A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells
Abstract
:1. Introduction
2. Results
2.1. Preparation of mPEG-b-PEPA Copolymers and Copolymer Complexes with Tenofovir Disopropoxil
2.2. Cytotoxicity of mPEG-b-PEPA Adducts of TFD
2.3. The Ability of TDF or TFD Adducts to Induce Apoptosis
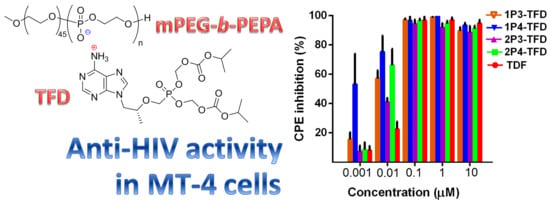
2.4. Antiviral Activity of mPEG-b-PEPA Adducts of TFD
2.5. Stability of mPEG-b-PEPA Adducts of TFD in Human Serum
2.6. Metabolic Conversions of TDF and mPEG-b-PEPA Adducts of TFD in MT-4 Cells: A Preliminary Study
3. Discussion
4. Materials and Methods
4.1. Synthesis of mPEG-b-PEPA and TFD Adducts
4.1.1. General Experimental Remarks
4.1.2. Synthesis of mPEG-b-poly(tBuOEP)
4.1.3. Preparation of mPEG-b-PEPA Solutions and TFD Adducts
- 1P3-TFD: 275 mg TFD, 1.220 g of P3 solution; 1.395 g of the solution obtained, dilution by 1.605 g H2O.
- 1P4-TFD: 225 mg TFD, 1.5 g of P4 solution; 1.755 g of the solution obtained, dilution by 1.245 g H2O.
- 2P3-TFD: 78 mg TFD, 2.92 g of P3 solution.
- 2P4-TFD: 78 mg TFD, 2.92 g of P4 solution.
4.2. Cells and Viruses
4.2.1. Cells
4.2.2. Virus and Virus Titration
4.3. Cytotoxicity Assay
4.4. Antiviral Activity Assay
4.5. Apoptosis Assay
4.6. Serum Treatment
4.7. Metabolism of TDF and mPEG-b-PEPA Adducts of TD in MT-4 Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global HIV & AIDS Statistics—2020 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 1 December 2020).
- Gulick, R.M.; Flexner, C. Long-acting HIV drugs for treatment and prevention. Annu. Rev. Med. 2019, 70, 137–150. [Google Scholar] [CrossRef]
- Singh, K.; Sarafianos, S.G.; Sönnerborg, A. Long-Acting Anti-HIV Drugs Targeting HIV-1 Reverse Transcriptase and Integrase. Pharmaceuticals 2019, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Tatham, L.M.; Savage, A.C.; Dwyer, A.; Siccardi, M.; Scott, T.; Vourvahis, M.; Clark, A.; Rannard, S.P.; Owen, A. Towards a Maraviroc long-acting injectable nanoformulation. Eur. J. Pharm. Biopharm. 2019, 138, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yant, S.R.; Ahmadyar, S.; Chan, T.Y.; Chiu, A.; Cihlar, T.; Link, J.O.; Lu, B.; Mwangi, J.; Rowe, W.; et al. GS-CA2: A Novel, Potent, and Selective First-In-class Inhibitor of HIV-1 Capsid Function Displays Nonclinical Pharmacokinetics Supporting Long-Acting Potential in Humans. Open Forum Infect. Dis. 2018, 5, S199–S200. [Google Scholar] [CrossRef]
- Li, M.; Cheng, S.; Ding, Y.; Wang, C.; Feng, Y.; Wang, W.; Ma, L.; Li, X. Polyethylene Glycol 40-Modified Peptide with High Therapeutic Efficacy in Simian-Human Immunodeficiency Virus-Acutely Infected Rhesus Monkeys. J. Virol. 2020, 94, 00386-20. [Google Scholar] [CrossRef] [PubMed]
- Krovi, S.A.; Gallovic, M.D.; Keller, A.M.; Bhat, M.; Tiet, P.; Chen, N.; Collier, M.A.; Gurysh, E.G.; Pino, E.N.; Johnson, M.M.; et al. Injectable long-acting human immunodeficiency virus antiretroviral prodrugs with improved pharmacokinetic profiles. Int. J. Pharm. 2018, 552, 371–377. [Google Scholar] [CrossRef]
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob. Agents Chemother. 2018, 62, 01058-18. [Google Scholar] [CrossRef] [Green Version]
- Owen, A.; Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 2016, 103, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Andersen, A.H.F.; Tolstrup, M. The Potential of Long-Acting, Tissue-Targeted Synthetic Nanotherapy for Delivery of Antiviral Therapy Against HIV Infection. Viruses 2020, 12, 412. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, J.; Mao, H.Q.; Leong, K.W. Polyphosphoesters in drug and gene delivery. Adv. Drug Deliv. Rev. 2003, 55, 483–499. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yuan, Y.-Y.; Du, J.-Z.; Yang, X.-Z.; Wang, J. Recent progress in polyphosphoesters: From controlled synthesis to biomedical applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Du, X.-J.; Mao, C.-Q.; Wang, J. Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef] [PubMed]
- Penczek, S.; Pretula, J.B.; Kaluzynski, K.; Lapienis, G. Polymers with Esters of Phosphoric Acid Units: From Synthesis, Models of Biopolymers to Polymer-Inorganic Hybrids. Isr. J. Chem. 2012, 52, 306–319. [Google Scholar] [CrossRef]
- Steinbach, T.; Wurm, F.R. Poly(phosphoester)s: A new platform for degradable polymers. Angew. Chem. Int. Ed. 2015, 54, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.E.; Jérôme, C. Polyphosphoesters: New trends in synthesis and drug delivery applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Pang, Y.; Yan, D. Hyperbranched polyphosphates: Synthesis, functionalization and biomedical applications. Chem. Soc. Rev. 2015, 44, 3942–3953. [Google Scholar] [CrossRef]
- Becker, G.; Ackermann, L.-M.; Schechtel, E.; Klapper, M.; Tremel, W.; Wurm, F.R. Joining Two Natural Motifs: Catechol-Containing Poly(phosphoester)s. Biomacromolecules 2017, 18, 767–777. [Google Scholar] [CrossRef]
- Bauer, K.N.; Liu, L.; Wagner, M.; Andrienko, D.; Wurm, F.R. Mechanistic study on the hydrolytic degradation of polyphosphates. Eur. Polym. J. 2018, 108, 286–294. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotech. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appukutti, N.; Serpell, C.J. High definition polyphosphoesters: Between nucleic acids and plastics. Polym. Chem. 2018, 9, 2210–2226. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Kosarev, M.A.; Gavrilov, D.E.; Komarov, P.D.; Ilyin, S.O.; Karchevsky, S.G.; Ivchenko, P.V. Mechanistic study of transesterification in TBD-catalyzed ring-opening polymerization of methyl ethylene phosphate. Eur. Polym. J. 2019, 118, 393–403. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Gavrilov, D.; Karchevsky, S.; Ivchenko, P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers 2019, 11, 1641. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, C.; Tinè, M.R.; Wurm, F.R. Main-chain water-soluble polyphosphoesters: Multi-functional polymers as degradable PEG-alternatives for biomedical applications. Eur. Polym. J. 2020, 141, 110079. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Synthesis and ring-opening polymerization of glycidyl ethylene phosphate with a formation of linear and branched polyphosphates. Mendeleev. Commun. 2018, 28, 155–157. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Tavtorkin, A.N.; Minyaev, M.E.; Kosarev, M.A.; Ivchenko, P.V. Synthesis in aqueous media of poly(ethylene phosphoric acids) by mild thermolysis of homopolymers and block copolymers based on tert-butyl ethylene phosphate. Eur. Polym. J. 2018, 106, 249–256. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Karchevsky, S.; Ivchenko, P. Mechanistic Insights of BHT-Mg-Catalyzed Ethylene Phosphate’s Coordination Ring-Opening Polymerization: DFT Modeling and Experimental Data. Polymers 2018, 10, 1105. [Google Scholar] [CrossRef] [Green Version]
- Kaluzynski, K.; Libisowski, J.; Penczek, S. A New Class of Synthetic Polyelectrolytes. Acidic Polyesters of Phosphoric Acid (Poly(hydroxyalkylene phosphates)). Macromolecules 1976, 9, 365–367. [Google Scholar] [CrossRef]
- Penczek, S.; Biela, T.; Klosinski, P.; Lapienis, G. Polymerization of phosphorus containing cyclic monomers: Synthesis of polymers related to biopolymers. Makromol. Chem. Macromol. Symp. 1986, 6, 123–153. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Mao, H.-Q.; Wang, S.; Phua, S.H.; Lee, G.P.; Pan, J.; Lu, S.; Wang, J.; Leong, K.W. Poly(phosphoester) ionomers as tissue-engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomat. 2004, 70B, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kawakita, T.; Yusa, S. Thermoresponsive Polyphosphoesters Bearing Enzyme-cleavable Side Chains. Chem. Lett. 2009, 38, 1054–1055. [Google Scholar] [CrossRef]
- Ergul Yilmaz, Z.; Debuigne, A.; Calvignac, B.; Boury, F.; Jerome, C. Double hydrophilic polyphosphoester containing copolymers as efficient templating agents for calcium carbonate microparticles. J. Mater. Chem. B 2015, 3, 7227–7236. [Google Scholar] [CrossRef]
- Hirano, Y.; Iwasaki, Y. Bone-specific poly(ethylene sodium phosphate)-bearing biodegradable nanoparticles. Coll. Surf. B Biointerfaces 2017, 153, 104–110. [Google Scholar] [CrossRef]
- Otaka, A.; Iwasaki, Y. Endocytosis of poly(ethylene sodium phosphate) by macrophages and the effect of polymer length on cellular uptake. J. Ind. Eng. Chem. 2019, 75, 115–122. [Google Scholar] [CrossRef]
- Yasuda, H.; Sumitani, M.; Nakamura, A. Novel Synthesis of Acidic Polyesters of Phosphoric Acid by Thermal Elimination of Isobutylene from Poly(alkylene tert-butyl phosphates). Macromolecules 1981, 14, 458–460. [Google Scholar] [CrossRef]
- Moriyama, R.; Iwasaki, Y.; Miyoshi, D. Stabilization of DNA Structures with Poly(ethylene sodium phosphate). J. Phys. Chem. B 2014, 119, 11969–11977. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yokota, A.; Otaka, A.; Inoue, N.; Yamaguchi, A.; Yoshitomi, T.; Yoshimotode, K.; Neo, M. Bone-targeting poly(ethylene sodium phosphate). Biomater. Sci. 2018, 6, 91–95. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Bukharova, T.; Dyakonov, A.; Goldshtein, D.; Galitsyna, E.; Kosarev, M.; Shlyakhtin, A.; Gavrilov, D.; Ivchenko, P. Osteogenic differentiation of human adipose tissue-derived MSCs by non-toxic calcium poly(ethylene phosphate)s. Int. J. Mol. Sci. 2019, 20, 6242. [Google Scholar] [CrossRef] [Green Version]
- Noree, S.; Iwasaki, Y. Thermally Assisted Generation of Protein–Poly(ethylene sodium phosphate) Conjugates with High Mineral Affinity. ACS Omega 2019, 4, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Noree, S.; Thongthai, P.; Kitagawa, H.; Imazato, S.; Iwasaki, Y. Reduction of Acidic Erosion and Oral Bacterial Adhesion through the Immobilization of Zwitterionic Polyphosphoesters on Mineral Substrates. Chem. Lett. 2019, 48, 1529–1532. [Google Scholar] [CrossRef]
- Iwasaki, Y. Bone Mineral Affinity of Polyphosphodiesters. Molecules 2020, 25, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walwyn, D. Patents and profits: A disparity of manufacturing margins in the tenofovir value chain. Afr. J. AIDS Res. 2013, 12, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Anandgaonkar, V.; Gupta, A.; Kona, S.; Talluri, M.V.N.K. Isolation, LC–MS/MS and 2D-NMR characterization of alkaline degradants of tenofovir disoproxil fumarate. J. Pharm. Biomed. Anal. 2015, 107, 175–185. [Google Scholar] [CrossRef]
- Guidance for Industry: Antiviral Product Development—Conducting and Submitting Virology Studies to the Agency; US Food and Drug Administration: Rockville, MD, USA, 2006. Available online: https://www.fda.gov/media/71223/download (accessed on 1 December 2020).
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, P.; Desfarges, S.; Bartha, I.; Joos, B.; Zangger, N.; Muñoz, M.; Günthard, H.F.; Beerenwinkel, N.; Telenti, A.; Ciuffi, A. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog. 2013, 9, 1003161. [Google Scholar] [CrossRef]
- Gallo, S.A.; Finnegan, C.M.; Viard, M.; Raviv, Y.; Dimitrov, A.; Rawat, S.S.; Puri, A.; Durell, S.; Blumenthal, R. The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 2003, 1614, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Raviv, Y.; Viard, M.; Bess, J., Jr.; Blumenthal, R. Quantitative measurement of fusion of HIV-1 and SIV with cultured cells using photosensitized labeling. Virology 2002, 293, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Shcherbatova, O.; Grebennikov, D.; Sazonov, I.; Meyerhans, A.; Bocharov, G. Modeling of the HIV-1 Life Cycle in Productively Infected Cells to Predict Novel Therapeutic Targets. Pathogens 2020, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Robbins, B.L.; Srinivas, R.V.; Kim, C.; Bischofberger, N.; Fridland, A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998, 42, 612–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taneva, E.; Crooker, K.; Park, S.H.; Su, J.T.; Ott, A.; Cheshenko, N.; Szleifer, I.; Kiser, P.F.; Frank, B.; Mesquita, P.M.; et al. Differential Mechanisms of Tenofovir and Tenofovir Disoproxil Fumarate Cellular Transport and Implications for Topical Preexposure Prophylaxis. Antimicrob. Agents Chemother. 2015, 60, 1667–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkus, G.; Kutty, N.; He, G.X.; Mulato, A.; Lee, W.; McDermott, M.; Cihlar, T. Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases. Mol. Pharmacol. 2008, 74, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Masters, M.C.; Krueger, K.M.; Williams, J.L.; Morrison, L.; Cohn, S.E. Beyond one pill, once daily: Current challenges of antiretroviral therapy management in the United States. Expert Rev. Clin. Pharmacol. 2019, 12, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Liu, W.F.; Megido, L.; Díez, P.; Fuentes, M.; Fager, C.; Olsson, E.; Gessner, I.; Mathur, S. Understanding and utilizing the biomolecule/nanosystems interface. In Nanotechnologies in Preventive and Regenerative Medicine; Uskoković, V., Uskoković, D.P., Eds.; Micro and Nano Technologies Series; Elsevier: Amsterdam, The Netherlands, 2018; pp. 207–297. [Google Scholar] [CrossRef]
- Lu, H.; Utama, R.H.; Kitiyotsawat, U.; Babiuch, K.; Jiang, Y.; Stenzel, M.H. Enhanced transcellular penetration and drug delivery by crosslinked polymeric micelles into pancreatic multicellular tumor spheroids. Biomater Sci. 2015, 3, 1085–1095. [Google Scholar] [CrossRef]
- Costamagna, F.; Hillaireau, H.; Vergnaud, J.; Clarisse, D.; Jamgotchian, L.; Loreau, O.; Denis, S.; Gravel, E.; Doris, E.; Fattal, E. Nanotoxicology at the particle/micelle frontier: Influence of core-polymerization on the intracellular distribution, cytotoxicity and genotoxicity of polydiacetylene micelles. Nanoscale 2020, 12, 2452–2463. [Google Scholar] [CrossRef]
- Kim, Y.; Pourgholami, M.H.; Morris, D.L.; Lu, H.; Stenzel, M.H. Effect of shell-crosslinking of micelles on endocytosis and exocytosis: Acceleration of exocytosis by crosslinking. Biomater. Sci. 2013, 1, 265–275. [Google Scholar] [CrossRef]
- Hong, W.; Gao, X.; Qiu, P.; Yang, J.; Qiao, M.; Shi, H.; Zhang, D.; Tian, C.; Niu, S.; Liu, M. Synthesis, construction, and evaluation of self-assembled nano-bacitracin A as an efficient antibacterial agent in vitro and in vivo. Int. J. Nanomed. 2017, 12, 4691–4708. [Google Scholar] [CrossRef] [Green Version]
- Duo, X.; Li, Q.; Wang, J.; Lv, J.; Hao, X.; Feng, Y.; Ren, X.; Shi, C.; Zhang, W. Core/Shell Gene Carriers with Different Lengths of PLGA Chains to Transfect Endothelial Cells. Langmuir 2017, 33, 13315–13325. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Breathing air as oxidant: Optimization of 2-chloro-2-oxo-1,3,2-dioxaphospholane synthesis as a precursor for phosphoryl choline derivatives and cyclic phosphate monomers. Tetrahedron 2017, 73, 3536–3540. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Ivchenko, P.V.; Borisov, R.S.; Churakov, A.V. Monomeric and dimeric magnesium mono-BHT complexes as effective ROP catalysts. Catal. Commun. 2016, 87, 106–111. [Google Scholar] [CrossRef]
- Pannecouque, C.; Daelemans, D.; De Clercq, E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: Revisited 20 years later. Nat. Protoc. 2008, 3, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Bam, R.A.; Birkus, G.; Babusis, D.; Cihlar, T.; Yant, S.R. Metabolism and antiretroviral activity of tenofovir alafenamide in CD4+ T-cells and macrophages from demographically diverse donors. Antivir. Ther. 2014, 19, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shailender, J.; Ravi, P.R.; Saha, P.; Dalvi, A.; Myneni, S. Tenofovir disoproxil fumarate loaded PLGA nanoparticles for enhanced oral absorption: Effect of experimental variables and in vitro, ex vivo and in vivo evaluation. Colloids Surf. B Biointerfaces 2017, 158, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.I.; Jensen, O.N.; Rogowska-Wrzesinska, A. FlashPack: Fast and Simple Preparation of Ultrahigh-performance Capillary Columns for LC-MS. Mol. Cell Proteom. 2019, 18, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.J.; Pratt, B.; Bose, N.; Dubois, L.G.; St John-Williams, L.; Perrott, K.M.; Ky, K.; Kapahi, P.; Sharma, V.; MacCoss, M.J.; et al. Alzheimer’s Disease Metabolomics Consortium. Skyline for Small Molecules: A Unifying Software Package for Quantitative Metabolomics. J. Proteome Res. 2020, 19, 1447–1458. [Google Scholar] [CrossRef]







| Compound | CC50 (μM) | 95% CI | R2 |
|---|---|---|---|
| TDF | 91.9 | 71.2–118.6 | 0.98 |
| 1P3-TFD | 152.4 | 143.9–161.4 | 0.99 |
| 1P4-TFD | 85.3 | 73.4–99.2 | 0.99 |
| 2P3-TFD | 72.5 | 61.1–85.9 | 0.96 |
| 2P4-TFD | 79.4 | 71.1–88.7 | 0.99 |
| Compound | IC50 (μM) | 95% CI |
|---|---|---|
| TDF | 0.019 | 0.011–0.03 |
| 1P3-TFD | 0.007 | 0.0053–0.0087 |
| 1P4-TFD | 0.0013 | 0.0006–0.0028 |
| 2P3-TFD | 0.011 | 0.01–0.017 |
| 2P4-TFD | 0.006 | 0.004–0.008 |
| Compound | SI | SIadd/SITDF |
|---|---|---|
| TDF | 4836.84 | n/a 1 |
| 1P3-TFD | 21,771.43 | 4.5 |
| 1P4-TFD | 65,615.38 | 13.57 |
| 2P3-TFD | 6590.91 | 1.36 |
| 2P4-TFD | 13,233.33 | 2.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Siniavin, A.; Karamov, E.; Kosarev, M.; Kovalchuk, S.; Turgiev, A.; Nametkin, S.; Bagrov, V.; Tavtorkin, A.; Ivchenko, P. A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells. Int. J. Mol. Sci. 2021, 22, 340. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010340
Nifant’ev I, Siniavin A, Karamov E, Kosarev M, Kovalchuk S, Turgiev A, Nametkin S, Bagrov V, Tavtorkin A, Ivchenko P. A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells. International Journal of Molecular Sciences. 2021; 22(1):340. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010340
Chicago/Turabian StyleNifant’ev, Ilya, Andrei Siniavin, Eduard Karamov, Maxim Kosarev, Sergey Kovalchuk, Ali Turgiev, Sergey Nametkin, Vladimir Bagrov, Alexander Tavtorkin, and Pavel Ivchenko. 2021. "A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells" International Journal of Molecular Sciences 22, no. 1: 340. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010340