Impairment of IGF-1 Signaling and Antioxidant Response Are Associated with Radiation Sensitivity and Mortality
Abstract
:1. Introduction
2. Results
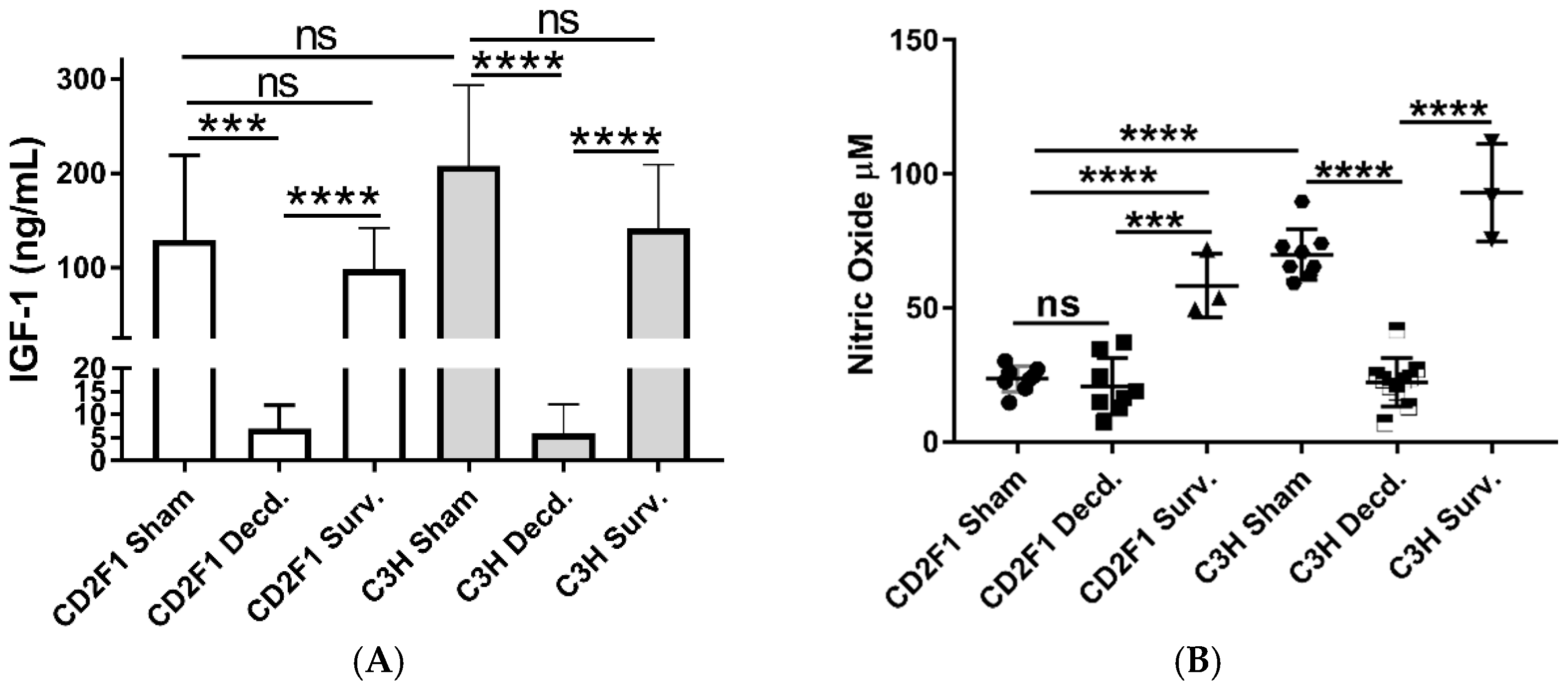
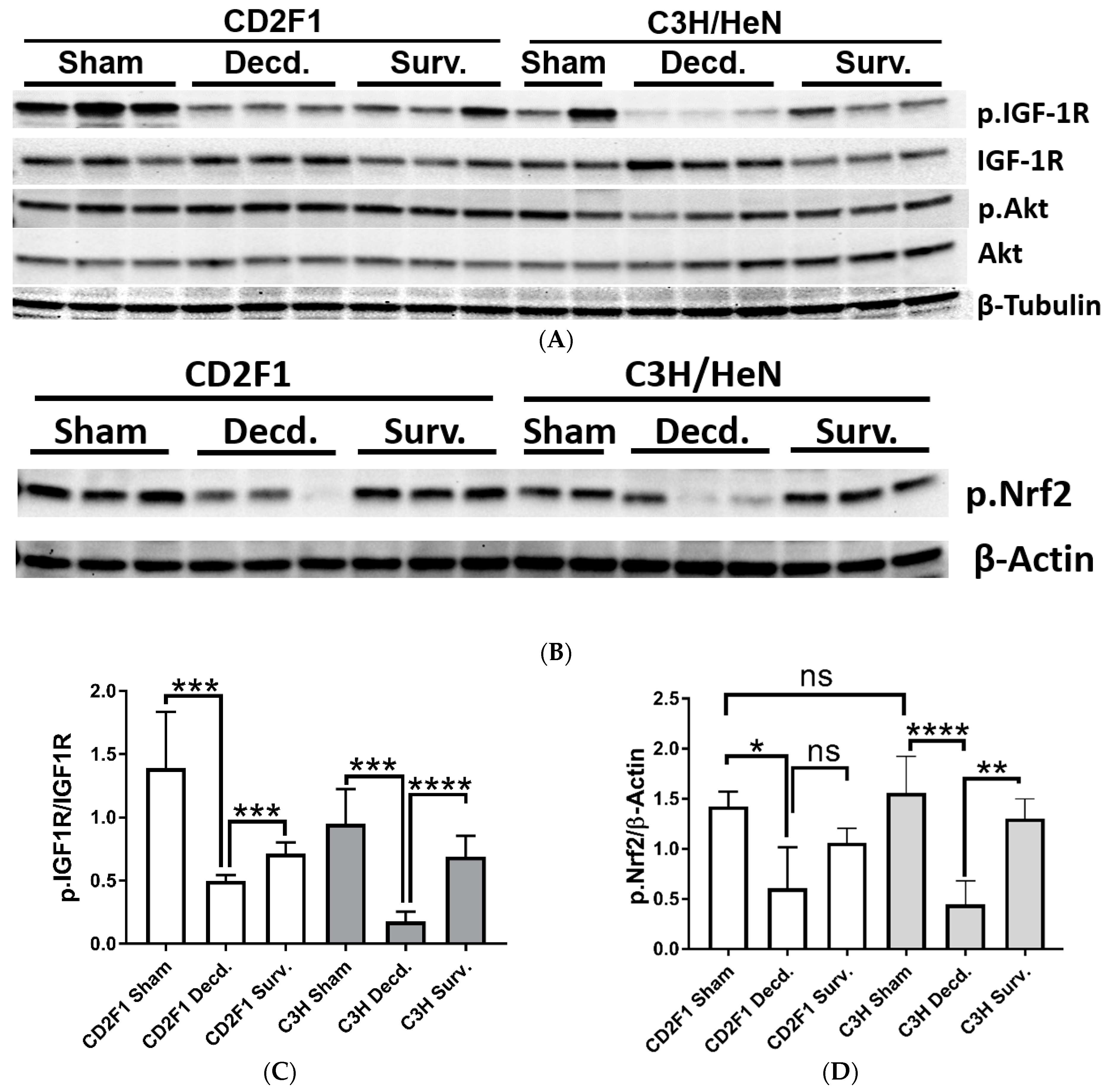
2.1. Systemic and Cardiac Impairment of IGF-1 and Nrf2 Signaling in Lethal Dose-70/30 (LD70/30) Irradiated CD2F1 and C3H/HeN Mice
2.2. Radiation Has Differential Effects on the Expression of Cardiac Genes Involved in Oxidative Stress and Mitochondrial Energy Metabolism in the LD70/30 Irradiated CD2F1 and C3H/HeN Mice
2.3. Radiation Disrupts the IGF-1 Signaling in the Lungs and Kidneys of the Irradiated Decedent C3H/HeN Strain and Inactivates the Nrf2 Signaling in the Lungs of Irradiated Decedent CD2F1 Mice
3. Discussion
4. Materials and Methods
4.1. Animal Strains, Radiation, Blood and Tissue Collection from Animals
4.2. Western Blot Analysis
4.3. ELISA, Peroxide, ATP and Nitric Oxide Measurements
4.4. Real-Time PCR Array Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H-ARS | Hematopoietic acute radiation syndrome |
| IGF-1 | Insulin-like Growth Factor-1 |
| IR | Insulin receptor |
| Ptx3 Slc38a1 | Pentraxin 3 Sodium-coupled neutral amino acid transporter 1 |
| Cdkn1a | Cyclin Dependent Kinase inhibitor 1A |
| NADH | Nicotinamide Adenine Dinucleotide Hydride |
| PTEN | Phosphatase and tensin homolog |
| ATP | Adenosine Triphosphate |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| Ucp2 | Uncoupling protein 2 |
| ELISA | Enzyme-linked immunosorbent assay |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
References
- Garau, M.M.I.; Calduch, A.L.; Lopez, E.C. Radiobiology of the acute radiation syndrome. Rep. Pract. Oncol. Radiother. 2011, 16, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroni, M.; Ngudiankama, B.F.; Christensen, C.; Olsen, C.H.; Owens, R.; Lombardini, E.D.; Holt, R.K.; Whitnall, M.H. The Göttingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body gamma-irradiation. Int. J. Radiat Oncol. Biol. Phys. 2013, 86, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Severson, G.V.N.; Gulani, J.; Bolduc, D.; Moroni, M. Development of a pediatric model of hematopoietic acute radiation syndrome (H-ARS) and countermeasure testing using the Göttingen minipigs. Radiat. Appl. 2017, 2, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGeoghegan, D.; Binks, K.; Gillies, M.; Jones, S.; Whaley, S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int. J. Epidemiol. 2008, 37, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, O.; Azizova, T.; Merl-Pham, J.; Subramanian, V.; Bakshi, M.V.; Moseeva, M.; Zubkova, O.; Hauck, S.M.; Anastasov, N.; Atkinson, M.J.; et al. A dose-dependent perturbation in cardiac energy metabolism is linked to radiation-induced ischemic heart disease in Mayak nuclear workers. Oncotarget 2016, 8, 9067–9078. [Google Scholar] [CrossRef] [PubMed]
- Cohn, K.E.; Stewart, J.R.; Fajardo, L.F.; Hancock, E.W. Heart Disease Following Radiation. Medicine 1967, 46, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Stewart, H.; Peto, R.; Baum, M.; Fisher, B.; Host, H.; Lythgoe, J.P.; Ribeiro, G.; Scheurlen, H.; Wallgren, A. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat. Rep. 1987, 71, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; McGale, P.; Taylor, C.W.; Peto, R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005, 6, 557–565. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443. [Google Scholar] [CrossRef] [Green Version]
- Salmon, W.D., Jr.; Daughaday, W.H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab Clin. Med. 1957, 49, 825–836. [Google Scholar] [PubMed]
- Ohlsson, C.; Mohan, S.; Sjogren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; Jansson, J.-O.; Svensson, J. The Role of Liver-Derived Insulin-Like Growth Factor-I. Endocr. Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenchegowda, D.; Legesse, B.; Hritzo, B.; Olsen, C.; Aghdam, S.; Kaur, A.; Culp, W.; Derrien-Colemyn, A.; Severson, G.; Moroni, M. Selective insulin-like growth factor resistance associated with heart hemorrhages and poor prognosis in a novel preclinical model of the hematopoietic acute radiation syndrome. Radiat Res. 2018, 190, 164–175. [Google Scholar] [CrossRef]
- Loscalzo, J.; Welch, G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995, 38, 87–104. [Google Scholar] [CrossRef]
- Troncoso, R.; Ibarra, C.; Vicencio, J.M.; Jaimovich, E.; Lavandero, S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 2014, 25, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, S.Y.; Kenchegowda, D.; Sharma, N.K.; Holmes-Hampton, G.P.; Legesse, B.; Moroni, M.; Ghosh, S.P. Dysregulated Cardiac IGF-1 Signaling and Antioxidant Response Are Associated with Radiation Sensitivity. Int. J. Mol. Sci. 2020, 21, 5049. [Google Scholar] [CrossRef] [PubMed]
- Sukhanov, S.; Higashi, Y.; Shai, S.-Y.; Vaughn, C.; Mohler, J.; Li, Y.; Song, Y.-H.; Titterington, J.; Delafontaine, P. IGF-1 Reduces Inflammatory Responses, Suppresses Oxidative Stress, and Decreases Atherosclerosis Progression in ApoE-Deficient Mice. Arter. Thromb. Vasc. Biol. 2007, 27, 2684–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey-Downs, L.C.; Mitschelen, M.; Sosnowska, D.; Toth, P.; Pinto, J.T.; Ballabh, P.; Valcarcel-Ares, M.N.; Farley, J.; Koller, A.; Henthorn, J.C.; et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2012, 67, 313–329. [Google Scholar] [CrossRef]
- Lyons, A.; Coleman, M.; Riis, S.; Favre, C.; O’Flanagan, C.H.; Zhdanov, A.V.; Papkovsky, D.B.; Hursting, S.D.; O’Connor, R. Insulin-like growth factor 1 signaling is essential for mitochondrial biogenesis and mitophagy in cancer cells. J. Biol. Chem. 2017, 292, 16983–16998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riis, S.; Murray, J.B.; O’Connor, R. IGF-1 signalling regulates mitochondria dynamics and turnover through a conserved GSK-3beta-Nrf2-BNIP3 pathway. Cells 2020, 9, 147. [Google Scholar] [CrossRef] [Green Version]
- Satyamitra, M.; Kumar, V.P.; Biswas, S.; Cary, L.; Dickson, L.; Venkataraman, S.; Ghosh, S.P. Impact of Abbreviated Filgrastim Schedule on Survival and Hematopoietic Recovery after Irradiation in Four Mouse Strains with Different Radiosensitivity. Radiat. Res. 2017, 187, 659–671. [Google Scholar] [CrossRef]
- Isenovic, E.R.; Divald, A.; Milivojevic, N.; Grgurevic, T.; Fisher, S.E.; Sowers, J.R. Interactive effects of insulin-like growth factor-1 and beta-estradiol on endothelial nitric oxide synthase activity in rat aortic endothelial cells. Metabolism 2003, 52, 482–487. [Google Scholar] [CrossRef]
- Repetto, S.; Salani, B.; Maggi, D.; Cordera, R. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochem. Biophys. Res. Commun. 2005, 337, 849–852. [Google Scholar] [CrossRef] [PubMed]
- González-Guerra, J.L.; Castilla-Cortazar, I.; Aguirre, G.A.; Muñoz, U.; Martin-Estal, I.; Ávila-Gallego, E.; Granado, M.; Puche, J.E.; García-Villalón, A.L. Partial IGF-1 deficiency is sufficient to reduce heart contractibility, angiotensin II sensibility, and alter gene expression of structural and functional cardiac proteins. PLoS ONE 2017, 12, e0181760. [Google Scholar] [CrossRef] [Green Version]
- Strijdom, H.; Chamane, N.; Lochner, A. Nitric oxide in the cardiovascular system: A simple molecule with complex actions. Cardiovasc. J. Afr. 2009, 20, 303–310. [Google Scholar] [PubMed]
- Kato, H.; Faria, T.N.; Stannard, B.; Roberts, C.T., Jr.; Leroith, D. Essential role of tyrosine residues 1131, 1135, and 1136 of the insulin-like growth factor-I (IGF-I) receptor in IGF-I action. Mol. Endocrinol. 1994, 8, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef]
- Jung, H.J.; Suh, Y. Regulation of IGF -1 signaling by microRNAs. Front. Genet. 2014, 5, 472. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, S.; Mettler, E.; Fottner, C.; Miederer, M.; Kaina, B.; Weber, M.M. The impact of the IGF-1 system of cancer cells on radiation response—An in vitro study. Clin. Transl. Radiat. Oncol. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, C.J.; Dimai, H.P.; Vereault, D.; Donahue, L.R.; Beamer, W.G.; Farley, J.; Linkhart, S.; Linkhart, T.; Mohan, S.; Baylink, D.J. Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone 1997, 21, 217–223. [Google Scholar] [CrossRef]
- Knacke, H.; Pietzner, M.; Do, K.T.; Römisch-Margl, W.; Kastenmüller, G.; Völker, U.; Völzke, H.; Krumsiek, J.; Artati, A.; Wallaschofski, H.; et al. Metabolic Fingerprints of Circulating IGF-1 and the IGF-1/IGFBP-3 Ratio: A Multifluid Metabolomics Study. J. Clin. Endocrinol. Metab. 2016, 101, 4730–4742. [Google Scholar] [CrossRef]
- Singh, K.; Maity, P.; Krug, L.; Meyer, P.; Treiber, N.; Lucas, T.; Basu, A.; Kochanek, S.; Wlaschek, M.; Geiger, H.; et al. Superoxide anion radicals induce IGF -1 resistance through concomitant activation of PTP 1 B and PTEN. EMBO Mol. Med. 2015, 7, 59–77. [Google Scholar] [CrossRef]
- Lopez-Mirabal, H.R.; Winther, J.R. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta 2008, 1783, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.D.; Eguchi, S.; Reynolds, C.M.; Eguchi, K.; Frank, G.D.; Motley, E.D. Hydrogen peroxide inhibits insulin signaling in vascular smooth muscle cells. Exp. Biol. Med. 2003, 228, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Lee, W.H. Hydrogen peroxide attenuates insulin-like growth factor-1 neuroprotective effect, prevented by minocycline. Neurochem. Int. 2007, 51, 398–404. [Google Scholar] [CrossRef]
- Verhaar, M.C.; Westerweel, P.E.; Van Zonneveld, A.J.; Rabelink, T.J. Free radical production by dysfunctional eNOS. Heart 2004, 90, 494–495. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [Green Version]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chida, J.; Yamane, K.; Takei, T.; Kido, H. An efficient extraction method for quantitation of adenosine triphosphate in mammalian tissues and cells. Anal. Chim. Acta 2012, 727, 8–12. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Y. Aghdam, S.; Kenchegowda, D.; Holmes-Hampton, G.P.; Moroni, M.; P. Ghosh, S. Impairment of IGF-1 Signaling and Antioxidant Response Are Associated with Radiation Sensitivity and Mortality. Int. J. Mol. Sci. 2021, 22, 451. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010451
Y. Aghdam S, Kenchegowda D, Holmes-Hampton GP, Moroni M, P. Ghosh S. Impairment of IGF-1 Signaling and Antioxidant Response Are Associated with Radiation Sensitivity and Mortality. International Journal of Molecular Sciences. 2021; 22(1):451. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010451
Chicago/Turabian StyleY. Aghdam, Saeed, Doreswamy Kenchegowda, Gregory P. Holmes-Hampton, Maria Moroni, and Sanchita P. Ghosh. 2021. "Impairment of IGF-1 Signaling and Antioxidant Response Are Associated with Radiation Sensitivity and Mortality" International Journal of Molecular Sciences 22, no. 1: 451. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010451



