Intracerebroventricular Treatment with 2-Hydroxypropyl-β-Cyclodextrin Decreased Cerebellar and Hepatic Glycoprotein Nonmetastatic Melanoma Protein B (GPNMB) Expression in Niemann–Pick Disease Type C Model Mice
Abstract
:1. Introduction
2. Results
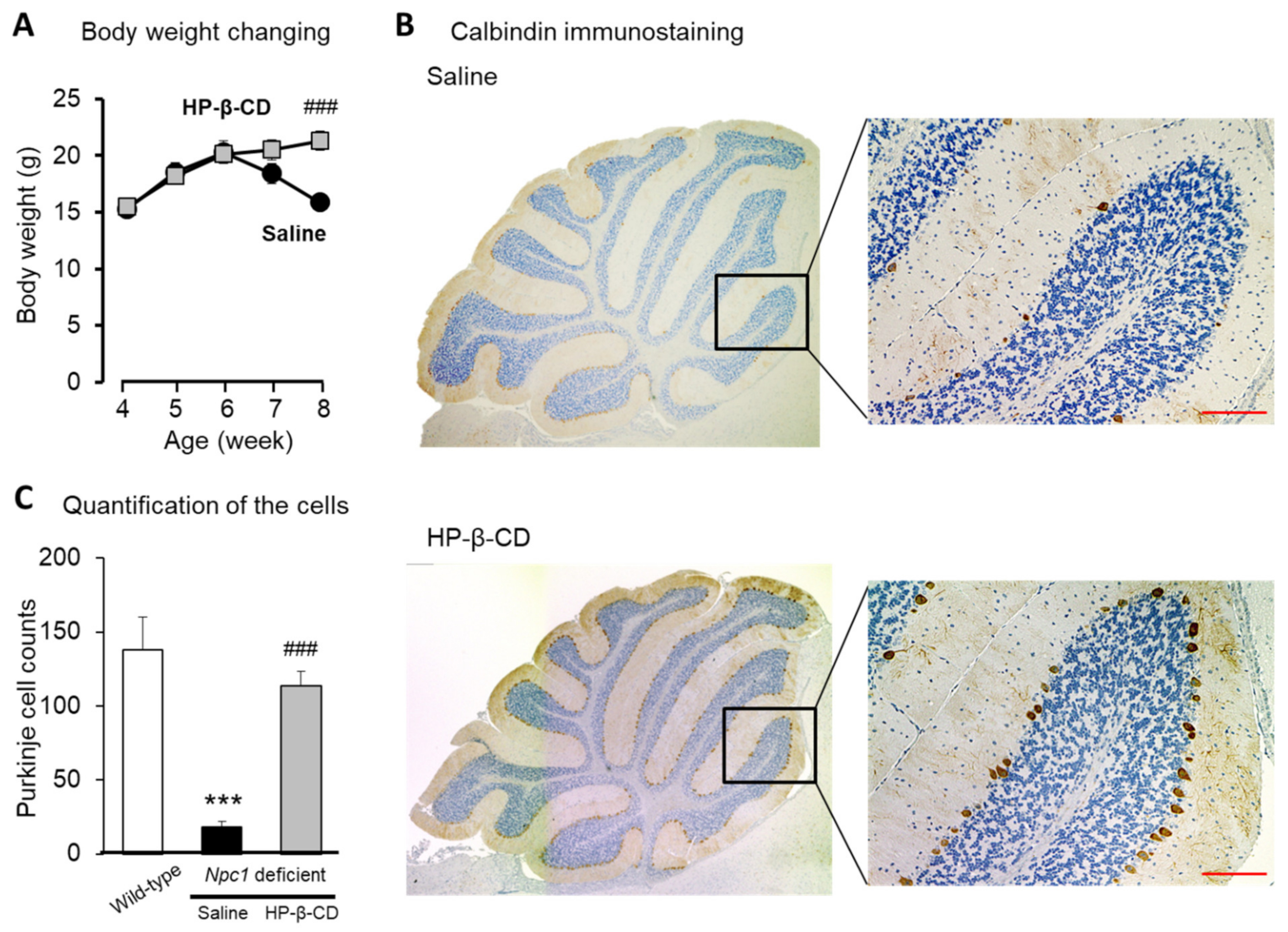
2.1. Protective Effects of Intracerebroventricular HP-β-CD Treatment on Neuronal Injury in Npc1-Deficient Mice
2.2. Evaluation of Soluble GPNMB (sGPNMB) Levels upon Intracerebroventricular HP-β-CD Treatment in Npc1−/− Mice
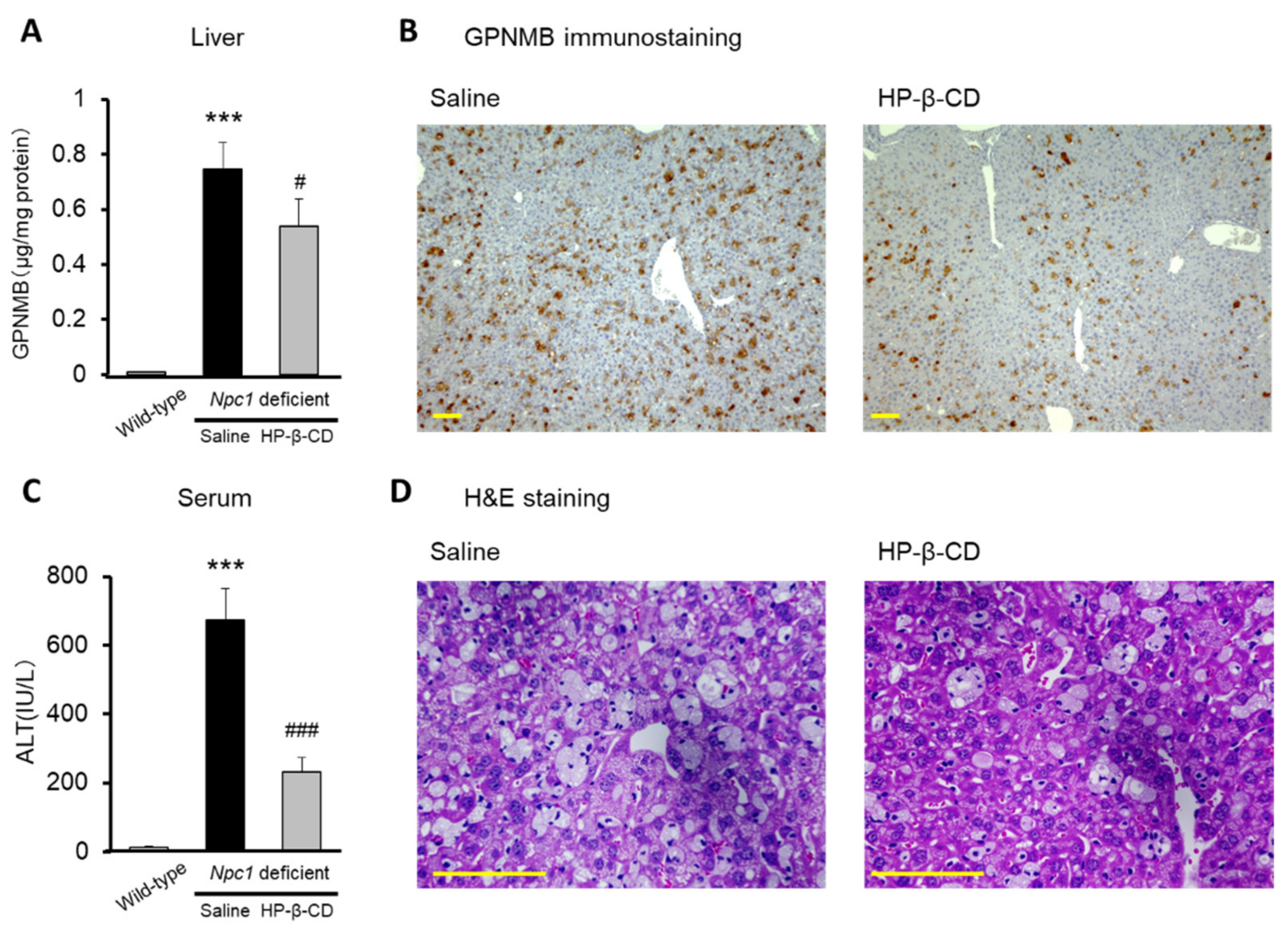
2.3. Attenuating Effect of Intracerebroventricular Injection of HP-β-CD on Abnormal Increases of GPNMB in Npc1−/− Mouse Cerebellum
2.4. Hepatoprotective Effects of Intracerebroventricular HP-β-CD Treatment in Npc1-Deficient Mice
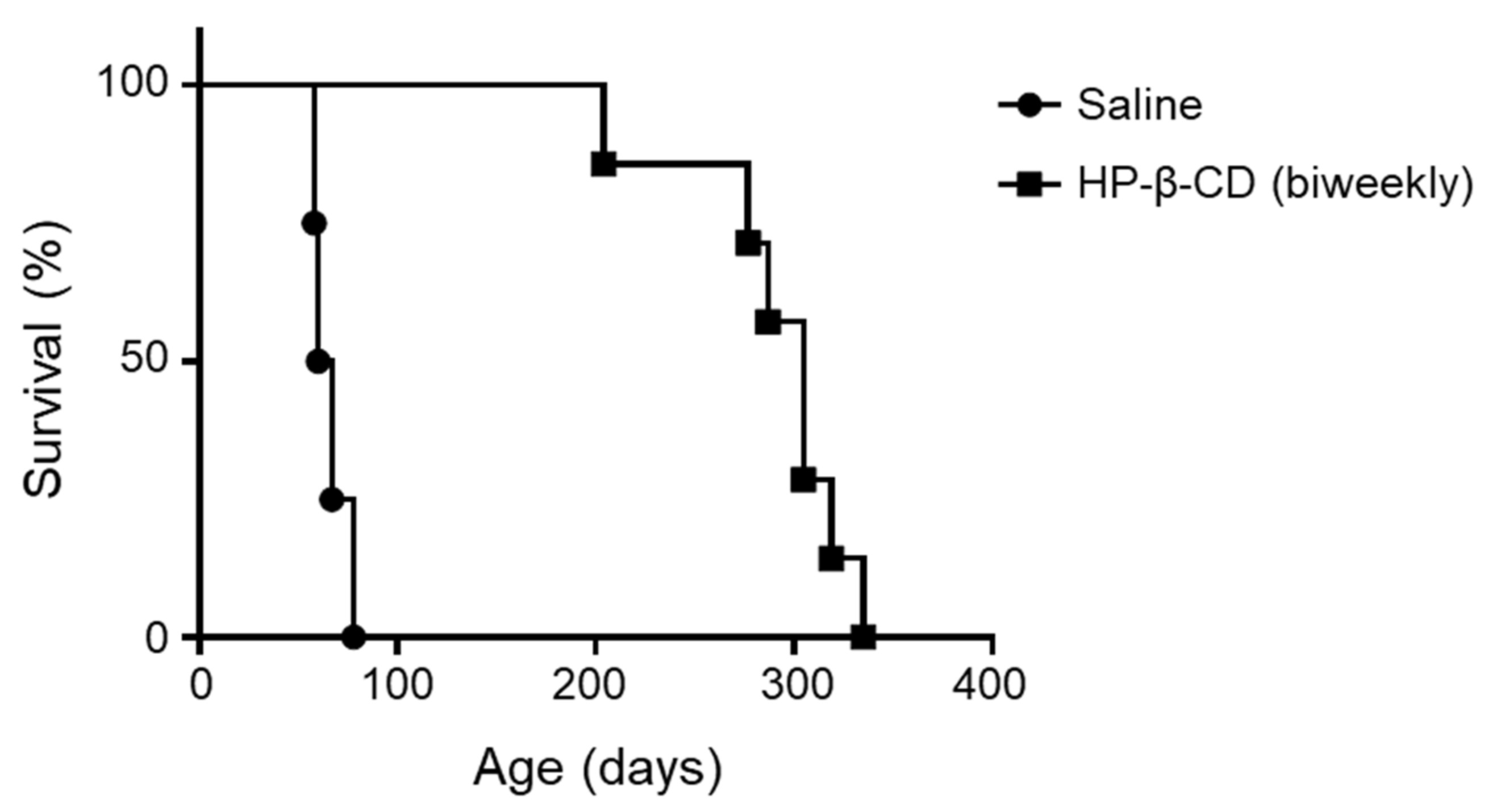
2.5. Repeated Doses of Intracerebroventricular HP-β-CD Drastically Prolonged the Lifespan of Npc1-Deficient Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Experiments
4.2.1. Intracerebroventricular Administration of HP-β-CD in Npc1-Deficient Mice
4.2.2. Evaluation of Purkinje Cell Loss in the Cerebellum of Npc1-Deficient Mice
4.2.3. Quantification of GPNMB Levels of Npc1-Deficient Mice
4.2.4. Evaluation of Hepatic Changes in Npc1-Deficient Mice
4.2.5. Evaluation of Lifespan in Npc1-Deficient Mice
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPC | Niemann–Pick disease type C |
| HP-β-CD | 2-Hydroxypropyl-β-cyclodextrin |
| WT | Wild type |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| H&E | Hematoxylin & eosin |
| IU/L | International unit per liter |
References
- Vanier, M.T. Niemann-Pick Disease Type C. Orphanet J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, A.I.; Maxfield, F.R. Niemann-Pick Type C Disease: Molecular Mechanisms and Potential Therapeutic Approaches: Therapeutics for NPC Disease. J. Neurochem. 2011, 116, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Peake, K.B.; Vance, J.E. Normalization of Cholesterol Homeostasis by 2-Hydroxypropyl-β-Cyclodextrin in Neurons and Glia from Niemann-Pick C1 (NPC1)-Deficient Mice. J. Biol. Chem. 2012, 287, 9290–9298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of Defective Lysosomal Transport in NPC Disease Ameliorates Liver Dysfunction and Neurodegeneration in the Npc1−/− Mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic Cyclodextrin Treatment of Murine Niemann-Pick C Disease Ameliorates Neuronal Cholesterol and Glycosphingolipid Storage and Disease Progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, C.M.; Liu, B.; Taylor, A.M.; Repa, J.J.; Burns, D.K.; Weinberg, A.G.; Turley, S.D.; Dietschy, J.M. Weekly Cyclodextrin Administration Normalizes Cholesterol Metabolism in Nearly Every Organ of the Niemann-Pick Type C1 Mouse and Markedly Prolongs Life. Pediatr. Res. 2010, 68, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-Hydroxypropyl-β-Cyclodextrin in Niemann–Pick Disease Type C Model Mice and Its Pharmacokinetic Analysis in a Patient with the Disease. Biol. Pharm. Bull. 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, M.; Togawa, M.; Hirabaru, K.; Mochinaga, S.; Narita, A.; Adachi, M.; Egashira, M.; Irie, T.; Ohno, K. Effects of Cyclodextrin in Two Patients with Niemann–Pick Type C Disease. Mol. Genet. Metab. 2013, 108, 76–81. [Google Scholar] [CrossRef]
- Matsuo, M.; Shraishi, K.; Wada, K.; Ishitsuka, Y.; Doi, H.; Maeda, M.; Mizoguchi, T.; Eto, J.; Mochinaga, S.; Arima, H.; et al. Effects of Intracerebroventricular Administration of 2-Hydroxypropyl-β-Cyclodextrin in a Patient with Niemann–Pick Type C Disease. Mol. Genet. Metab. Rep. 2014, 1, 391–400. [Google Scholar] [CrossRef]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-Hydroxypropyl-β-Cyclodextrin Decreases Neurological Disease Progression in Niemann-Pick Disease, Type C1: A Non-Randomised, Open-Label, Phase 1–2 Trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef] [Green Version]
- Van der Lienden, M.; Gaspar, P.; Boot, R.; Aerts, J.; van Eijk, M. Glycoprotein Non-Metastatic Protein B: An Emerging Biomarker for Lysosomal Dysfunction in Macrophages. Int. J. Mol. Sci. 2018, 20, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.S.; Getz, M.; Safeukui, I.; Yi, S.; Tamez, P.; Shin, J.; Velázquez, P.; Haldar, K. Genomic Expression Analyses Reveal Lysosomal, Innate Immunity Proteins, as Disease Correlates in Murine Models of a Lysosomal Storage Disorder. PLoS ONE 2012, 7, e48273. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.R.A.; Gabriel, T.L.; Aten, J.; van Roomen, C.P.A.A.; Ottenhoff, R.; Claessen, N.; Alfonso, P.; Irún, P.; Giraldo, P.; Aerts, J.M.F.G.; et al. Gpnmb Is a Potential Marker for the Visceral Pathology in Niemann-Pick Type C Disease. PLoS ONE 2016, 11, e0147208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarna, J.R.; Larouche, M.; Marzban, H.; Sillitoe, R.V.; Rancourt, D.E.; Hawkes, R. Patterned Purkinje Cell Degeneration in Mouse Models of Niemann-Pick Type C Disease. J. Comp. Neurol. 2003, 456, 279–291. [Google Scholar] [CrossRef]
- Elrick, M.J.; Pacheco, C.D.; Yu, T.; Dadgar, N.; Shakkottai, V.G.; Ware, C.; Paulson, H.L.; Lieberman, A.P. Conditional Niemann-Pick C Mice Demonstrate Cell Autonomous Purkinje Cell Neurodegeneration. Hum. Mol. Genet. 2010, 19, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Vite, C.H.; Bagel, J.H.; Swain, G.P.; Prociuk, M.; Sikora, T.U.; Stein, V.M.; O’Donnell, P.; Ruane, T.; Ward, S.; Crooks, A.; et al. Intracisternal Cyclodextrin Prevents Cerebellar Dysfunction and Purkinje Cell Death in Feline Niemann-Pick Type C1 Disease. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Aqul, A.; Liu, B.; Ramirez, C.M.; Pieper, A.A.; Estill, S.J.; Burns, D.K.; Liu, B.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Unesterified Cholesterol Accumulation in Late Endosomes/Lysosomes Causes Neurodegeneration and Is Prevented by Driving Cholesterol Export from This Compartment. J. Neurosci. 2011, 31, 9404–9413. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Wegdam, W.; Donker-Koopman, W.; Ottenhoff, R.; Gaspar, P.; Verhoek, M.; Nelson, J.; Gabriel, T.; Kallemeijn, W.; Boot, R.G.; et al. Elevation of Glycoprotein Nonmetastatic Melanoma Protein B in Type 1 Gaucher Disease Patients and Mouse Models. FEBS Open Biol. 2016, 6, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jullg, M.; Middleditch, M.J.; Cooper, G.J.S. Modelling Atherosclerosis by Proteomics: Molecular Changes in the Ascending Aortas of Cholesterol-Fed Rabbits. Atherosclerosis 2015, 242, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Katayama, A.; Nakatsuka, A.; Eguchi, J.; Murakami, K.; Teshigawara, S.; Kanzaki, M.; Nunoue, T.; Hida, K.; Wada, N.; Yasunaka, T.; et al. Beneficial Impact of Gpnmb and Its Significance as a Biomarker in Nonalcoholic Steatohepatitis. Sci. Rep. 2015, 5, 16920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, K.; Tabu, K.; Sasaki, F.; Takami, Y.; Morinaga, Y.; Mawatari, S.; Hashimoto, S.; Tanoue, S.; Kanmura, S.; Tamai, T.; et al. Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 2015, 10, e0143413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, T.L.; Tol, M.J.; Ottenhof, R.; van Roomen, C.; Aten, J.; Claessen, N.; Hooibrink, B.; de Weijer, B.; Serlie, M.J.; Argmann, C.; et al. Lysosomal Stress in Obese Adipose Tissue Macrophages Contributes to MITF-Dependent Gpnmb Induction. Diabetes 2014, 63, 3310–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irie, T.; Fukunaga, K.; Pitha, J. Hydroxypropylcyclodextrins in Parenteral Use. I: Lipid Dissolution and Effects on Lipid Transfers in Vitro. J. Pharm. Sci. 1992, 81, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.; Kondo, Y.; Sakata, N.; Yamada, Y.; Fukaura, M.; Higashi, T.; Motoyama, K.; Arima, H.; Higaki, K.; Hayashi, A.; et al. Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells. Int. J. Mol. Sci. 2020, 21, 898. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.R.A.; Aten, J.; Ottenhoff, R.; van Roomen, C.P.A.A.; Herrera Moro, D.; Claessen, N.; Vinueza Veloz, M.F.; Zhou, K.; Lin, Z.; Mirzaian, M.; et al. Reducing GBA2 Activity Ameliorates Neuropathology in Niemann-Pick Type C Mice. PLoS ONE 2015, 10, e0135889. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukaura, M.; Ishitsuka, Y.; Shirakawa, S.; Ushihama, N.; Yamada, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; Motoyama, K.; Higashi, T.; et al. Intracerebroventricular Treatment with 2-Hydroxypropyl-β-Cyclodextrin Decreased Cerebellar and Hepatic Glycoprotein Nonmetastatic Melanoma Protein B (GPNMB) Expression in Niemann–Pick Disease Type C Model Mice. Int. J. Mol. Sci. 2021, 22, 452. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010452
Fukaura M, Ishitsuka Y, Shirakawa S, Ushihama N, Yamada Y, Kondo Y, Takeo T, Nakagata N, Motoyama K, Higashi T, et al. Intracerebroventricular Treatment with 2-Hydroxypropyl-β-Cyclodextrin Decreased Cerebellar and Hepatic Glycoprotein Nonmetastatic Melanoma Protein B (GPNMB) Expression in Niemann–Pick Disease Type C Model Mice. International Journal of Molecular Sciences. 2021; 22(1):452. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010452
Chicago/Turabian StyleFukaura, Madoka, Yoichi Ishitsuka, Seiichi Shirakawa, Naoki Ushihama, Yusei Yamada, Yuki Kondo, Toru Takeo, Naomi Nakagata, Keiichi Motoyama, Taishi Higashi, and et al. 2021. "Intracerebroventricular Treatment with 2-Hydroxypropyl-β-Cyclodextrin Decreased Cerebellar and Hepatic Glycoprotein Nonmetastatic Melanoma Protein B (GPNMB) Expression in Niemann–Pick Disease Type C Model Mice" International Journal of Molecular Sciences 22, no. 1: 452. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010452





