Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism
Abstract
:1. Introduction
Demographics of β-Endorphin Levels
2. Effects of β-Endorphins
2.1. β-Endorphins Analgesia
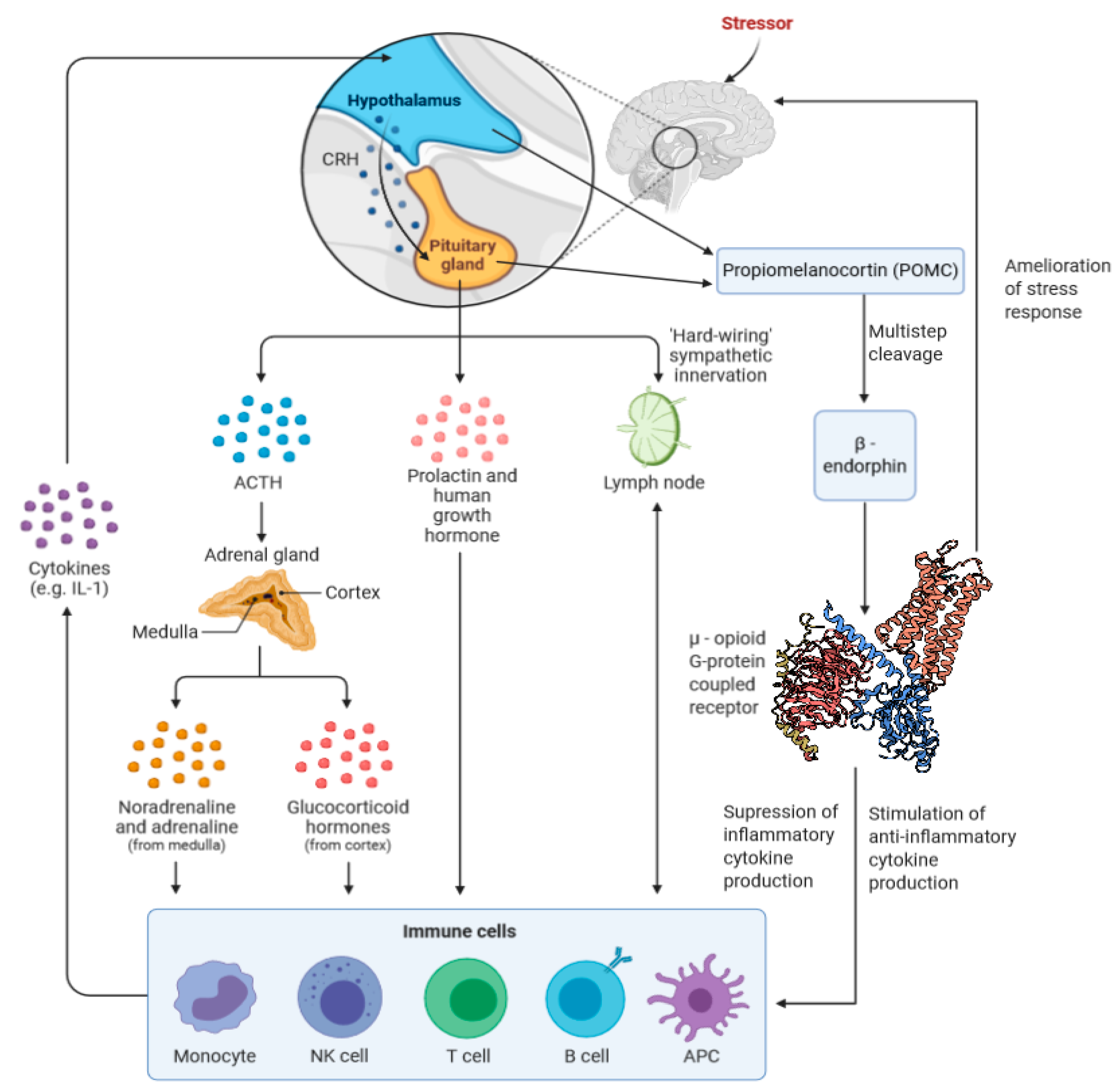
2.2. β-Endorphins Effect on the Immune System
2.3. β-Endorphins in the Holistic Stress Response
2.4. β-Endorphins and Behavior
2.4.1. Addiction
2.4.2. Food Consumption
2.4.3. Sexual Behavior
2.4.4. Other Effects
2.5. β-Endorphins in Sleep/Sleep Cycles
2.6. β-Endorphins in Depression and Stress/Anxiety Disorders
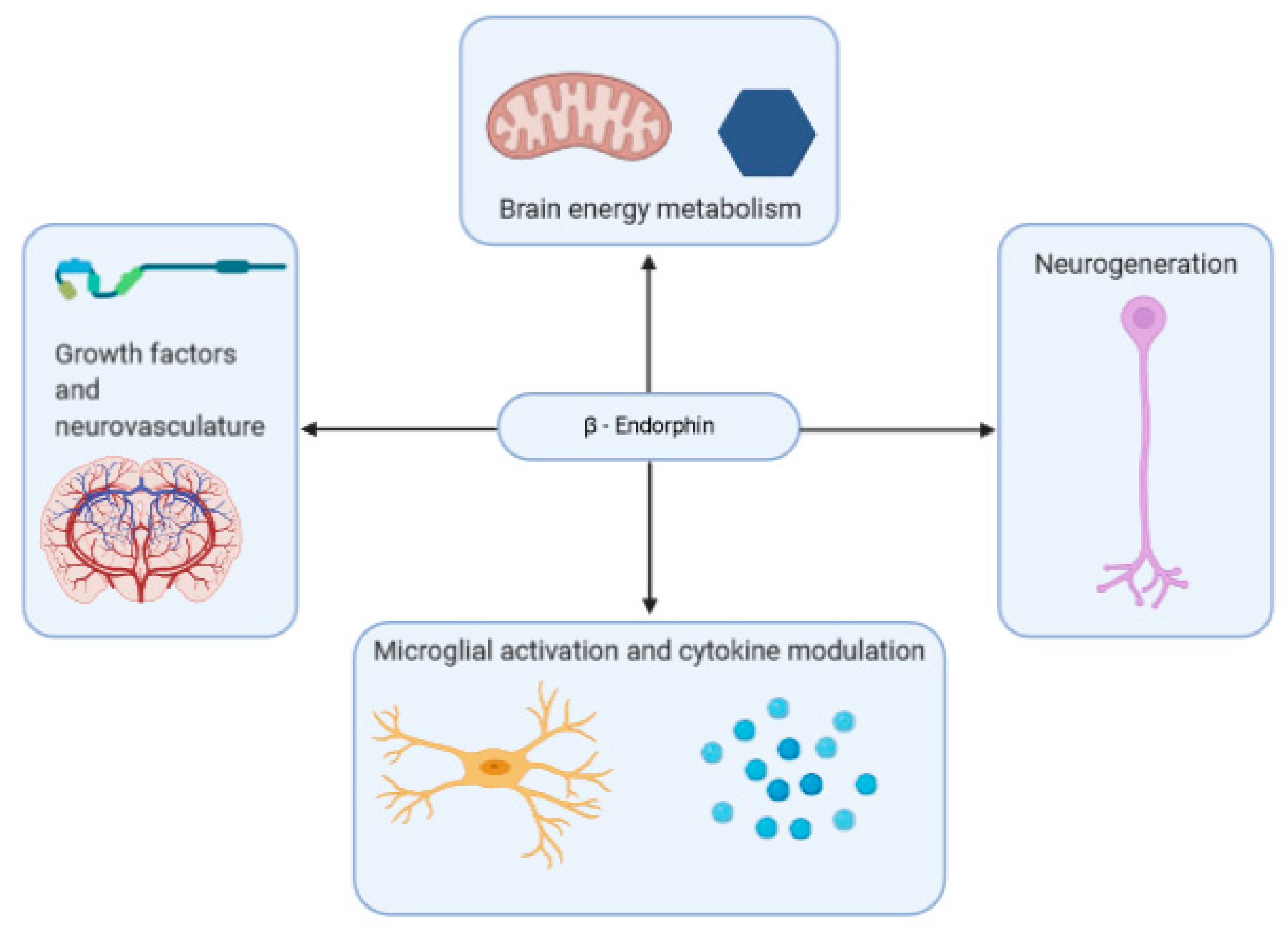
2.7. β-Endorphins in Brain Health and Neurodegenerative Disorder
2.7.1. Neuroinflammation
2.7.2. Metabolism
2.7.3. Growth-Factors and Neurovasculature
2.7.4. β-Endorphin-Based Treatment
3. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AD | Alzheimer’s disease |
| Aβ | Amyloid beta |
| CB1R | Cannabinoid receptor 1 |
| eGFR | Epidermal growth factor receptors |
| GABA | Gamma-aminobutyric acid |
| GLP-1 | Glucagon-like-peptide-1 |
| HPA | Hypothalamic–pituitary–adrenal |
| IFN | Interferon |
| IGFR | Insulin-like growth factor receptor |
| IL | Interleukin |
| MAD | Major depression |
| MD | Myeloid differentiation factor |
| NK | Natural killer cell |
| PC | Prohormone Convertase |
| POMC | Proopiomelanocortin |
| PTSD | Post-traumatic stress disorder |
| REM | Rapid eye movement |
| ROS | Reactive oxygen species |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
References
- Smyth, D.G. 60 years of pomc: Lipotropin and beta-endorphin: A perspective. J. Mol. Endocrinol. 2016, 56, T13–T25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, A.C. Peripheral beta-endorphin and pain modulation. Anesth. Prog. 1991, 38, 75–78. [Google Scholar] [PubMed]
- Veening, J.G.; Gerrits, P.O.; Barendregt, H.P. Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 2012, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S. Comparative aspects of intracellular proteolytic processing of peptide hormone precursors: Studies of proopiomelanocortin processing. Zool. Sci. 2003, 20, 1183–1198. [Google Scholar] [CrossRef]
- Ji, L.; Wu, H.-T.; Qin, X.-Y.; Lan, R. Dissecting carboxypeptidase E: Properties, functions and pathophysiological roles in disease. Endocr. Connect. 2017, 6, R18–R38. [Google Scholar] [CrossRef] [Green Version]
- Cawley, N.X.; Li, Z.; Loh, Y.P. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol. 2016, 56, T77–T97. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.; Sierra, S.; Lueptow, L.; Gupta, A.; Gouty, S.; Margolis, E.B.; Cox, B.M.; Devi, L.A. Biased signaling by endogenous opioid peptides. Proc. Natl. Acad. Sci. USA 2020, 117, 11820–11828. [Google Scholar] [CrossRef]
- Fricker, L.D.; Margolis, E.B.; Gomes, I.; Devi, L.A. Five decades of research on opioid peptides: Current knowledge and unanswered questions. Mol. Pharmacol. 2020, 98, 96–108. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Funasaka, Y.; Slominski, A.; Ermak, G.; Hwang, J.; Pawelek, J.M.; Ichihashi, M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet B. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1996, 1313, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A. Identification of β-endorphin, α-MSH and ACTH peptides in cultured human melanocytes, melanoma and squamous cell carcinoma cells by RP-HPLC. Exp. Dermatol. 1998, 7, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Tuckey, R.C.; Paus, R. Differential expression of HPA axis homolog in the skin. Mol. Cell Endocrinol. 2007, 265–266, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blalock, J. Proopiomelanocortin-derived peptides in the immune system. Clin. Endocrinol. 1985, 22, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.; Linner, K. What do we know about the expression of proopiomelanocortin transcripts and related peptides in lymphoid tissue? Endocrinology 1993, 133, 1921a–1921b. [Google Scholar] [CrossRef]
- Ermisch, A.; Rühle, H.-J.; Landgraf, R.; Hess, J. Blood—Brain barrier and peptides. J. Cereb. Blood Flow Metab. 1985, 5, 350–357. [Google Scholar] [CrossRef] [Green Version]
- King, M.; Su, W.; Chang, A.; Zuckerman, A.; Pasternak, G.W. Transport of opioids from the brain to the periphery by P-glycoprotein: Peripheral actions of central drugs. Nat. Neurosci. 2001, 4, 268–274. [Google Scholar] [CrossRef]
- Mains, R.E.; Eipper, B.A. Differences in the post-translational processing of beta-endorphin in rat anterior and intermediate pituitary. J. Biol. Chem. 1981, 256, 5683–5688. [Google Scholar]
- Zakarian, S.; Smyth, D. Distribution of β-endorphin-related peptides in rat pituitary and brain. Biochem. J. 1982, 202, 561–571. [Google Scholar] [CrossRef]
- Hammonds, R.G.; Nicolas, P.; Li, C.H. beta-endorphin-(1-27) is an antagonist of beta-endorphin analgesia. Proc. Natl. Acad. Sci. USA 1984, 81, 1389–1390. [Google Scholar] [CrossRef] [Green Version]
- Suh, H.H.; Tseng, L.-F.; Li, C.H. Beta-endorphin-(1–27) antagonizes beta-endorphin-induced hypothermia in mice. Peptides 1987, 8, 123–126. [Google Scholar] [CrossRef]
- Nicolas, P.; Li, C.H. Beta-endorphin-(1-27) is a naturally occurring antagonist to etorphine-induced analgesia. Proc. Natl. Acad. Sci. USA 1985, 82, 3178–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, M.D.; Millington, W.R. Endoproteolytic conversion of β-endorphin-1-31 to β-endorphin-1-27 potentiates its central cardioregulatory activity. Brain Res. 1991, 550, 61–68. [Google Scholar] [CrossRef]
- Alt, A.; Mansour, A.; Akil, H.; Medzihradsky, F.; Traynor, J.R.; Woods, J.H. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J. Pharmacol. Exp. Ther. 1998, 286, 282–288. [Google Scholar] [PubMed]
- Koneru, A.; Satyanarayana, S.; Rizwan, S. Endogenous opioids: Their physiological role and receptors. Glob. J. Pharmacol. 2009, 3, 149–153. [Google Scholar]
- Facchinetti, F.; Petraglia, F.; Genazzani, A.R. Localization and expression of the three opioid systems. Semin. Reprod. Endocrinol. 1987, 5, 103–113. [Google Scholar] [CrossRef]
- Raynor, K.; Kong, H.; Chen, Y.; Yasuda, K.; Yu, L.; Bell, G.I.; Reisine, T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol. Pharmacol. 1994, 45, 330–334. [Google Scholar]
- Gong, J.; Strong, J.A.; Zhang, S.; Yue, X.; DeHaven, R.N.; Daubert, J.D.; Cassel, J.A.; Yu, G.; Mansson, E.; Yu, L. Endomorphins fully activate a cloned human mu opioid receptor. FEBS Lett. 1998, 439, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I.E.; Rossi, G.C.; Letchworth, S.R.; Mathis, J.P.; Ryan-Moro, J.; Leventhal, L.; Su, W.; Emmel, D.; Bolan, E.A.; Pasternak, G.W. Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J. Pharmacol. Exp. Ther. 1998, 286, 1007–1013. [Google Scholar]
- Hackler, L.; Zadina, J.E.; Ge, L.-J.; Kastin, A.J. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides 1997, 18, 1635–1639. [Google Scholar] [CrossRef]
- Zadina, J.E.; MARTIN-SCHILD, S.; Gerall, A.A.; Kastin, A.J.; Hackler, L.; GE, L.J.; Zhang, X. Endomorphins: Novel endogenous μ-opiate receptor agonists in regions of high μ-opiate receptor density. Ann. N. Y. Acad. Sci. 1999, 897, 136–144. [Google Scholar] [CrossRef]
- Schreff, M.; Schulz, S.; Wiborny, D.; Höllt, V. Immunofluorescent identification of endomorphin-2-containing nerve fibers and terminals in the rat brain and spinal cord. Neuroreport 1998, 9, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Jessop, D.S.; Major, G.N.; Coventry, T.L.; Kaye, S.J.; Fulford, A.J.; Harbuz, M.S.; De Bree, F.M. Novel opioid peptides endomorphin-1 and endomorphin-2 are present in mammalian immune tissues. J. Neuroimmunol. 2000, 106, 53–59. [Google Scholar] [CrossRef]
- Terskiy, A.; Wannemacher, K.M.; Yadav, P.N.; Tsai, M.; Tian, B.; Howells, R.D. Search of the human proteome for endomorphin-1 and endomorphin-2 precursor proteins. Life Sci. 2007, 81, 1593–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Harbuz, M.; Lightman, S. Stress and the hypothalamo-pituitary-adrenal axis: Acute, chronic and immunological activation. J. Endocrinol. 1992, 134, 327–339. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar]
- Gianoulakis, C. Alcohol-seeking behavior: The roles of the hypothalamic-pituitary-adrenal axis and the endogenous opioid system. Alcohol Health Res. World 1998, 22, 202–210. [Google Scholar]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Barden, N.; Dupont, A.; Labrie, F.; Me, Y.; Rouleau, D.; Vaudry, H.; Boissier, J. Age-dependent changes in the β-endorphin content of discrete rat brain nuclei. Brain Res. 1981, 208, 209–212. [Google Scholar] [CrossRef]
- Gambert, S.R. Interaction of age and thyroid hormone status on beta-endorphin content in rat corpus striatum and hypothalamus. Neuroendocrinology 1981, 32, 114–117. [Google Scholar] [CrossRef]
- Kowalski, C.; Micheau, J.; Corder, R.; Gaillard, R.; Conte-Devolx, B. Age-related changes in cortico-releasing factor, somatostatin, neuropeptide Y, methionine enkephalin and β-endorphin in specific rat brain areas. Brain Res. 1992, 582, 38–46. [Google Scholar] [CrossRef]
- Malinowski, K.; Shock, E.; Rochelle, P.; Kearns, C.; Guirnalda, P.; McKeever, K. Plasma β-endorphin, cortisol and immune responses to acute exercise are altered by age and exercise training in horses. Equine Vet. J. 2006, 38, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Breda, M.; Barcellini, W.; Meroni, P.L.; Panerai, A.E. Age-related changes of beta-endorphin and cholecystokinin in human and rat mononuclear cells. Peptides 1991, 12, 1353–1356. [Google Scholar] [CrossRef]
- Panerai, A.E.; Manfredi, B.; Sacerdote, P. Beta-endorphin concentrations in resting peripheral mononuclear cells and after treatment with PHA or serotoninergic drugs in human aging, Alzheimer’s disease, and Down’s syndrome. Ann. N. Y. Acad. Sci. 1992, 663, 311–318. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Austin, M.P.; Curran, S.M.; Ross, M.; Murray, C.; Prentice, N.; Ebmeier, K.P.; Bennie, J.; Carroll, S.; Dick, H.; et al. The elevation of plasma beta-endorphin levels in major depression. J. Affect. Disord. 1993, 29, 281–289. [Google Scholar] [CrossRef]
- Forman, L.; Cavalieri, T.; Fitzharris, J.; Chopra, A.; Marquis, D.; Estilow, S. Plasma levels of beta-endorphin in young and aged human males during the morning hours. Horm. Metab. Res. 1987, 19, 38–39. [Google Scholar] [CrossRef]
- Alessio, L.; Govoni, S.; Salar, G.; Battaini, F.; Iavicoli, R.; Trabucchi, M. Age-related changes of methionine-enkephalin and beta-endorphin/beta-lipotropin immunoreactivity in human CSF. Life Sci. 1988, 43, 1545–1550. [Google Scholar] [CrossRef]
- Lloyd, J.M.; Scarbrough, K.; Weiland, N.G.; Wise, P.M. Age-related changes in proopiomelanocortin (POMC) gene expression in the periarcuate region of ovariectomized rats. Endocrinology 1991, 129, 1896–1902. [Google Scholar] [CrossRef]
- Gruenewald, D.A.; Matsumoto, A.M. Age-related decrease in proopiomelanocortin gene expression in the arcuate nucleus of the male rat brain. Neurobiol. Aging 1991, 12, 113–121. [Google Scholar] [CrossRef]
- Pain, S.; Dezutter, C.; Reymermier, C.; Vogelgesang, B.; Delay, E.; André, V. Age-related changes in pro-opiomelanocortin (POMC) and related receptors in human epidermis. Int. J. Cosmet. Sci. 2010, 32, 266–275. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; Jamurtas, A.Z.; Kamimori, G.H.; Hegde, S.; Otterstetter, R.; Brown, D.A. Gender effect on beta-endorphin response to exercise. Med. Sci. Sports Exerc. 1998, 30, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Gianoulakis, C.; Dai, X.; Brown, T. Effect of Chronic Alcohol Consumption on the Activity of the Hypothalamic-Pituitary-Adrenal Axis and Pituitary β-Endorphin as a Function of Alcohol Intake, Age, and Gender. Alcohol. Clin. Exp. Res. 2003, 27, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Leuschen, M.P.; Willett, L.D.; Bolam, D.L.; NELSON JR, R.M. Plasma β-endorphin in neonates: Effect of prematurity, gender, and respiratory status. J. Clin. Endocrinol. Metab. 1991, 73, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Sacerdote, P.; Albonetti, M.E.; Carli, G. Sex-related effects on behaviour and β-endorphin of different intensities of formalin pain in rats. Brain Res. 1995, 699, 242–249. [Google Scholar] [CrossRef]
- Krzanowska, E.K.; Bodnar, R.J. Analysis of sex and gonadectomy differences in β-endorphin antinociception elicited from the ventrolateral periaqueductal gray in rats. Eur. J. Pharmacol. 2000, 392, 157–161. [Google Scholar] [CrossRef]
- Kepler, K.L.; Standifer, K.M.; Paul, D.; Kest, B.; Pasternak, G.W.; Bodnar, R.J. Gender effects and central opioid analgesia. Pain 1991, 45, 87–94. [Google Scholar] [CrossRef]
- Loh, H.H.; Tseng, L.F.; Wei, E.; Li, C.H. beta-endorphin is a potent analgesic agent. Proc. Natl. Acad. Sci. USA 1976, 73, 2895–2898. [Google Scholar] [CrossRef] [Green Version]
- Chong, D.; Shao, L.; Yang, Y.; Wang, R.; Yang, C.; Zhang, B. Correlations of cancer pain degree with levels of β-EP, CGRP and PGE2 and the effects of oxycontin on them. J. BUON 2018, 23, 1552–1557. [Google Scholar]
- Rasmussen, N.A.; Farr, L.A. Effects of morphine and time of day on pain and beta-endorphin. Biol. Res. Nurs. 2003, 5, 105–116. [Google Scholar] [CrossRef]
- Bruehl, S.; Burns, J.W.; Gupta, R.; Buvanendran, A.; Chont, M.; Orlowska, D.; Schuster, E.; France, C.R. Do Resting Plasma β-Endorphin Levels Predict Responses to Opioid Analgesics? Clin. J. Pain 2017, 33, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.M.; Schmidt, E.A.; Mueller, G.P.; Dionne, R.A. Dexamethasone alters plasma levels of beta-endorphin and postoperative pain. Clin. Pharmacol. Ther. 1987, 42, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Dabo, F.; Nyberg, F.; Qin, Z.; Sundström-Poromaa, I.; Akerud, H. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod. Sci. 2010, 17, 742–747. [Google Scholar] [CrossRef]
- Feldreich, A.; Ernberg, M.; Rosén, A. Reduction in maximum pain after surgery in temporomandibular joint patients is associated with decreased beta-endorphin levels—A pilot study. Int. J. Oral Maxillofac. Surg. 2017, 46, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Raisanen, I.; Paatero, H.; Salminen, K.; Laatikainen, T. Pain and plasma beta-endorphin level during labor. Obstet. Gynecol. 1984, 64, 783–786. [Google Scholar] [PubMed]
- Zhang, S.; Tang, H.; Zhou, J.; Gu, Y. Electroacupuncture attenuates neuropathic pain after brachial plexus injury. Neural Regen. Res. 2014, 9, 1365–1370. [Google Scholar] [CrossRef]
- Kaada, B.; Torsteinbo, O. Increase of plasma beta-endorphins in connective tissue massage. Gen. Pharmacol. 1989, 20, 487–489. [Google Scholar] [CrossRef]
- Day, J.A.; Mason, R.R.; Chesrown, S.E. Effect of massage on serum level of beta-endorphin and beta-lipotropin in healthy adults. Phys. Ther. 1987, 67, 926–930. [Google Scholar] [CrossRef]
- Morhenn, V.; Beavin, L.E.; Zak, P.J. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern. Ther. Health Med. 2012, 18, 11. [Google Scholar]
- Zai, F.L.; Wu, R.L.; Zheng, M.F.; Guo, L.Y. Warming Needle Moxibustion Relieves Symptoms of Lumbar Disc Herniation Patients and Upregulates Plasma beta-endorphin. Zhen Ci Yan Jiu 2018, 43, 512–515. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, C.-H. Can Beta-Endorphin Be Used as a Biomarker for Chronic Low Back Pain? A Meta-analysis of Randomized Controlled Trials. Pain Med. 2019, 20, 28–36. [Google Scholar] [CrossRef]
- Andersson, S.; Lundeberg, T. Acupuncture--from empiricism to science: Functional background to acupuncture effects in pain and disease. Med. Hypotheses 1995, 45, 271–281. [Google Scholar] [CrossRef]
- Mohammed, N.; Allam, H.; Elghoroury, E.; Zikri, E.N.; Helmy, G.A.; Elgendy, A. Evaluation of serum beta-endorphin and substance P in knee osteoarthritis patients treated by laser acupuncture. J. Complement. Integr. Med. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Harbach, H.; Moll, B.; Boedeker, R.H.; Vigelius-Rauch, U.; Otto, H.; Muehling, J.; Hempelmann, G.; Markart, P. Minimal immunoreactive plasma beta-endorphin and decrease of cortisol at standard analgesia or different acupuncture techniques. Eur. J. Anaesthesiol. 2007, 24, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, A.H.; Jamurtas, A.Z. Beta-endorphin response to exercise. An update. Sports Med. 1997, 24, 8–16. [Google Scholar] [CrossRef]
- Polaski, A.M.; Phelps, A.L.; Kostek, M.C.; Szucs, K.A.; Kolber, B.J. Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain. PLoS ONE 2019, 14, e0210418. [Google Scholar] [CrossRef] [Green Version]
- Paungmali, A.; Joseph, L.H.; Punturee, K.; Sitilertpisan, P.; Pirunsan, U.; Uthaikhup, S. Immediate Effects of Core Stabilization Exercise on β-Endorphin and Cortisol Levels Among Patients with Chronic Nonspecific Low Back Pain: A Randomized Crossover Design. J. Manip. Physiol. Ther. 2018, 41, 181–188. [Google Scholar] [CrossRef]
- Gisslinger, H.; Svoboda, T.; Clodi, M.; Gilly, B.; Ludwig, H.; Havelec, L.; Luger, A. Interferon-α stimulates the hypothalamic-pituitary-adrenal axis in vivo and in vitro. Neuroendocrinology 1993, 57, 489–495. [Google Scholar] [CrossRef]
- Mastorakos, G.; Chrousos, G.P.; Weber, J.S. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J. Clin. Endocrinol. Metab. 1993, 77, 1690–1694. [Google Scholar]
- Späth-Schwalbe, E.; Born, J.; Schrezenmeier, H.; Bornstein, S.; Stromeyer, P.; Drechsler, S.; Fehm, H.; Porzsolt, F. Interleukin-6 stimulates the hypothalamus-pituitary-adrenocortical axis in man. J. Clin. Endocrinol. Metab. 1994, 79, 1212–1214. [Google Scholar]
- Yeager, M.P.; Pioli, P.A.; Guyre, P.M. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose-response 2011, 9, 10–013. [Google Scholar] [CrossRef]
- Panerai, A.E.; Manfredi, B.; Granucci, F.; Sacerdote, P. The beta-endorphin inhibition of mitogen-induced splenocytes proliferation is mediated by central and peripheral paracrine/autocrine effects of the opioid. J. Neuroimmunol. 1995, 58, 71–76. [Google Scholar] [CrossRef]
- Manfredi, B.; Sacerdote, P.; Bianchi, M.; Locatelli, L.; Veljic-Radulovic, J.; Panerai, A.E. Evidence for an opioid inhibitory effect on T cell proliferation. J. Neuroimmunol. 1993, 44, 43–48. [Google Scholar] [CrossRef]
- Sacerdote, P.; di San Secondo, V.E.; Sirchia, G.; Manfredi, B.; Panerai, A.E. Endogenous opioids modulate allograft rejection time in mice: Possible relation with Th1/Th2 cytokines. Clin. Exp. Immunol. 1998, 113, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef]
- Pacifici, R.; di Carlo, S.; Bacosi, A.; Pichini, S.; Zuccaro, P. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int. J. Immunopharmacol. 2000, 22, 603–614. [Google Scholar] [CrossRef]
- Bessler, H.; Sztein, M.B.; Serrate, S.A. β-Endorphin modulation of IL-1-induced IL-2 production. Immunopharmacology 1990, 19, 5–14. [Google Scholar] [CrossRef]
- Nelson, B.H. IL-2, Regulatory T Cells, and Tolerance. J. Immunol. 2004, 172, 3983–3988. [Google Scholar] [CrossRef] [Green Version]
- van den Bergh, P.; Rozing, J.; Nagelkerken, L. Two opposing modes of action of beta-endorphin on lymphocyte function. Immunology 1991, 72, 537–543. [Google Scholar]
- Carr, D.J.; Klimpel, G.R. Enhancement of the generation of cytotoxic T cells by endogenous opiates. J. Neuroimmunol. 1986, 12, 75–87. [Google Scholar] [CrossRef]
- Mandler, R.N.; Biddison, W.E.; Mandler, R.; Serrate, S.A. beta-Endorphin augments the cytolytic activity and interferon production of natural killer cells. J. Immunol. 1986, 136, 934–939. [Google Scholar] [PubMed]
- Johnston, M.F.; Ortiz Sánchez, E.; Vujanovic, N.L.; Li, W. Acupuncture May Stimulate Anticancer Immunity via Activation of Natural Killer Cells. Evid. Based Complement. Alternat. Med. 2011, 2011, 481625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, S.A.; Knight, R.A.; Lightman, S.L.; Hobbs, J.R. Effects of beta endorphin on specific immune responses in man. Immunology 1988, 65, 47–51. [Google Scholar] [PubMed]
- Philippe, D.; Dubuquoy, L.; Groux, H.; Brun, V.; Van Chuoï-Mariot, M.T.; Gaveriaux-Ruff, C.; Colombel, J.-F.; Kieffer, B.L.; Desreumaux, P. Anti-inflammatory properties of the μ opioid receptor support its use in the treatment of colon inflammation. J. Clin. Investig. 2003, 111, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Wiedermann, C.J.; Sacerdote, P.; Mur, E.; Kinigadner, U.; Wicker, T.; Panerai, A.E.; Braunsteiner, H. Decreased immunoreactive beta-endorphin in mononuclear leucocytes from patients with rheumatic diseases. Clin. Exp. Immunol. 1992, 87, 178–182. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Payne, J.K. State of the science: Stress, inflammation, and cancer. Oncol. Nurs. Forum 2014, 41, 533–540. [Google Scholar] [CrossRef]
- Powell, N.D.; Tarr, A.J.; Sheridan, J.F. Psychosocial stress and inflammation in cancer. Brain Behav. Immun. 2013, 30, S41–S47. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation-Associated Co-morbidity Between Depression and Cardiovascular Disease. Curr. Top. Behav. Neurosci. 2017, 31, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanovich, L.; Marisavljevich, D. Stress as a trigger of autoimmune disease. Autoimmun. Rev. 2008, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Benamar, K.; Yondorf, M.; Barreto, V.T.; Geller, E.B.; Adler, M.W. Deletion of mu-opioid receptor in mice alters the development of acute neuroinflammation. J. Pharmacol. Exp. Ther. 2007, 323, 990–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anttila, J.E.; Albert, K.; Wires, E.S.; Mätlik, K.; Loram, L.C.; Watkins, L.R.; Rice, K.C.; Wang, Y.; Harvey, B.K.; Airavaara, M. Post-stroke Intranasal (+)-Naloxone Delivery Reduces Microglial Activation and Improves Behavioral Recovery from Ischemic Injury. eNeuro 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, M.R.; Bland, S.T.; Johnson, K.W.; Rice, K.C.; Maier, S.F.; Watkins, L.R. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci. World J. 2007, 7, 98–111. [Google Scholar] [CrossRef]
- Wang, X.; Loram, L.C.; Ramos, K.; de Jesus, A.J.; Thomas, J.; Cheng, K.; Reddy, A.; Somogyi, A.A.; Hutchinson, M.R.; Watkins, L.R.; et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. USA 2012, 109, 6325–6330. [Google Scholar] [CrossRef] [Green Version]
- Veening, J.G.; Barendregt, H.P. The effects of beta-endorphin: State change modification. Fluids Barriers CNS 2015, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Vaanholt, L.M.; Turek, F.W.; Meerlo, P. Beta-endorphin modulates the acute response to a social conflict in male mice but does not play a role in stress-induced changes in sleep. Brain Res. 2003, 978, 169–176. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Barfield, E.T.; Barry, S.M.; Hodgin, H.B.; Thompson, B.M.; Allen, S.S.; Grisel, J.E. Beta-endorphin mediates behavioral despair and the effect of ethanol on the tail suspension test in mice. Alcohol. Clin. Exp. Res. 2010, 34, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Barfield, E.T.; Moser, V.A.; Hand, A.; Grisel, J.E. beta-endorphin modulates the effect of stress on novelty-suppressed feeding. Front. Behav. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Komori, T.; Matsumoto, T.; Zhang, K.; Miyahara, S.; Shizuya, K.; Okazaki, Y. Effects of single and repeated prolonged stress on mu-opioid receptor mRNA expression in rat gross hypothalamic and midbrain homogenates. Brain Res. 2003, 980, 191–196. [Google Scholar] [CrossRef]
- Dubois, M.; Pickar, D.; Cohen, M.R.; Roth, Y.F.; Macnamara, T.; Bunney, W.E. Surgical stress in humans is acompanied by an increase in plasma beta-endorphin immunoreactivity. Life Sci. 1981, 29, 1249–1254. [Google Scholar] [CrossRef]
- Mirilas, P.; Mentessidou, A.; Kontis, E.; Antypa, E.; Makedou, A.; Petropoulos, A.S. Serum beta-endorphin response to stress before and after operation under fentanyl anesthesia in neonates, infants and preschool children. Eur. J. Pediatric Surg. 2010, 20, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Carlson, J.M.; Cha, J.; Rubin, D. The fine line between ‘brave’ and ‘reckless’: Amygdala reactivity and regulation predict recognition of risk. NeuroImage 2014, 103, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schedlowski, M.; Flüge, T.; Richter, S.; Tewes, U.; Schmidt, R.E.; Wagner, T.O. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology 1995, 20, 103–110. [Google Scholar] [CrossRef]
- Malarkey, W.B.; Pearl, D.K.; Demers, L.M.; Kiecolt-Glaser, J.K.; Glaser, R. Influence of academic stress and season on 24-h mean concentrations of ACTH, cortisol, and β-endorphin. Psychoneuroendocrinology 1995, 20, 499–508. [Google Scholar] [CrossRef]
- Hernandez, L.; Hoebel, B.G. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988, 42, 1705–1712. [Google Scholar] [CrossRef]
- Söderpalm, B.; Löf, E.; Ericson, M. Mechanistic studies of ethanol’s interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 2009, 42, S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Taber, K.H.; Black, D.N.; Porrino, L.J.; Hurley, R.A. Neuroanatomy of dopamine: Reward and addiction. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G.; Bassareo, V. Reward system and addiction: What dopamine does and doesn’t do. Curr. Opin. Pharmacol. 2007, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.; Pfaus, J.; Blaha, C. Dopamine and motivated behavior: Insights provided by in vivo analyses. Mesolimbic Dopamine Syst. Motiv. Action 1991, 199–224. [Google Scholar]
- Loh, H.; Brase, D.; Sampath-Khanna, S.; Mar, J.; Way, E.; Li, C. beta-Endorphin in vitro inhibition of striatal dopamine release. Nature 1976, 264, 567. [Google Scholar] [CrossRef]
- Deyo, S.; Swift, R.; Miller, R. Morphine and endorphins modulate dopamine turnover in rat median eminence. Proc. Natl. Acad. Sci. USA 1979, 76, 3006. [Google Scholar] [CrossRef] [Green Version]
- van Loon, G.; Ho, D.; Kim, C. Beta-endorphin-induced decrease in hypothalamic dopamine turnover. Endocrinology 1980, 106, 76. [Google Scholar] [CrossRef]
- Van Loon, G. Plasma dopamine: Regulation and significance. Fed. Proc. 1983, 42, 3012. [Google Scholar] [CrossRef]
- Rasmussen, D.D.; Liu, J.H.; Wolf, P.L.; Yen, S.S. Neurosecretion of human hypothalamic immunoreactive beta-endorphin: In vitro regulation by dopamine. Neuroendocrinology 1987, 45, 197–200. [Google Scholar] [CrossRef]
- Farah, J.M., Jr.; Malcolm, D.S.; Mueller, G.P. Dopaminergic inhibition of pituitary beta-endorphin-like immunoreactivity secretion in the rat. Endocrinology 1982, 110, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Sprouse-Blum, A.S.; Smith, G.; Sugai, D.; Parsa, F.D. Understanding endorphins and their importance in pain management. Hawaii Med. J. 2010, 69, 70–71. [Google Scholar] [PubMed]
- Cryan, J.F.; Kaupmann, K. Don’t worry ‘B’ happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 2005, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.C. Opioid peptides. Alcohol Health Res. World 1997, 21, 132–136. [Google Scholar]
- Olive, M.F.; Koenig, H.N.; Nannini, M.A.; Hodge, C.W. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J. Neurosci. 2001, 21, RC184. [Google Scholar] [CrossRef]
- Bardo, M.T.; Bevins, R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Amalric, M.; Cline, E.J.; Martinez, J.L., Jr.; Bloom, F.E.; Koob, G.F. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology 1987, 91, 14–19. [Google Scholar] [CrossRef]
- Dum, J.; Gramsch, C.; Herz, A. Activation of hypothalamic beta-endorphin pools by reward induced by highly palatable food. Pharmacol. Biochem. Behav. 1983, 18, 443–447. [Google Scholar] [CrossRef]
- Roth-Deri, I.; Green-Sadan, T.; Yadid, G. Beta-endorphin and drug-induced reward and reinforcement. Prog. Neurobiol. 2008, 86, 1–21. [Google Scholar] [CrossRef]
- Moldow, R.L.; Fischman, A.J. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides 1987, 8, 819–822. [Google Scholar] [CrossRef]
- Bilsky, E.J.; Montegut, M.J.; Delong, C.L.; Reid, L.D. Opioidergic modulation of cocaine conditioned place preferences. Life Sci. 1992, 50, Pl85–P190. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Marquez, P.; Hamid, A.; Kieffer, B.; Friedman, T.C.; Lutfy, K. The rewarding action of acute cocaine is reduced in β-endorphin deficient but not in μ opioid receptor knockout mice. Eur. J. Pharmacol. 2012, 686, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikshtein, Y.; Barnea, R.; Kronfeld, N.; Lax, E.; Roth-Deri, I.; Friedman, A.; Gispan, I.; Elharrar, E.; Levy, S.; Ben-Tzion, M.; et al. β-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology 2013, 38, 2508–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaliwal, A.; Gupta, M. Physiology, Opioid Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Weiss, R.D.; Rao, V. The Prescription Opioid Addiction Treatment Study: What have we learned. Drug Alcohol Depend. 2017, 173 (Suppl. S1), S48–S54. [Google Scholar] [CrossRef]
- Dunne, R.B. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018, 8, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, E.A.; Lundahl, L.H.; Burmeister, M.; Greenwald, M.K. Functional mu opioid receptor polymorphism (OPRM1 A(118) G) associated with heroin use outcomes in Caucasian males: A pilot study. Am. J. Addict. 2015, 24, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Zalewska-Kaszubska, J.; Czarnecka, E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides 2005, 26, 701–705. [Google Scholar] [CrossRef]
- Ulm, R.R.; Volpicelli, J.R.; Volpicelli, L.A. Opiates and alcohol self-administration in animals. J. Clin. Psychiatry 1995, 56 (Suppl. S7), 5–14. [Google Scholar]
- Berczik, K.; Griffiths, M.D.; Szabó, A.; Kurimay, T.; Urban, R.; Demetrovics, Z. Exercise addiction. In Behavioral Addictions; Elsevier: Amsterdam, The Netherlands, 2014; pp. 317–342. [Google Scholar]
- Nogueira, A.; Molinero, O.; Salguero, A.; Márquez, S. Exercise addiction in practitioners of endurance sports: A literature review. Front. Psychol. 2018, 9, 1484. [Google Scholar] [CrossRef] [Green Version]
- Nogueiras, R.; Romero-Pico, A.; Vazquez, M.J.; Novelle, M.G.; Lopez, M.; Dieguez, C. The opioid system and food intake: Homeostatic and hedonic mechanisms. Obes. Facts 2012, 5, 196–207. [Google Scholar] [CrossRef]
- Holtzman, S.G. Suppression of appetitive behavior in the rat by naloxone: Lack of effect of prior morphine dependence. Life Sci. 1979, 24, 219–226. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Zhang, D.D.; Chen, X.D.; Lu, M.; Lin, R.Y.; Wen, H.; Jin, L.; Wang, X.F. OPRM1 gene is associated with BMI in Uyghur population. Obesity (Silver Spring) 2009, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, F.; Kieffer, B.L.; Tabarin, A.; Contarino, A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur. J. Neurosci. 2007, 25, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Mizushige, T.; Saitoh, K.; Manabe, Y.; Nishizuka, T.; Taka, Y.; Eguchi, A.; Yoneda, T.; Matsumura, S.; Tsuzuki, S.; Inoue, K.; et al. Preference for dietary fat induced by release of beta-endorphin in rats. Life Sci. 2009, 84, 760–765. [Google Scholar] [CrossRef]
- Appleyard, S.M.; Hayward, M.; Young, J.I.; Butler, A.A.; Cone, R.D.; Rubinstein, M.; Low, M.J. A role for the endogenous opioid β-endorphin in energy homeostasis. Endocrinology 2003, 144, 1753–1760. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.S.; Morley, J.; Gosnell, B.A.; Billington, C.J.; Bartness, T. Opioids and consummatory behavior. Brain Res. Bull. 1985, 14, 663–672. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Gorzalka, B.B. Opioids and sexual behavior. Neurosci. Biobehav. Rev. 1987, 11, 1–34. [Google Scholar] [CrossRef]
- Gessa, G.L.; Paglietti, E.; Quarantotti, B.P. Induction of copulatory behavior in sexually inactive rats by naloxine. Science 1979, 204, 203–205. [Google Scholar] [CrossRef]
- Fabbri, A.; Jannini, E.A.; Gnessi, L.; Moretti, C.; Ulisse, S.; Franzese, A.; Lazzari, R.; Fraioli, F.; Frajese, G.; Isidori, A. Endorphins in male impotence: Evidence for naltrexone stimulation of erectile activity in patient therapy. Psychoneuroendocrinology 1989, 14, 103–111. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Vallano, M.L.; Barfield, R.J. Effects of morphine, beta-endorphin and naloxone on catecholamine levels and sexual behavior in the male rat. Pharmacol. Biochem. Behav. 1980, 13, 435–441. [Google Scholar] [CrossRef]
- Miller, R.; Baum, M. Naloxone inhibits mating and conditioned place preference for an estrous female in male rats soon after castration. Pharmacol. Biochem. Behav. 1987, 26, 781–789. [Google Scholar] [CrossRef]
- Wiesner, J.B.; Moss, R.L. Suppression of receptive and proceptive behavior in ovariectomized, estrogen-progesterone-primed rats by intraventricular beta-endorphin: Studies of behavioral specificity. Neuroendocrinology 1986, 43, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sirinathsinghji, D.J. Regulation of lordosis behaviour in the female rat by corticotropin-releasing factor, beta-endorphin/corticotropin and luteinizing hormone-releasing hormone neuronal systems in the medial preoptic area. Brain Res. 1986, 375, 49–56. [Google Scholar] [CrossRef]
- Torii, M.; Kubo, K.; Sasaki, T. Facilitatory and Inhibitory Effects of β-Endorphin on Lordosis in Female Rats: Relation to Time of Administration. Horm. Behav. 1999, 35, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Gorzalka, B.B. Selective activation of opioid receptors differentially affects lordosis behavior in female rats. Peptides 1987, 8, 309–317. [Google Scholar] [CrossRef]
- Meglio, M.; Hosobuchi, Y.; Loh, H.H.; Adams, J.E.; Li, C.H. beta-Endorphin: Behavioral and analgesic activity in cats. Proc. Natl. Acad. Sci. USA 1977, 74, 774–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, F.; Segal, D.; Ling, N.; Guillemin, R. Endorphins: Profound behavioral effects in rats suggest new etiological factors in mental illness. Science (New York, N.Y.) 1976, 194, 630–632. [Google Scholar] [CrossRef]
- Loh, H.H.; Li, C.H. Unique behavioral effects of β endorphin and their relationship to thermoregulation and hypothalamic function. Life Sci. 1978, 22, 1525–1535. [Google Scholar]
- Glickman-Weiss, E.L.; Nelson, A.G.; Hearon, C.M.; Goss, F.L.; Robertson, R.J. Are beta-endorphins and thermoregulation during cold-water immersion related? Undersea Hyperb. Med. 1993, 20, 205–213. [Google Scholar]
- King, C.; Masserano, J.M.; Codd, E.; Byrne, W.L. Effects of beta-endorphin and morphine on the sleep-wakefulness behavior of cats. Sleep 1981, 4, 259–262. [Google Scholar] [CrossRef]
- Wang, Q.; Yue, X.F.; Qu, W.M.; Tan, R.; Zheng, P.; Urade, Y.; Huang, Z.L. Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats. Neuropsychopharmacology 2013, 38, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myer, E.C.; Morris, D.L.; Brase, D.A.; Dewey, W.L.; Zimmerman, A.W. Naltrexone therapy of apnea in children with elevated cerebrospinal fluid beta-endorphin. Ann. Neurol. 1990, 27, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gessa, G.L.; Pani, L.; Fadda, P.; Fratta, W. Sleep deprivation in the rat: An animal model of mania. Eur. Neuropsychopharmacol. 1995, 5, 89–93. [Google Scholar] [CrossRef]
- Fratta, W.; Collu, M.; Martellotta, M.C.; Pichiri, M.; Muntoni, F.; Gessa, G.L. Stress-induced insomnia: Opioid-dopamine interactions. Eur. J. Pharmacol. 1987, 142, 437–440. [Google Scholar] [CrossRef]
- Przewlocka, B.; Mogilnicka, E.; Lason, W.; van Luijtelaar, E.L.; Coenen, A.M. Deprivation of REM sleep in the rat and the opioid peptides beta-endorphin and dynorphin. Neurosci. Lett. 1986, 70, 138–142. [Google Scholar] [CrossRef]
- Song, J.; Um, Y.H.; Kim, T.W.; Kim, S.M.; Kwon, S.Y.; Hong, S.-C. Sleep and Anesthesia. Sleep Med. Res. 2018, 9, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.; Mortier, P.; Auerbach, R.P.; Bruffaerts, R.; Vilagut, G.; Cuijpers, P.; Demyttenaere, K.; Ebert, D.D.; Ennis, E.; Gutiérrez-García, R.A.; et al. Severe role impairment associated with mental disorders: Results of the WHO World Mental Health Surveys International College Student Project. Depress. Anxiety 2018, 35, 802–814. [Google Scholar] [CrossRef]
- Darko, D.F.; Irwin, M.R.; Risch, S.C.; Gillin, J.C. Plasma beta-endorphin and natural killer cell activity in major depression: A preliminary study. Psychiatry Res. 1992, 43, 111–119. [Google Scholar] [CrossRef]
- Panerai, A.E.; Vecchiet, J.; Panzeri, P.; Meroni, P.; Scarone, S.; Pizzigallo, E.; Giamberardino, M.A.; Sacerdote, P. Peripheral blood mononuclear cell beta-endorphin concentration is decreased in chronic fatigue syndrome and fibromyalgia but not in depression: Preliminary report. Clin. J. Pain 2002, 18, 270–273. [Google Scholar] [CrossRef]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef]
- Lobstein, D.D.; Rasmussen, C.L.; Dunphy, G.E.; Dunphy, M.J. Beta-endorphin and components of depression as powerful discriminators between joggers and sedentary middle-aged men. J. Psychosom. Res. 1989, 33, 293–305. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Sulkava, R.; Erkinjuntti, T.; Laatikainen, T. CSF beta-endorphin and beta-lipotropin in Alzheimer’s disease and multi-infarct dementia. Neurology 1985, 35, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, B.; Szczudlik, A.; Lypka, A. Beta-endorphin levels in the blood and cerebrospinal fluid in humans. Neurol. Neurochir. Pol. 1985, 19, 281–285. [Google Scholar]
- Baker, D.G.; West, S.A.; Orth, D.N.; Hill, K.K.; Nicholson, W.E.; Ekhator, N.N.; Bruce, A.B.; Wortman, M.D.; Keck, P.E., Jr.; Geracioti, T.D., Jr. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinology 1997, 22, 517–529. [Google Scholar] [CrossRef]
- Adeodu, O.O.; Olorunmoteni, O.E.; Oseni, S.B.A.; Obuotor, E.M. Plasma and Cerebrospinal Fluid Beta-Endorphin Levels Show a Strong Association in Children with Cerebral Malaria. J. Pediatric Neurosci. 2018, 13, 416–422. [Google Scholar] [CrossRef]
- Nappi, G.; Facchinetti, F.; Martignoni, E.; Petraglia, F.; Bono, G.; Genazzani, A.R. CSF beta-EP in headache and depression. Cephalalgia 1985, 5, 99–101. [Google Scholar] [CrossRef]
- France, R.D.; Urban, B.J. Cerebrospinal fluid concentrations of beta-endorphin in chronic low back pain patients. Influence of depression and treatment. Psychosomatics 1991, 32, 72–77. [Google Scholar] [CrossRef]
- Savic, D.; Knezevic, G.; Matic, G.; Damjanovic, S.; Spiric, Z. Posttraumatic and depressive symptoms in β-endorphin dynamics. J. Affect. Disord. 2015, 181, 61–66. [Google Scholar] [CrossRef]
- Volpicelli, J.; Balaraman, G.; Hahn, J.; Wallace, H.; Bux, D. The role of uncontrollable trauma in the development of PTSD and alcohol addiction. Alcohol Res. Health 1999, 23, 256–262. [Google Scholar]
- Merenlender-Wagner, A.; Dikshtein, Y.; Yadid, G. The β-Endorphin Role in Stress-Related Psychiatric Disorders. Curr. Drug Targets 2009, 10, 1096–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu-Kia, A.M.; Fan, L.Q.; Kreek, M.J.; Simon, E.J.; Hiller, J.M. Mu-, delta- and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer’s disease patients. Brain Res. 2001, 893, 121–134. [Google Scholar] [CrossRef]
- Kim, D.J.; Blossom, S.J.; Delgado, P.L.; Carbajal, J.M.; Caceda, R. Examination of pain threshold and neuropeptides in patients with acute suicide risk. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109705. [Google Scholar] [CrossRef] [PubMed]
- Traskman-Bendz, L.; Ekman, R.; Regnell, G.; Ohman, R. HPA-related CSF neuropeptides in suicide attempters. Eur. Neuropsychopharmacol. 1992, 2, 99–106. [Google Scholar] [CrossRef]
- Scarone, S.; Gambini, O.; Calabrese, G.; Sacerdote, P.; Bruni, M.; Carucci, M.; Panerai, A.E. Asymmetrical distribution of beta-endorphin in cerebral hemispheres of suicides: Preliminary data. Psychiatry Res. 1990, 32, 159–166. [Google Scholar] [CrossRef]
- Hishimoto, A.; Cui, H.; Mouri, K.; Nushida, H.; Ueno, Y.; Maeda, K.; Shirakawa, O. A functional polymorphism of the micro-opioid receptor gene is associated with completed suicides. J. Neural Transm (Vienna) 2008, 115, 531–536. [Google Scholar] [CrossRef]
- Alzheimer’s-Association. 2019 Alzheimer’s Disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Husain, M.; Nemeroff, C. Neuropeptides and Alzheimer’s disease. J. Am. Geriatr. Soc. 1990, 38, 918. [Google Scholar] [CrossRef]
- Tariot, P.N.; Upadhyaya, A.; Sunderland, T.; Cox, C.; Cohen, R.M.; Murphy, D.L.; Loy, R. Physiologic and neuroendocrine responses to intravenous naloxone in subjects with Alzheimer’s disease and age-matched controls. Biol. Psychiatry 1999, 46, 412–419. [Google Scholar] [CrossRef]
- Jolkkonen, J.T.; Soininen, H.S.; Riekkinen, P.J. beta-Endorphin-like immunoreactivity in cerebrospinal fluid of patients with Alzheimer’s disease and Parkinson’s disease. J. Neurol. Sci. 1987, 77, 153–159. [Google Scholar] [CrossRef]
- Kaiya, H.; Tanaka, T.; Takeuchi, K.; Morita, K.; Adachi, S.; Shirakawa, H.; Ueki, H.; Namba, M. Decreased level of β-endorphin-like immunoreactivity in cerebrospinal fluid of patients with senile dementia of Alzheimer type. Life Sci. 1983, 33, 1039–1043. [Google Scholar] [CrossRef]
- Lee, S.; Chiba, T.; Kitahama, T.; Kaieda, R.; Hagiwara, M.; Nagazumi, A.; Terashi, A. CSF beta-endorphin, HVA and 5-HIAA of dementia of the Alzheimer type and Binswanger’s disease in the elderly. J. Neural Transm. 1990, 30, 45–55. [Google Scholar] [CrossRef]
- Koehl, M.; Meerlo, P.; Gonzales, D.; Rontal, A.; Turek, F.W.; Abrous, D.N. Exercise-induced promotion of hippocampal cell proliferation requires beta-endorphin. FASEB J. 2008, 22, 2253–2262. [Google Scholar] [CrossRef] [Green Version]
- Foster, P.P.; Rosenblatt, K.P.; Kuljiš, R.O. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front. Neurol. 2011, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Lyketsos, C.G.; Olin, J. Depression in Alzheimer’s disease: Overview and treatment. Biol. Psychiatry 2002, 52, 243–252. [Google Scholar] [CrossRef]
- Santos, L.E.; Beckman, D.; Ferreira, S.T. Microglial dysfunction connects depression and Alzheimer’s disease. Brain Behav. Immun. 2016, 55, 151–165. [Google Scholar] [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Varela, L.; Kim, J.G.; Kim, J.D.; Hernandez-Nuno, F.; Simonds, S.E.; Castorena, C.M.; Vianna, C.R.; Elmquist, J.K.; Morozov, Y.M.; et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 2015, 519, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Kravtsov, G.M.; Riazhskii, G.G.; Orlov, S.N. Transport of calcium to synaptosomes and subcellular membrane fractions of the brain: Effects of opioid peptides. Biokhimiia 1982, 47, 2006–2014. [Google Scholar] [PubMed]
- Boveris, A.; Navarro, A. Brain mitochondrial dysfunction in aging. IUBMB Life 2008, 60, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes—evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, D.; Cozzolino, D.; Salvatore, T.; Ceriello, A.; Torella, R.; Franchimont, P.; Lefebvre, P.J.; D’Onofrio, F. Physiological elevations of plasma β-endorphin alter glucose metabolism in obese, but not normal-weight, subjects. Metabolism 1992, 41, 184–190. [Google Scholar] [CrossRef]
- Matsumura, M.; Fukushima, T.; Saito, H.; Saito, S. In vivo and in vitro effects of β-endorphin on glucose metabolism in the rat. Horm. Metab. Res. 1984, 16, 27–31. [Google Scholar] [CrossRef]
- Gupta, K. Iatrogenic Angiogenesis. In Morphine Metastasis; Springer: Dordrecht, The Netherlands, 2012; p. 63. [Google Scholar]
- Zammit, A.R.; Katz, M.J.; Zimmerman, M.E.; Bitzer, M.; Lipton, R.B. Low eGFR is associated with dysexecutive and amnestic mild cognitive impairment. Alzheimers Dement. 2015, 1, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Joosten, H.; Izaks, G.J.; Slaets, J.P.J.; de Jong, P.E.; Visser, S.T.; Bilo, H.J.G.; Gansevoort, R.T. Association of cognitive function with albuminuria and eGFR in the general population. Clin. J. Am. Soc. Nephrol. 2011, 6, 1400–1409. [Google Scholar] [CrossRef]
- Pan, Z.-G.; Mao, Y.; Sun, F.-Y. VEGF enhances reconstruction of neurovascular units in the brain after injury. Sheng Li Xue Bao 2017, 69, 96–108. [Google Scholar]
- Li, W.L.; Fraser, J.L.; Yu, S.P.; Zhu, J.; Jiang, Y.J.; Wei, L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp. Brain Res. 2011, 214, 503–513. [Google Scholar] [CrossRef]
- James, J.M.; Gewolb, C.; Bautch, V.L. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 2009, 136, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storkebaum, E.; Lambrechts, D.; Carmeliet, P. VEGF: Once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 2004, 26, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Shingo, T.; Kobayashi, K.; Takeuchi, A.; Yano, A.; Muraoka, K.; Matsui, T.; Miyoshi, Y.; Hamada, H.; Date, I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2004, 19, 1494–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Xie, Z.H.; Guo, Y.J.; Zhao, C.P.; Jiang, H.; Song, Y.; Zhu, Z.Y.; Lai, C.; Xu, S.L.; Bi, J.Z. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2011, 411, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Religa, P.; Cao, R.; Religa, D.; Xue, Y.; Bogdanovic, N.; Westaway, D.; Marti, H.H.; Winblad, B.; Cao, Y. VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Sci. Rep. 2013, 3, 2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, I.; Llorca, J.; Infante, J.; Rodriguez-Rodriguez, E.; Fernandez-Viadero, C.; Pena, N.; Berciano, J.; Combarros, O. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol. Scand. 2007, 116, 56–58. [Google Scholar] [CrossRef]
- Chiappelli, M.; Borroni, B.; Archetti, S.; Calabrese, E.; Corsi, M.M.; Franceschi, M.; Padovani, A.; Licastro, F. VEGF gene and phenotype relation with Alzheimer’s disease and mild cognitive impairment. Rejuvenation Res. 2006, 9, 485–493. [Google Scholar] [CrossRef]
- Gontier, G.; George, C.; Chaker, Z.; Holzenberger, M.; Aid, S. Blocking IGF Signaling in Adult Neurons Alleviates Alzheimer’s Disease Pathology through Amyloid-beta Clearance. J. Neurosci. 2015, 35, 11500–11513. [Google Scholar] [CrossRef]
- Freude, S.; Hettich, M.M.; Schumann, C.; Stohr, O.; Koch, L.; Kohler, C.; Udelhoven, M.; Leeser, U.; Muller, M.; Kubota, N.; et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer’s disease. FASEB J. 2009, 23, 3315–3324. [Google Scholar] [CrossRef]
- Zheng, W.H.; Kar, S.; Dore, S.; Quirion, R. Insulin-like growth factor-1 (IGF-1): A neuroprotective trophic factor acting via the Akt kinase pathway. J. Neural Transm 2000, 261–272. [Google Scholar] [CrossRef]
- Naia, L.; Ribeiro, M.; Rodrigues, J.; Duarte, A.I.; Lopes, C.; Rosenstock, T.R.; Hayden, M.R.; Rego, A.C. Insulin and IGF-1 regularize energy metabolites in neural cells expressing full-length mutant huntingtin. Neuropeptides 2016, 58, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Triguero, D.; Buciak, J.L. Beta-endorphin chimeric peptides: Transport through the blood-brain barrier in vivo and cleavage of disulfide linkage by brain. Endocrinology 1990, 126, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Faletti, A.G.; Mastronardi, C.A.; Lomniczi, A.; Seilicovich, A.; Gimeno, M.; McCann, S.M.; Rettori, V. beta-Endorphin blocks luteinizing hormone-releasing hormone release by inhibiting the nitricoxidergic pathway controlling its release. Proc. Natl. Acad. Sci. USA 1999, 96, 1722–1726. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.S.; Cervos-Navarro, J.; Dey, P.K. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci. Res. 1991, 10, 211–221. [Google Scholar] [CrossRef]
- Cai, Z.; Ratka, A. Opioid system and Alzheimer’s disease. Neuromolecular Med. 2012, 14, 91–111. [Google Scholar] [CrossRef]
- Anthony, I.C.; Norrby, K.E.; Dingwall, T.; Carnie, F.W.; Millar, T.; Arango, J.C.; Robertson, R.; Bell, J.E. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain 2010, 133, 3685–3698. [Google Scholar] [CrossRef] [Green Version]
- Hyman, B.T.; Eslinger, P.J.; Damasio, A.R. Effect of naltrexone on senile dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatry 1985, 48, 1169–1171. [Google Scholar] [CrossRef]
- Tariot, P.N.; Sunderland, T.; Murphy, D.L.; Cohen, M.R.; Welkowitz, J.A.; Weingartner, H.; Newhouse, P.A.; Cohen, R.M. Design and interpretation of opiate antagonist trials in dementia. Prog. Neuropsychopharmacol. Biol. Psychiatry 1986, 10, 611–626. [Google Scholar] [CrossRef]
- Henderson, V.; Roberts, E.; Wimer, C.; Bardolph, E.; Chui, H.; Damasio, A.; Eslinger, P.; Folstein, M.F.; Schneider, L.; Teng, E. Multicenter trial of naloxone in Alzheimer’s disease. Ann. Neurol. 1989, 25, 404–406. [Google Scholar] [CrossRef]
- Serby, M.; Resnick, R.; Jordan, B.; Adler, J.; Corwin, J.; Rotrosen, J.P. Naltrexone and Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1986, 10, 587–590. [Google Scholar] [CrossRef]
- Pope, S.K.; Shue, V.M.; Beck, C. Will a healthy lifestyle help prevent Alzheimer’s disease? Annu. Rev. Public Health 2003, 24, 111–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intlekofer, K.A.; Cotman, C.W. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis. 2013, 57, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimers Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-Y.; Tang, X.-Q.; Mao, X.-F.; Wang, Y.-X. Autocrine Interleukin-10 Mediates Glucagon-Like Peptide-1 Receptor-Induced Spinal Microglial β-Endorphin Expression. J. Neurosci. 2017, 37, 11701–11714. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J. Pharmacol. Exp. Ther. 2002, 302, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Gong, N.; Li, T.-F.; Zhu, B.; Wang, Y.-X. Peptidic exenatide and herbal catalpol mediate neuroprotection via the hippocampal GLP-1 receptor/β-endorphin pathway. Pharmacol. Res. 2015, 102, 276–285. [Google Scholar] [CrossRef]
- Hölscher, C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s disease models. Neuropharmacology 2018, 136, 251–259. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2021, 22, 338. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010338
Pilozzi A, Carro C, Huang X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. International Journal of Molecular Sciences. 2021; 22(1):338. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010338
Chicago/Turabian StylePilozzi, Alexander, Caitlin Carro, and Xudong Huang. 2021. "Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism" International Journal of Molecular Sciences 22, no. 1: 338. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010338





