhibition in Cortical Neurons. Neuropharmacology 2018, 139, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, N.E.; McCoy, K.L.; Mezey, É.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by Cannabinoids Is Absent in Mice Deficient for the Cannabinoid CB2 Receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar] [CrossRef]
- Wotherspoon, G.; Fox, A.; McIntyre, P.; Colley, S.; Bevan, S.; Winter, J. Peripheral Nerve Injury Induces Cannabinoid Receptor 2 Protein Expression in Rat Sensory Neurons. Neuroscience 2005, 135, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O’Donnell, D. Induction of CB2 Receptor Expression in the Rat Spinal Cord of Neuropathic but Not Inflammatory Chronic Pain Models. Eur. J. Neurosci. 2003, 17, 2750–2754. [Google Scholar] [CrossRef]
- Derbenev, A.V.; Stuart, T.C.; Smith, B.N. Cannabinoids Suppress Synaptic Input to Neurones of the Rat Dorsal Motor Nucleus of the Vagus Nerve. J. Physiol. 2004, 559, 923–938. [Google Scholar] [CrossRef]
- Schatz, A.R.; Lee, M.; Condie, R.B.; Pulaski, J.T.; Kaminski, N.E. Cannabinoid Receptors CB1 and CB2: A Characterization of Expression and Adenylate Cyclase Modulation within the Immune System. Toxicol. Appl. Pharmacol. 1997, 142, 278–287. [Google Scholar] [CrossRef]
- McCoy, K.L.; Matveyeva, M.; Carlisle, S.J.; Cabral, G.A. Cannabinoid Inhibition of the Processing of Intact Lysozyme by Macrophages: Evidence for CB2 Receptor Participation. J. Pharmacol. Exp. Ther. 1999, 289, 1620–1625. [Google Scholar]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LeFur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Herkenham, M. Cannabinoid Receptors Undergo Axonal Flow in Sensory Nerves. Neuroscience 1999, 92, 1171–1175. [Google Scholar] [CrossRef]
- Price, T.J.; Helesic, G.; Parghi, D.; Hargreaves, K.M.; Flores, C.M. The Neuronal Distribution of Cannabinoid Receptor Type 1 in the Trigeminal Ganglion of the Rat. Neuroscience 2003, 120, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D.; Buriani, A.; Dal Toso, R.; Petrelii, L.; Romanello, S.; Facci, L.; Leon, A. The ALIAmide Palmitoylethanolamide and Cannabinoids, but Not Anandamide, Are Protective in a Delayed Postglutamate Paradigm of Excitotoxic Death in Cerebellar Granule Neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 3984–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoS ONE 2008, 3, e1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.-R.; Pan, C.-H.; Hishimoto, A.; Li, C.-Y.; Xi, Z.-X.; Berzal, A.L.; Viveros, M.-P.; Ishiguro, H.; Arinami, T.; Onaivi, E.S.; et al. Species Differences in Cannabinoid Receptor 2 (CNR2) Gene: Identification of Novel Human and Rodent CB2 Isoforms, Differential Tissue Expression, and Regulation by Cannabinoid Receptor Ligands. Genes Brain Behav. 2009, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Gao, M.; Liu, Q.-R.; Bi, G.-H.; Li, X.; Yang, H.-J.; Gardner, E.L.; Wu, J.; Xi, Z.-X. Cannabinoid CB2 Receptors Modulate Midbrain Dopamine Neuronal Activity and Dopamine-Related Behavior in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, E5007–E5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Kim, J. Neuronal Expression of CB2 Cannabinoid Receptor MRNAs in the Mouse Hippocampus. Neuroscience 2015, 311, 253–267. [Google Scholar] [CrossRef] [Green Version]
- den Boon, F.S.; Chameau, P.; Zhao, Q.S.; van Aken, W.; Bari, M.; Oddi, S.; Kruse, C.G.; Maccarrone, M.; Wadman, W.J.; Werkmana, T.R. Excitability of Prefrontal Cortical Pyramidal Neurons Is Modulated by Activation of Intracellular Type-2 Cannabinoid Receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 3534–3539. [Google Scholar] [CrossRef] [Green Version]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid CB2 Receptors Are Expressed by Perivascular Microglial Cells in the Human Brain: An Immunohistochemical Study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef]
- Atwood, B.K.; MacKie, K. CB 2: A Cannabinoid Receptor with an Identity Crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.H.; Darlington, C.L.; Smith, P.F.; Ashton, J.C. Antibody Testing for Brain Immunohistochemistry: Brain Immunolabeling for the Cannabinoid CB2 Receptor. J. Neurosci. Methods 2013, 216, 87–95. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Shen, H.; Jordan, C.J.; Liu, Q.R.; Gardner, E.L.; Bonci, A.; Xi, Z.X. CB2 Receptor Antibody Signal Specificity: Correlations with the Use of Partial CB 2 -Knockout Mice and Anti-Rat CB2 Receptor Antibodies. Acta Pharmacol. Sin. 2019, 40, 398–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlisle, S.J.; Cabral, F.M.; Staab, A.; Ludwick, C.; Cabral, G.A. Differential Expression of the CB2 Cannabinoid Receptor by Rodent Macrophages and Macrophage-like Cells in Relation to Cell Activation. Int. Immunopharmacol. 2002, 2, 69–82. [Google Scholar] [CrossRef]
- López, A.; Aparicio, N.; Pazos, M.R.; Grande, M.T.; Manso, M.A.B.; Cuesta, I.B.; Vázquez, C.; Amores, M.; Pérez, G.R.; García, E.G.; et al. Cannabinoid CB2 Receptors in the Mouse Brain: Relevance for Alzheimer’s Disease. J. Neuroinflamm. 2018, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmöle, A.C.; Lundt, R.; Gennequin, B.; Schrage, H.; Beins, E.; Krämer, A.; Zimmer, T.; Limmer, A.; Zimmer, A.; Otte, D.M. Expression Analysis of CB2-GFP BAC Transgenic Mice. PLoS ONE 2015, 10, e0145472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A Novel in Situ RNA Analysis Platform for Formalin-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Y.; Bi, G.H.; Li, X.; Li, J.; Qu, H.; Zhang, S.J.; Li, C.Y.; Onaivi, E.S.; Gardner, E.L.; Xi, Z.X.; et al. Species Differences in Cannabinoid Receptor 2 and Receptor Responses to Cocaine Self-Administration in Mice and Rats. Neuropsychopharmacology 2015, 40, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-Y.; Gao, M.; Shen, H.; Bi, G.-H.; Yang, H.-J.; Liu, Q.-R.; Wu, J.; Gardner, E.L.; Bonci, A.; Xi, Z.-X. Expression of Functional Cannabinoid CB 2 Receptor in VTA Dopamine Neurons in Rats. Addict. Biol. 2017, 22, 752–765. [Google Scholar] [CrossRef] [Green Version]
- Jordan, C.J.; Xi, Z.-X. Progress in Brain Cannabinoid CB2 Receptor Research: From Genes to Behavior. Neurosci. Biobehav. Rev. 2019, 176, 139–148. [Google Scholar] [CrossRef]
- Franklin, A.; Stella, N. Arachidonylcyclopropylamide Increases Microglial Cell Migration through Cannabinoid CB2 and Abnormal-Cannabidiol-Sensitive Receptors. Eur. J. Pharmacol. 2003, 474, 195–198. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the Cannabinoid CB2 Receptor in Microglial Cells in Response to Inflammatory Stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Schmöle, A.-C.; Lundt, R.; Ternes, S.; Albayram, Ö.; Ulas, T.; Schultze, J.L.; Bano, D.; Nicotera, P.; Alferink, J.; Zimmer, A. Cannabinoid Receptor 2 Deficiency Results in Reduced Neuroinflammation in an Alzheimer’s Disease Mouse Model. Neurobiol. Aging 2015, 36, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, D. Stimulation of Cannabinoid Receptor 2 (CB2) Suppresses Microglial Activation. J. Neuroinflamm. 2005, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, B.G.; Blazquez, C.; del Pulgar, T.G.; Guzman, M.; de Ceballos, M.L. Prevention of Alzheimer’s Disease Pathology by Cannabinoids: Neuroprotection Mediated by Blockade of Microglial Activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; di Marzo, V.; Guaza, C. Anandamide Enhances IL-10 Production in Activated Microglia by Targeting CB2 Receptors: Roles of ERK1/2, JNK, and NF-ΚB. Glia 2010, 58, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jia, J.; Liu, X.; Bai, F.; Wang, Q.; Xiong, L. Activation of Murine Microglial N9 Cells Is Attenuated through Cannabinoid Receptor CB2 Signaling. Biochem. Biophys. Res. Commun. 2015, 458, 92–97. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Salinas, F.J.C.; Zubiaurre, A.R.; Gutiérrez, S.O.; de Sola, R.G.; Guaza, C. Endocannabinoids Drive the Acquisition of an Alternative Phenotype in Microglia. Brain Behav. Immun. 2015, 49, 233–245. [Google Scholar] [CrossRef]
- Benito, C.; Tolón, R.M.; Pazos, M.R.; Núñez, E.; Castillo, A.I.; Romero, J. Cannabinoid CB2 Receptors in Human Brain Inflammation. Br. J. Pharmacol. 2008, 153, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia Constitute a Barrier That Prevents Neurotoxic Protofibrillar Aβ42 Hotspots around Plaques. Nat. Commun. 2015, 6, 6176. [Google Scholar] [CrossRef] [Green Version]
- Perea, J.R.; Martín, M.L.; Ávila, J.; Bolós, M. The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Front. Cell. Neurosci. 2018, 1, 172. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; O’Banion, M.K.; Terwel, D.; Kummer, M.P. Neuroinflammatory Processes in Alzheimer’s Disease. J. Neural Transm. 2010, 117, 919–947. [Google Scholar] [CrossRef]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horti, A.G.; Gao, Y.; Ravert, H.T.; Finley, P.; Valentine, H.; Wong, D.F.; Endres, C.J.; Savonenko, A.V.; Dannals, R.F. Synthesis and Biodistribution of [11C]A-836339, a New Potential Radioligand for PET Imaging of Cannabinoid Type 2 Receptors (CB2). Bioorg. Med. Chem. 2010, 18, 5202–5207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savonenko, A.V.; Melnikova, T.; Wang, Y.; Ravert, H.; Gao, Y.; Koppel, J.; Lee, D.; Pletnikova, O.; Cho, E.; Sayyida, N.; et al. Cannabinoid CB2 Receptors in a Mouse Model of Aβ Amyloidosis: Immunohistochemical Analysis and Suitability as a PET Biomarker of Neuroinflammation. PLoS ONE 2015, 10, e0129618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 Receptor and Amyloid Pathology in Frontal Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef]
- Aso, E.; Juvés, S.; Maldonado, R.; Ferrer, I. CB2cannabinoid Receptor Agonist Ameliorates Alzheimer-like Phenotype in AβPP/PS1 Mice. J. Alzheimer Dis. 2013, 35, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Brits, K.B.; He, G.; Cai, F.; Zhang, X.; Song, W. Effect of Synthetic Cannabinoid HU210 on Memory Deficits and Neuropathology in Alzheimers Disease Mouse Model. Curr. Alzheimer Res. 2010, 7, 255–261. [Google Scholar] [CrossRef]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic Cannabidiol Treatment Improves Social and Object Recognition in Double Transgenic APPswe/PS1ΔE9 Mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- Parker, L.A.; Mechoulam, R.; Schlievert, C. Cannabidiol, a Non-Psychoactive Component of Cannabis and Its Synthetic Dimethylheptyl Homolog Suppress Nausea in an Experimental Model with Rats. Neuroreport 2002, 13, 567–570. [Google Scholar] [CrossRef]
- Moreno, A.M.M.; Brera, B.; Spuch, C.; Carro, E.; García, L.G.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged Oral Cannabinoid Administration Prevents Neuroinflammation, Lowers β-Amyloid Levels and Improves Cognitive Performance in Tg APP 2576 Mice. J. Neuroinflamm. 2012, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Shi, J.; Wang, B.; Li, J.; Jia, H. CB2 Cannabinoid Receptor Agonist Ameliorates Novel Object Recognition but Not Spatial Memory in Transgenic APP/PS1 Mice. Neurosci. Lett. 2019, 707, 134286. [Google Scholar] [CrossRef]
- Aso, E.; Benito, P.A.; Carmona, M.; Maldonado, R.; Ferrer, I. Cannabinoid Receptor 2 Participates in Amyloid-β Processing in a Mouse Model of Alzheimer’s Disease but Plays a Minor Role in the Therapeutic Properties of a Cannabis-Based Medicine. J. Alzheimer Dis. 2016, 51, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppel, J.; Vingtdeux, V.; Marambaud, P.; d’Abramo, C.; Jimenez, H.; Stauber, M.; Friedman, R.; Davies, P. CB2 Receptor Deficiency Increases Amyloid Pathology and Alters Tau Processing in a Transgenic Mouse Model of Alzheimer’s Disease. Mol. Med. 2013, 19, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Schmöle, A.-C.; Lundt, R.; Toporowski, G.; Hansen, J.N.; Beins, E.; Halle, A.; Zimmer, A. Cannabinoid Receptor 2-Deficiency Ameliorates Disease Symptoms in a Mouse Model with Alzheimer’s Disease-Like Pathology. J. Alzheimer Dis. 2018, 64, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.H.P.; van Hal, M.; den Kelder, I.C.O.; Meier, R.T.; de Ruiter, A.A.; Schut, M.H.; Smith, D.L.; Grit, C.; Brouwer, N.; Kamphuis, W.; et al. Frequency of Nuclear Mutant Huntingtin Inclusion Formation in Neurons and Glia Is Cell-Type-Specific. Glia 2017, 65, 50–61. [Google Scholar] [CrossRef]
- Sapp, E.; Kegel, K.B.; Aronin, N.; Hashikawa, T.; Uchiyama, Y.; Tohyama, K.; Bhide, P.G.; Vonsattel, J.P.; Difiglia, M. Early and Progressive Accumulation of Reactive Microglia in the Huntington Disease Brain. J. Neuropathol. Exp. Neurol. 2001, 60, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.H.; Wu, Y.R.; Chen, Y.C.; Chen, C.M. Plasma Inflammatory Biomarkers for Huntington’s Disease Patients and Mouse Model. Brain Behav. Immun. 2015, 44, 121–127. [Google Scholar] [CrossRef]
- Björkqvist, M.; Wild, E.J.; Thiele, J.; Silvestroni, A.; Andre, R.; Lahiri, N.; Raibon, E.; Lee, R.V.; Benn, C.L.; Soulet, D.; et al. A Novel Pathogenic Pathway of Immune Activation Detectable before Clinical Onset in Huntington’s Disease. J. Exp. Med. 2008, 205, 1869–1877. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.B.; Byrne, L.M.; McColgan, P.; Robertson, N.; Tabrizi, S.J.; Zetterberg, H.; Wild, E.J. Cerebrospinal Fluid Inflammatory Biomarkers Reflect Clinical Severity in Huntington’s Disease. PLoS ONE 2016, 11, e0163479. [Google Scholar] [CrossRef]
- Gu, M.; Gash, M.T.; Mann, V.M.; Agid, F.J.; Cooper, J.M.; Schapira, A.H.V. Mitochondrial Defect in Huntington’s Disease Caudate Nucleus. Ann. Neurol. 1996, 39, 385–389. [Google Scholar] [CrossRef]
- Wright, E.D.; Robertson, H. Cannabinoid Receptor Messenger RNA Levels Decrease in a Subset of Neurons of the Lateral Striatum, Cortex and Hippocampus of Transgenic Huntington’s Disease Mice. Neuroscience 2000, 98, 705–713. [Google Scholar] [CrossRef]
- Glass, M.; Dragunow, M.; Faull, R.L.M. The Pattern of Neurodegeneration in Huntington’s Disease: A Comparative Study of Cannabinoid, Dopamine, Adenosine and GABA(A) Receptor Alterations in the Human Basal Ganglia in Huntington’s Disease. Neuroscience 2000, 97, 505–519. [Google Scholar] [CrossRef]
- van Laere, K.; Casteels, C.; Dhollander, I.; Goffin, K.; Grachev, I.; Bormans, G.; Vandenberghe, W. Widespread Decrease of Type 1 Cannabinoid Receptor Availability in Huntington Disease in Vivo. J. Nucl. Med. 2010, 51, 1413–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez, C.; Chiarlone, A.; Sagredo, O.; Aguado, T.; Pazos, M.R.; Resel, E.; Palazuelos, J.; Julien, B.; Salazar, M.; Börner, C.; et al. Loss of Striatal Type 1 Cannabinoid Receptors Is a Key Pathogenic Factor in Huntington’s Disease. Brain 2011, 134, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; Rincón, D.G.; Sendtner, M.; Timmusk, T.; Lutz, B.; Roperh, I.G.; et al. The CB1 Cannabinoid Receptor Signals Striatal Neuroprotection via a PI3K/Akt/MTORC1/BDNF Pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Chiarlone, A.; Bellocchio, L.; Blázquez, C.; Resel, E.; Gómez, E.S.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A Restricted Population of CB1 Cannabinoid Receptors with Neuroprotective Activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, J.; Truong, J.; Bouchard, K.; Dunkelberger, D.; Desrayaud, S.; Moussaoui, S.; Tabrizi, S.J.; Stella, N.; Muchowski, P.J. Cannabinoid Receptor 2 Signaling in Peripheral Immune Cells Modulates Disease Onset and Severity in Mouse Models of Huntington’s Disease. J. Neurosci. 2012, 35, 18259–18268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid Receptors Are Neuroprotective in Huntington’s Disease Excitotoxicity. Brain 2009, 132, 3152–3164. [Google Scholar] [CrossRef] [Green Version]
- Sagredo, O.; González, S.; Aroyo, I.; Pazos, M.R.; Benito, C.; Becker, I.L.; Romero, J.P.; Tolón, R.M.; Mechoulam, R.; Brouillet, E.; et al. Cannabinoid CB2 Receptor Agonists Protect the Striatum against Malonate Toxicity: Relevance for Huntington’s Disease. Glia 2009, 57, 1154–1167. [Google Scholar] [CrossRef] [Green Version]
- Dowie, M.J.; Grimsey, N.L.; Hoffman, T.; Faull, R.L.M.; Glass, M. Cannabinoid Receptor CB2 Is Expressed on Vascular Cells, but Not Astroglial Cells in the Post-Mortem Human Huntington’s Disease Brain. J. Chem. Neuroanat. 2014, 59–60, 62–71. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Landry, R.P.; Martinez, E.; Deleo, J.A.; Sandoval, E.A.R. Spinal Cannabinoid Receptor Type 2 Agonist Reduces Mechanical Allodynia and Induces Mitogen-Activated Protein Kinase Phosphatases in a Rat Model of Neuropathic Pain. J. Pain 2012, 13, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svíženská, I.H.; Brázda, V.; Klusáková, I.; Dubový, P. Bilateral Changes of Cannabinoid Receptor Type 2 Protein and MRNA in the Dorsal Root Ganglia of a Rat Neuropathic Pain Model. J. Histochem. Cytochem. 2013, 61, 529–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, A.R.; Eisenach, J.C. Spinal Cannabinoid Receptor Type 2 Activation Reduces Hypersensitivity and Spinal Cord Glial Activation after Paw Incision. Anesthesiology 2007, 106, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leichsenring, A.; Andriske, M.; Bäcker, I.; Stichel, C.C.; Lübbert, H. Analgesic and Antiinflammatory Effects of Cannabinoid Receptor Agonists in a Rat Model of Neuropathic Pain. Naunyn. Schmiedebergs. Arch. Pharmacol. 2009, 379, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The Cannabinoid CB2 Receptor-Selective Phytocannabinoid Beta-Caryophyllene Exerts Analgesic Effects in Mouse Models of Inflammatory and Neuropathic Pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Adan, A.G.; Sanguino, E.; Manzanares, J.; et al. Crucial Role of CB2 Cannabinoid Receptor in the Regulation of Central Immune Responses during Neuropathic Pain. J. Neurosci. 2008, 28, 12125–12135. [Google Scholar] [CrossRef] [Green Version]
- Nent, E.; Nozaki, C.; Schmöle, A.C.; Otte, D.; Zimmer, A. CB2 Receptor Deletion on Myeloid Cells Enhanced Mechanical Allodynia in a Mouse Model of Neuropathic Pain. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Cabañero, D.; López, A.R.; Drews, E.; Schmöle, A.; Otte, D.M.; Bargiela, A.W.; Encabo, H.H.; Kummer, S.; Montiel, A.F.; Przewlocki, R.; et al. Protective Role of Neuronal and Lymphoid Cannabinoid CB2 Receptors in Neuropathic Pain. Elife 2020, 9, 1–24. [Google Scholar] [CrossRef]
- Wofford, K.L.; Loane, D.J.; Cullen, D.K. Acute Drivers of Neuroinflammation in Traumatic Brain Injury. Neural Regen. Res. 2019, 14, 1481–1489. [Google Scholar] [CrossRef]
- Panikashvili, D.; Simeonidou, C.; Shabat, S.B.; Hanuš, L.; Breuer, A.; Mechoulam, R.; Shohami, E. An Endogenous Cannabinoid (2-AG) Is Neuroprotective after Brain Injury. Nature 2001, 413, 527–531. [Google Scholar] [CrossRef]
- Rodriguez, A.B.L.; Mela, V.; Fonseca, E.A.; Segura, L.M.G.; Viveros, M.P. CB2 Cannabinoid Receptor Is Involved in the Anti-Inflammatory Effects of Leptin in a Model of Traumatic Brain Injury. Exp. Neurol. 2016, 279, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.B.L.; Fonseca, E.A.; Viveros, M.P.; Segura, L.M.G. Changes in Cannabinoid Receptors, Aquaporin 4 and Vimentin Expression after Traumatic Brain Injury in Adolescent Male Mice. Association with Edema and Neurological Deficit. PLoS ONE 2015, 10, e0128782. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective Activation of Cannabinoid Receptor-2 Reduces Neuroinflammation after Traumatic Brain Injury via Alternative Macrophage Polarization. Brain Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Honig, M.G.; del Mar, N.A.; Henderson, D.L.; Ragsdale, T.D.; Doty, J.B.; Driver, J.H.; Li, C.; Fortugno, A.P.; Mitchell, W.M.; Perry, A.M.; et al. Amelioration of Visual Deficits and Visual System Pathology after Mild TBI via the Cannabinoid Type-2 Receptor Inverse Agonism of Raloxifene. Exp. Neurol. 2019, 322, 113063. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Cannabinoid Receptors as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2006, 12, 1751–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guley, N.M.; del Mar, N.A.; Ragsdale, T.; Li, C.; Perry, A.M.; Moore, B.M.; Honig, M.G.; Reiner, A. Amelioration of Visual Deficits and Visual System Pathology after Mild TBI with the Cannabinoid Type-2 Receptor Inverse Agonist SMM-189. Exp. Eye Res. 2019, 182, 109–124. [Google Scholar] [CrossRef]
- Shoemaker, J.L.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. Agonist-Directed Trafficking of Response by Endocannabinoids Acting at CB2 Receptorsitle. J. Pharmacol. Exp. Ther. 2005, 315, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB 1 and CB 2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017, 2, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Rhee, M.H.; Bayewitch, M.; Reiss, T.A.; Levy, R.; Vogel, Z. Cannabinoid Receptor Activation Differentially Regulates the Various Adenylyl Cyclase Isozymes. J. Neurochem. 1998, 71, 1525–1534. [Google Scholar] [CrossRef]
- Börner, C.; Smida, M.; Höllt, V.; Schraven, B.; Kraus, J. Cannabinoid Receptor Type 1- and 2-Mediated Increase in Cyclic AMP Inhibits T Cell Receptor-Triggered Signaling. J. Biol. Chem. 2009, 284, 35450–35460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaikwad, S.M.; Thakur, B.; Sakpal, A.; Singh, R.K.; Ray, P. Differential Activation of NF-ΚB Signaling Is Associated with Platinum and Taxane Resistance in MyD88 Deficient Epithelial Ovarian Cancer Cells. Int. J. Biochem. Cell Biol. 2015, 61, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Zhang, P.; Bhat, A.N. Cytokine Induction of Inducible Nitric Oxide Synthase in an Oligodendrocyte Cell Line: Role of P38 Mitogen-Activated Protein Kinase Activation. J. Neurochem. 1999, 72, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Klemke, R.L.; Cai, S.; Giannini, A.L.; Gallagher, P.J.; de Lanerolle, P.; Cheresh, D.A. Regulation of Cell Motility by Mitogen-Activated Protein Kinase. J. Cell Biol. 1997, 137, 481–492. [Google Scholar] [CrossRef]
- Bouaboula, M.; Chazel, C.P.; Marchand, J.; Canat, X.; Bourrié, B.; Carmona, M.R.; Calandra, B.; Le Fur, G.; Casellas, P. Signaling Pathway Associated with Stimulation of CB2 Peripheral Cannabinoid Receptor: Involvement of Both Mitogen-Activated Protein Kinase and Induction of Krox-24 Expression. Eur. J. Biochem. 1996, 237, 704–711. [Google Scholar] [CrossRef]
- Montecucco, F.; Burger, F. CB 2 Cannabinoid Receptor Agonist JWH-015 Modulates Human Monocyte Migration through Defined Intracellular Signaling Pathways. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, E.A.R.; Horvath, R.; Landry, R.P.; de Leo, J.A. Cannabinoid Receptor Type 2 Activation Induces a Microglial Anti-Inflammatory Phenotype and Reduces Migration via MKP Induction and ERK Dephosphorylation. Mol. Pain 2009, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Holgado, F.M.; Pinteaux, E.; Moore, J.D.; Holgado, E.M.; Guaza, C.; Gibson, R.M.; Rothwell, N.J. Endogenous Interleukin-1 Receptor Antagonist Mediates Anti-Inflammatory and Neuroprotective Actions of Cannabinoids in Neurons and Glia. J. Neurosci. 2003, 23, 6470–6474. [Google Scholar] [CrossRef]
- Mestre, L.; Correa, F.; Martín, A.A.; Holgado, E.M.; Valenti, M.; Ortar, G.; Di Marzo, V.; Guaza, C. Pharmacological Modulation of the Endocannabinoid System in a Viral Model of Multiple Sclerosis. J. Neurochem. 2005, 96, 1327–1339. [Google Scholar] [CrossRef]
- Henry, C.J.; Huang, Y.; Wynne, A.M.; Godbout, J.P. Peripheral LPS Challenge Promotes Microglial Hyperactivity in Aged Mice That Is Associated with Exaggerated Induction of Both Proinflammatory IL1β and Antiinflammatory IL10 Cytokines. Brain Behav. Immun. 2009, 23, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Flannery, L.E.; Henry, R.J.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH, but Not MAGL, Inhibition Modulates Acute TLR3-Induced Neuroimmune Signaling in the Rat, Independent of Sex. J. Neurosci. Res. 2018, 96, 989–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, R.J.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH-Mediated Modulation of TLR3-Induced Neuroinflammation in the Rat Hippocampus. J. Neuroimmunol. 2014, 276, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.E. The Peripheral Cannabinoid Receptor Knockout Mice: An Update. Br. J. Pharmacol. 2008, 153, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.R.; Alba, A.C.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Neto, J.C.E.; Ishiguro, H.; et al. Cannabinoid Type 2 Receptors in Dopamine Neurons Inhibits Psychomotor Behaviors, Alters Anxiety, Depression and Alcohol Preference. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sisay, S.; Pryce, G.; Jackson, S.J.; Tanner, C.; Ross, R.A.; Michael, G.J.; Selwood, D.L.; Giovannoni, G.; Baker, D. Genetic Background Can Result in a Marked or Minimal Effect of Gene Knockout (GPR55 and CB2 Receptor) in Experimental Autoimmune Encephalomyelitis Models of Multiple Sclerosis. PLoS ONE 2013, 8, e76907. [Google Scholar] [CrossRef]
- Navarrete, F.; Arias, M.R.; García, E.M.; Navarro, D.; Gutiérrez, M.S.G.; Aguilar, M.A.; Fernández, A.A.; Berbel, P.; Miñarro, J.; Maldonado, R.; et al. Role of CB2 Cannabinoid Receptors in the Rewarding, Reinforcing, and Physical Effects of Nicotine. Neuropsychopharmacology 2013, 38, 2515–2524. [Google Scholar] [CrossRef] [Green Version]
- Alvaro, A.O.; Fernández, A.A.; Gutiérrez, M.S.G.; Navarrete, F.; Manzanares, J. Deletion of CB2 Cannabinoid Receptor Induces Schizophrenia-Related Behaviors in Mice. Neuropsychopharmacology 2011, 36, 1489–1504. [Google Scholar] [CrossRef] [Green Version]
- LaPorta, C.; Bura, S.A.; Fernández, A.A.; Manzanares, J.; Maldonado, R. Role of CB1 and CB2 Cannabinoid Receptors in the Development of Joint Pain Induced by Monosodium Iodoacetate. Pain 2013, 154, 160–174. [Google Scholar] [CrossRef]
- Sun, L.; Dong, R.; Xu, X.; Yang, X.; Peng, M. Activation of Cannabinoid Receptor Type 2 Attenuates Surgery-Induced Cognitive Impairment in Mice through Anti-Inflammatory Activity. J. Neuroinflammation 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Lou, J.; Teng, Z.; Zhang, L.; Yang, J.; Ma, L.; Wang, F.; Tian, X.; An, R.; Yang, M.; Zhang, Q.; et al. β-Caryophyllene/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Improves Cognitive Deficits in Rats With Vascular Dementia Through the Cannabinoid Receptor Type 2-Mediated Pathway. Front. Pharmacol. 2017, 8, e00002. [Google Scholar] [CrossRef] [Green Version]
- Jayant, S.; Sharma, B.M.; Bansal, R.; Sharma, B. Pharmacological Benefits of Selective Modulation of Cannabinoid Receptor Type 2 (CB2) in Experimental Alzheimer’s Disease. Pharmacol. Biochem. Behav. 2016, 140, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 Cannabinoid Receptor in Mice Improves Insulin Sensitivity but Increases Food Intake and Obesity with Age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verty, A.N.A.; Stefanidis, A.; McAinch, A.J.; Hryciw, D.H.; Oldfield, B. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS ONE 2015, 10, e0140592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, H.; Carpio, O.; Horiuchi, Y.; Shu, A.; Higuchi, S.; Schanz, N.; Benno, R.; Arinami, T.; Onaivi, E.S. A Nonsynonymous Polymorphism in Cannabinoid CB2 Receptor Gene Is Associated with Eating Disorders in Humans and Food Intake Is Modified in Mice by Its Ligands. Synapse 2010, 64, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.S.G.; Álvaro, A.O.; García, A.B.; Ortiz, J.M.P.; Caltana, L.; Ricatti, M.J.; Brusco, A.; Maldonado, R.; Manzanares, J. Synaptic Plasticity Alterations Associated with Memory Impairment Induced by Deletion of CB2 Cannabinoid Receptors. Neuropharmacology 2013, 73, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J. Deletion of CB2 Cannabinoid Receptors Reduces Synaptic Transmission and Long-Term Potentiation in the Mouse Hippocampus. Hippocampus 2016, 26, 275–281. [Google Scholar] [CrossRef]
- Ratano, P.; Petrella, C.; Forti, F.; Passeri, P.P.; Morena, M.; Palmery, M.; Trezza, V.; Severini, C.; Campolongo, P. Pharmacological Inhibition of 2-Arachidonoilglycerol Hydrolysis Enhances Memory Consolidation in Rats through CB2 Receptor Activation and MTOR Signaling Modulation. Neuropharmacology 2018, 138, 210–218. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J. Distinct Roles of Neuronal and Microglial CB2 Cannabinoid Receptors in the Mouse Hippocampus. Neuroscience 2017, 363, 11–25. [Google Scholar] [CrossRef]
- Jankowska, B.M.I.; Muldoon, P.P.; Lichtman, A.H.; Damaj, M.I. The Cannabinoid CB2 Receptor Is Necessary for Nicotine-Conditioned Place Preference, but Not Other Behavioral Effects of Nicotine in Mice. Psychopharmacology 2013, 229, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.X.; Peng, X.Q.; Li, X.; Song, R.; Zhang, H.Y.; Liu, Q.R.; Yang, H.J.; Bi, G.H.; Li, J.; Gardner, E.L. Brain Cannabinoid CB2receptors Modulate Cocaine’s Actions in Mice. Nat. Neurosci. 2011, 14, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.B.; Bastos, J.R.; Costa, R.B.; Aguiar, D.C.; Moreira, F.A. The Roles of Cannabinoid CB1 and CB2 Receptors in Cocaine-Induced Behavioral Sensitization and Conditioned Place Preference in Mice. Psychopharmacology 2020, 237, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Gamaleddin, I.; Zvonok, A.; Makriyannis, A.; Goldberg, S.R.; LeFoll, B. Effects of a Selective Cannabinoid CB2 Agonist and Antagonist on Intravenous Nicotine Self Administration and Reinstatement of Nicotine Seeking. PLoS ONE 2012, 7, e29900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, P.; Miszkiel, J.; McCreary, A.C.; Filip, M.; Papp, M.; Przegaliński, E. The Effects of Cannabinoid CB1, CB2 and Vanilloid TRPV1 Receptor Antagonists on Cocaine Addictive Behavior in Rats. Brain Res. 2012, 1444, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.; Franklin, A.; Witting, A.; Möller, T.; Stella, N. Astrocytes in Culture Produce Anandamide and Other Acylethanolamides. J. Biol. Chem. 2002, 277, 20869–20876. [Google Scholar] [CrossRef] [Green Version]
- Carrier, E.J.; Kearn, C.S.; Barkmeier, A.J.; Breese, N.M.; Yang, W.; Nithipatikom, K.; Pfister, S.L.; Campbell, W.B.; Hillard, C.J. Cultured Rat Microglial Cells Synthesize the Endocannabinoid 2-Arachidonylglycerol, Which Increases Proliferation via a CB2 Receptor-Dependent Mechanism. Mol. Pharmacol. 2004, 65, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Walter, L.; Stella, N. Cannabinoids and Neuroinflammation. Br. J. Pharmacol. 2004, 141, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Muccioli, G.G.; Xu, C.; Odah, E.; Cudaback, E.; Cisneros, J.A.; Lambert, D.M.; Rodríguez, M.L.L.; Bajjalieh, S.; Stella, N. Identification of a Novel Endocannabinoid-Hydrolyzing Enzyme Expressed by Microglial Cells. J. Neurosci. 2007, 27, 2883–2889. [Google Scholar] [CrossRef] [Green Version]
- Eyo, U.B.; Wu, L.J. Bidirectional Microglia-Neuron Communication in the Healthy Brain. Neural Plast. 2013, 2013, 456857. [Google Scholar] [CrossRef]
- Chavarría, A.; Cárdenas, G. Neuronal Influence behind the Central Nervous System Regulation of the Immune Cells. Front. Integr. Neurosci. 2013, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Hoek, R.H.; Ruuls, S.R.; Murphy, C.A.; Wright, G.J.; Goddard, R.; Zurawski, S.M.; Blom, B.; Homola, M.E.; Streit, W.J.; Brown, M.H.; et al. Down-Regulation of the Macrophage Lineage through Interaction with OX2 (CD200). Science 2000, 290, 1768–1771. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.R.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of Microglial Neurotoxicity by the Fractalkine Receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Bessis, A.; Béchade, C.; Bernard, D.; Roumier, A. Microglial Control of Neuronal Death and Synaptic Properties. Glia 2007, 55, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ativie, F.; Komorowska, J.A.; Beins, E.; Albayram, Ö.; Zimmer, T.; Zimmer, A.; Tejera, D.; Heneka, M.; Gorzo, A.B. Cannabinoid 1 Receptor Signaling on Hippocampal GABAergic Neurons Influences Microglial Activity. Front. Mol. Neurosci. 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielli, M.; Battista, N.; Riganti, L.; Prada, I.; Antonucci, F.; Cantone, L.; Lombardi, M.; Matteoli, M.; Maccarrone, M.; Verderio, C. Active Endocannabinoids Are Secreted on the Surface of Microglial Microvesicles. Springerplus 2015, 4, L29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, E.L.; Gallopin, T.; Férézou, I.; Cauli, B.; Rossier, J.; Schweitzer, P.; Lambolez, B. Functional CB1 Receptors Are Broadly Expressed in Neocortical GABAergic and Glutamatergic Neurons. J. Neurophysiol. 2007, 97, 2580–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsicano, G.; Lutz, B. Expression of the Cannabinoid Receptor CB1 in Distinct Neuronal Subpopulations in the Adult Mouse Forebrain. Eur. J. Neurosci. 1999, 11, 4213–4225. [Google Scholar] [CrossRef] [PubMed]
- Kaindl, A.M.; Degos, V.; Peineau, S.; Gouadon, E.; Chhor, V.; Loron, G.; LeCharpentier, T.; Josserand, J.; Ali, C.; Vivien, D. Activation of Microglial N-Methyl-D-Aspartate Receptors Triggers Inflammation and Neuronal Cell Death in the Developing and Mature Brain. Ann. Neurol. 2012, 72, 536–549. [Google Scholar] [CrossRef]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [Green Version]
- Luongo, L.; Palazzo, E.; de Novellis, V.; Maione, S. Role of Endocannabinoids in Neuron-Glial Crosstalk. Open Pain J. 2010, 3, 29–36. [Google Scholar] [CrossRef]
- Thacker, M.A.; Clark, A.K.; Bishop, T.; Grist, J.; Yip, P.K.; Moon, L.D.F.; Thompson, S.W.N.; Marchand, F.; McMahon, S.B. CCL2 Is a Key Mediator of Microglia Activation in Neuropathic Pain States. Eur. J. Pain 2009, 13, 263–272. [Google Scholar] [CrossRef]
- Abbadie, C.; Lindia, J.A.; Cumiskey, A.M.; Peterson, L.B.; Mudgett, J.S.; Bayne, E.K.; deMartino, J.A.; MacIntyre, D.E.; Forrest, M.J. Impaired Neuropathic Pain Responses in Mice Lacking the Chemokine Receptor CCR2. Proc. Natl. Acad. Sci. USA 2003, 100, 7947–7952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stella, N. Endocannabinoid Signaling in Microglial Cells. Neuropharmacology 2009, 56, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, J.M.; Achua, J.K.; Smith, J.P.; Prince, M.A.; Staton, C.D.; Ronan, P.J.; Summers, T.R.; Summersa, C.H. Anxious Behavior Induces Elevated Hippocampal Cb2 Receptor Gene Expression. Neuroscience 2017, 352, 273–284. [Google Scholar] [CrossRef]
- Du, J.J.; Liu, Z.Q.; Yan, Y.; Xiong, J.; Jia, X.T.; Di, Z.L.; Ren, J.J. The Cannabinoid WIN 55,212-2 Reduces Delayed Neurologic Sequelae After Carbon Monoxide Poisoning by Promoting Microglial M2 Polarization Through ST2 Signaling. J. Mol. Neurosci. 2020, 70, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, Y. Chronic Activation of CB2 Cannabinoid Receptors in the Hippocampus Increases Excitatory Synaptic Transmission. J. Physiol. 2015, 593, 871–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorowska-Müller, J.A.; Schmöle, A.-C. CB2 Receptor in Microglia: The Guardian of Self-Control. Int. J. Mol. Sci. 2021, 22, 19. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010019
Komorowska-Müller JA, Schmöle A-C. CB2 Receptor in Microglia: The Guardian of Self-Control. International Journal of Molecular Sciences. 2021; 22(1):19. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010019
Chicago/Turabian StyleKomorowska-Müller, Joanna Agnieszka, and Anne-Caroline Schmöle. 2021. "CB2 Receptor in Microglia: The Guardian of Self-Control" International Journal of Molecular Sciences 22, no. 1: 19. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010019