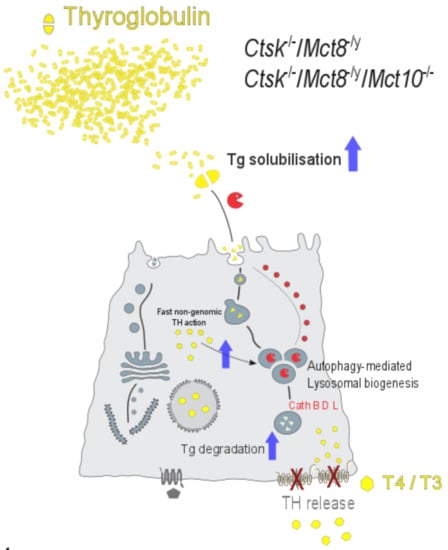
The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy
Abstract
:1. Introduction
2. Results
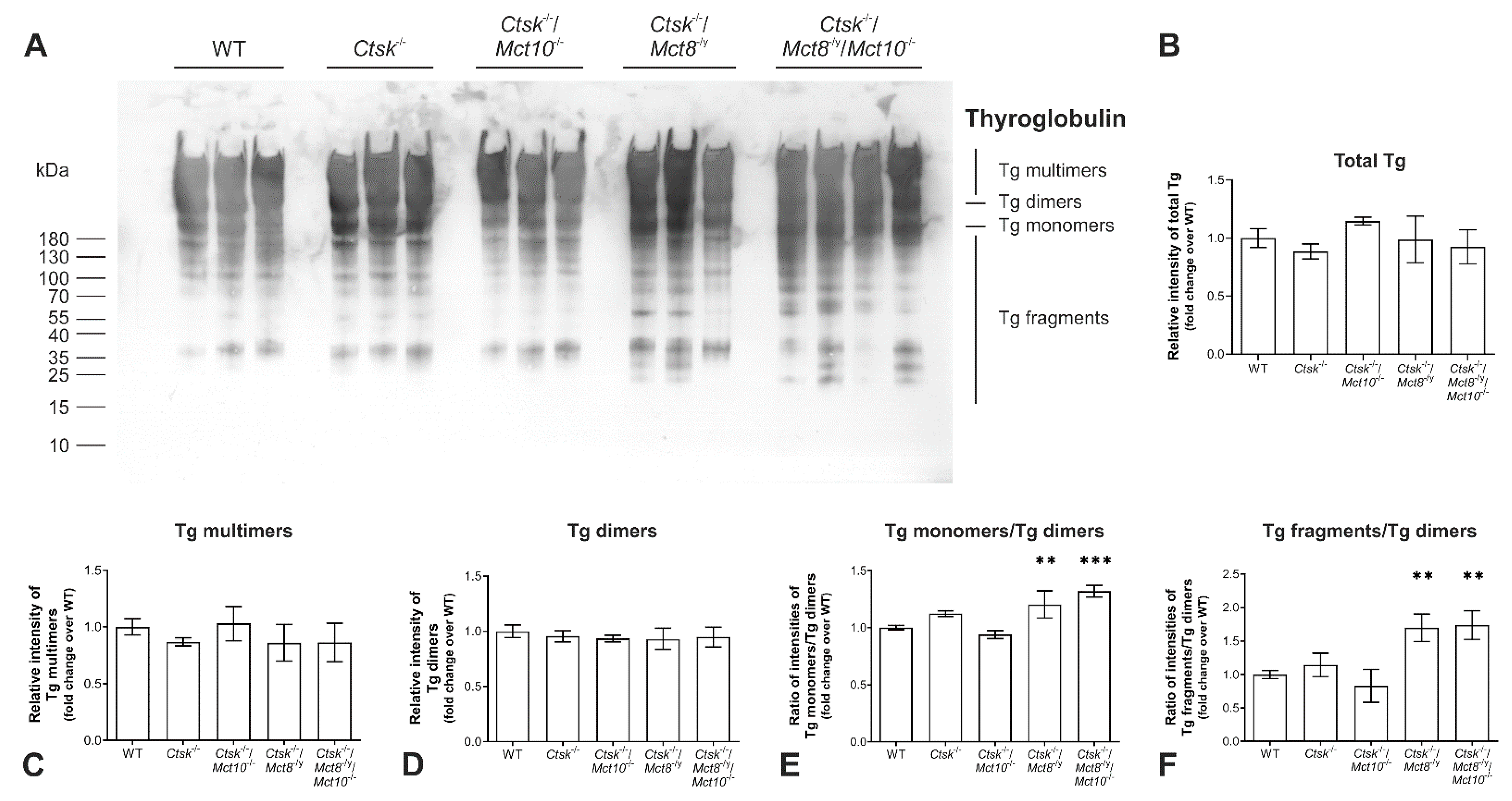
2.1. Tg Storage Status Is Altered in Combined Cathepsin K and TH Transporter Deficiency
2.2. Tg Degradation Remains Normal upon Cathepsin K Deficiency, but Is Enhanced in Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Mice
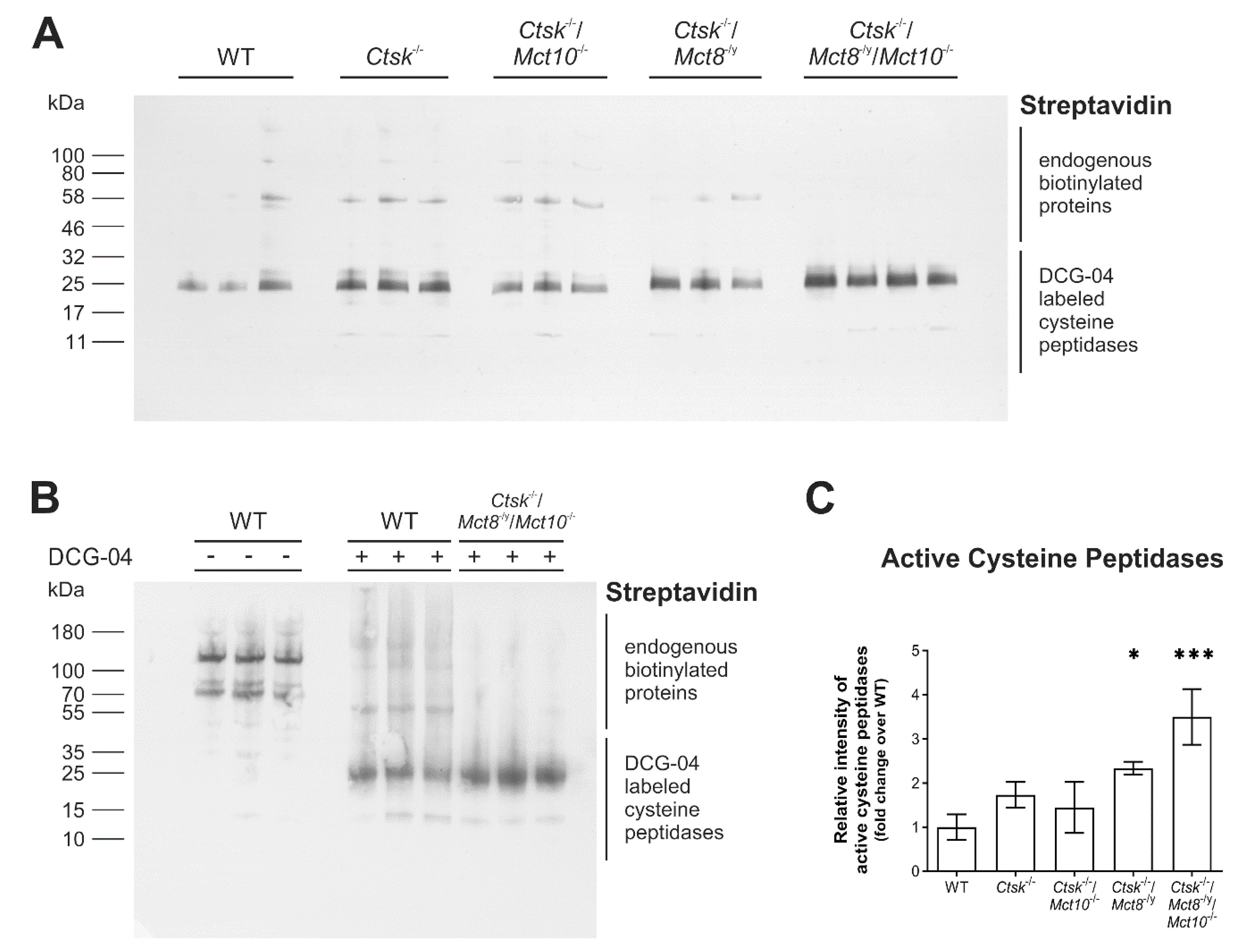
2.3. Proteolytic Activities of Cysteine Peptidases Are Enhanced in Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Mice
2.4. Increased Proteolytic Activity of Cysteine Peptidases Is Not Due to an Imbalance of Proteolytic and Anti-Proteolytic Factors
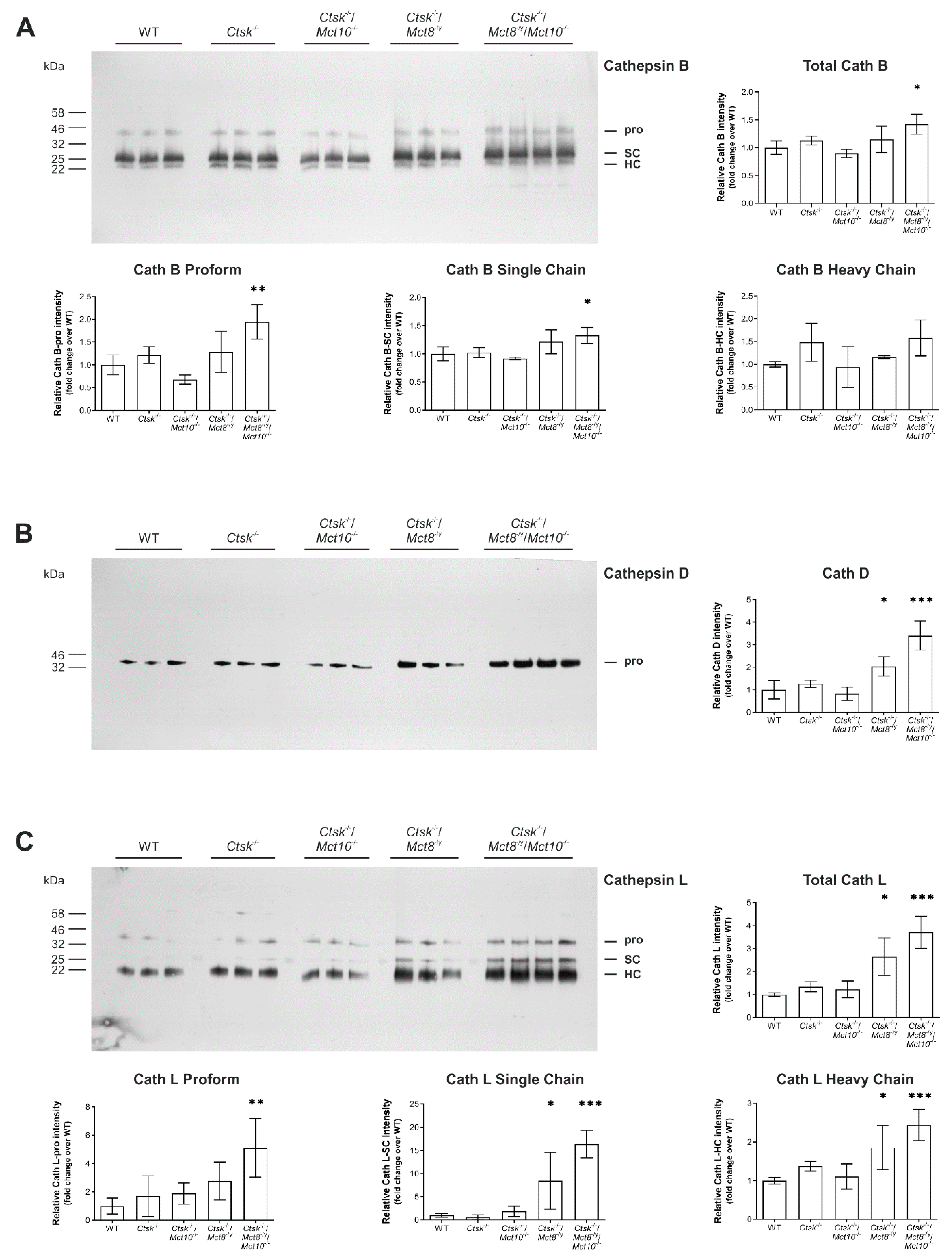
2.5. Processing States of Tg-Processing Cathepsins Remain Unaltered in Thyroid Tissue of Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Mice
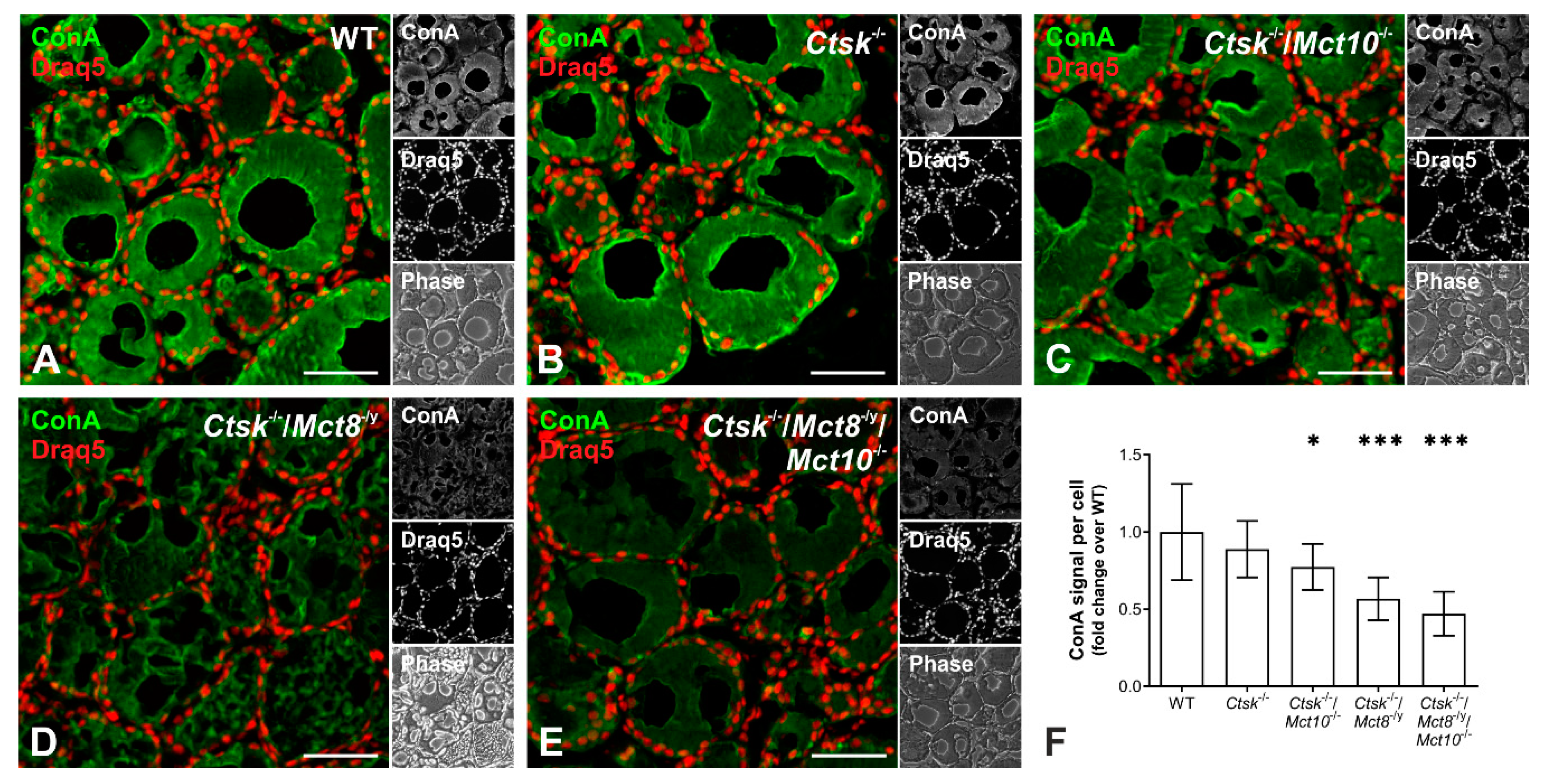
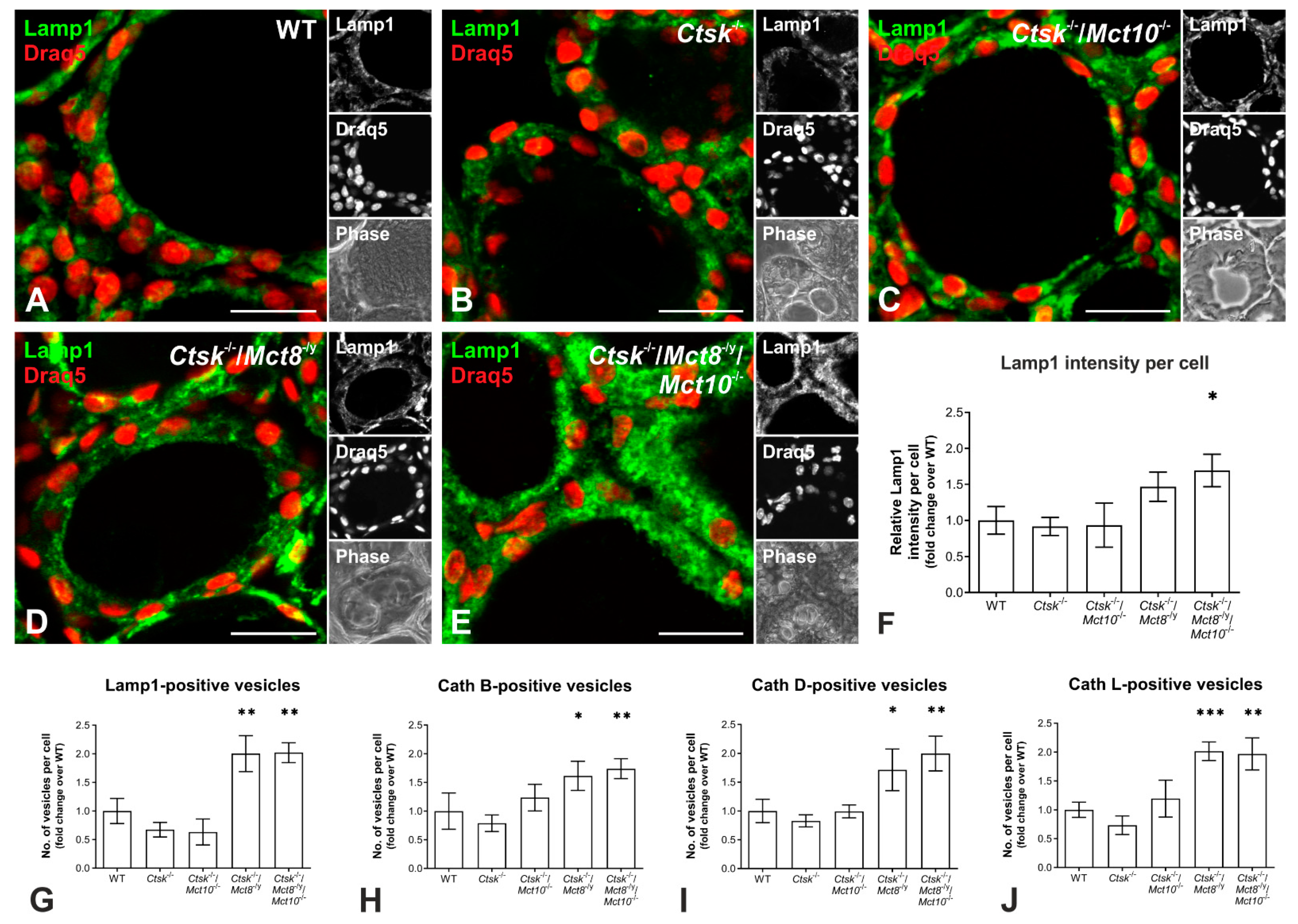
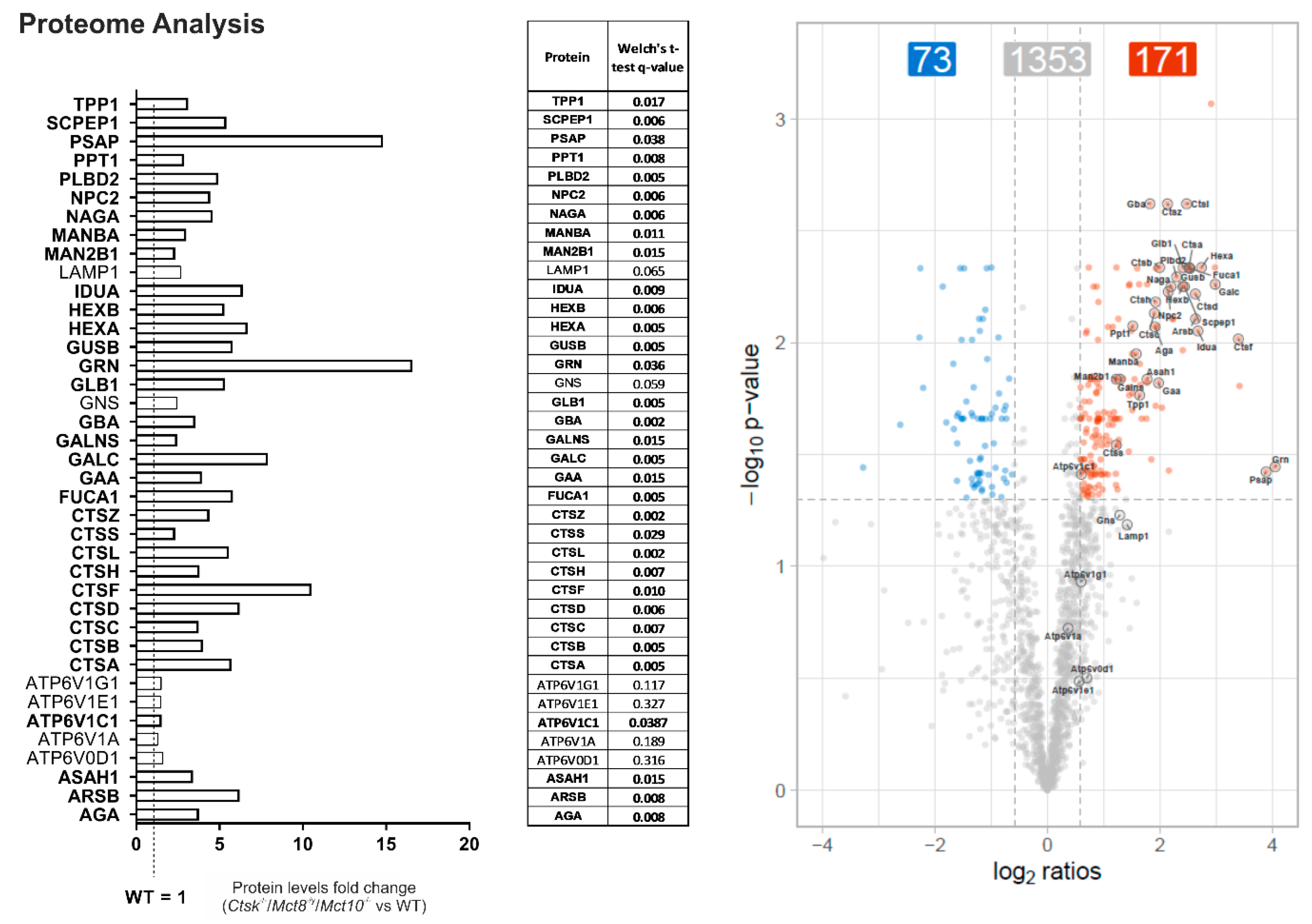
2.6. Enhanced Lysosomal Biogenesis Results in Higher Cathepsin Protein Amounts in Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Mice
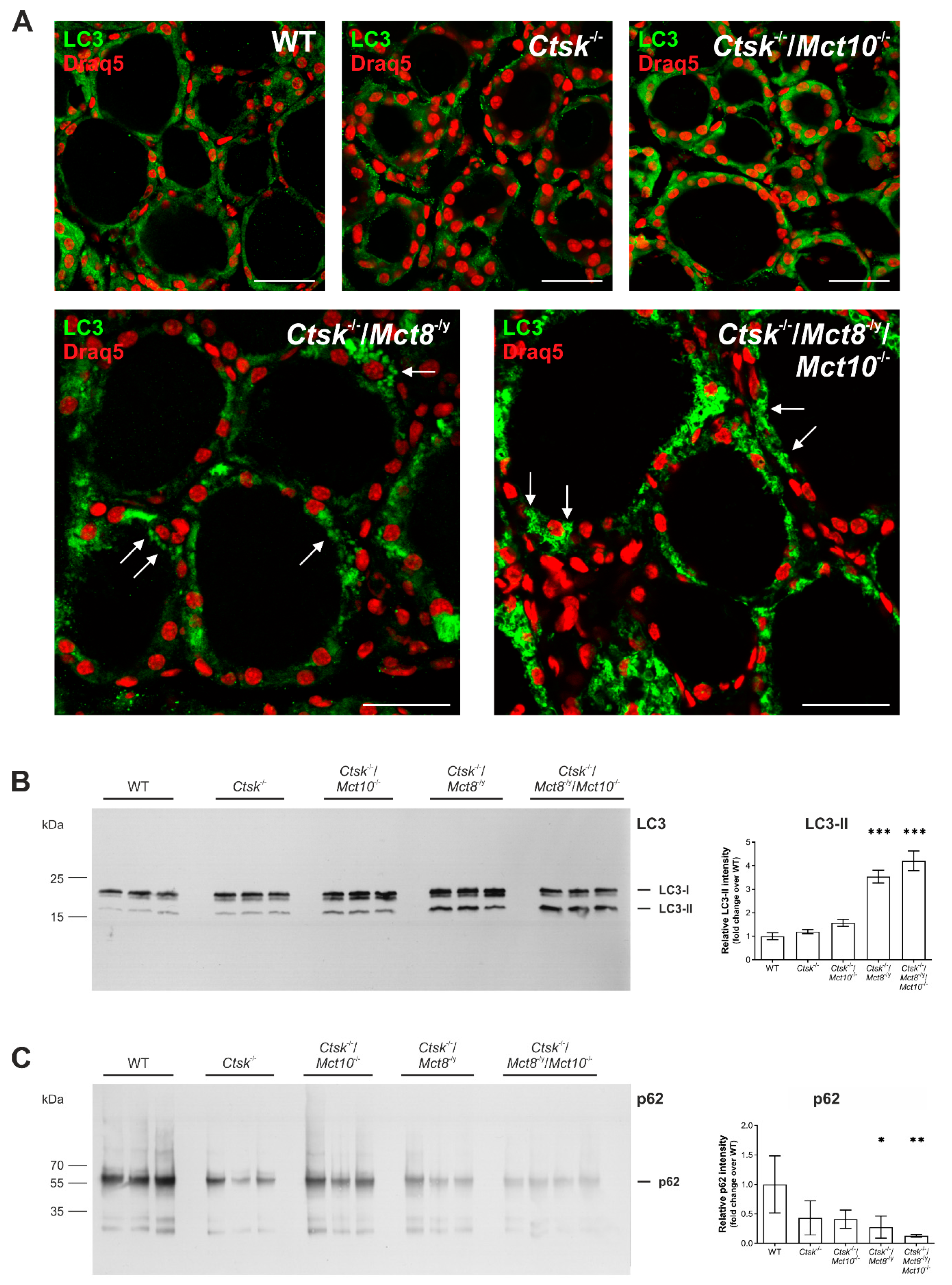
2.7. Autophagy Is Induced in the Thyroid Glands of Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Mice
2.8. Intrathyroidal Iodine Levels Are Enhanced in Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Mice
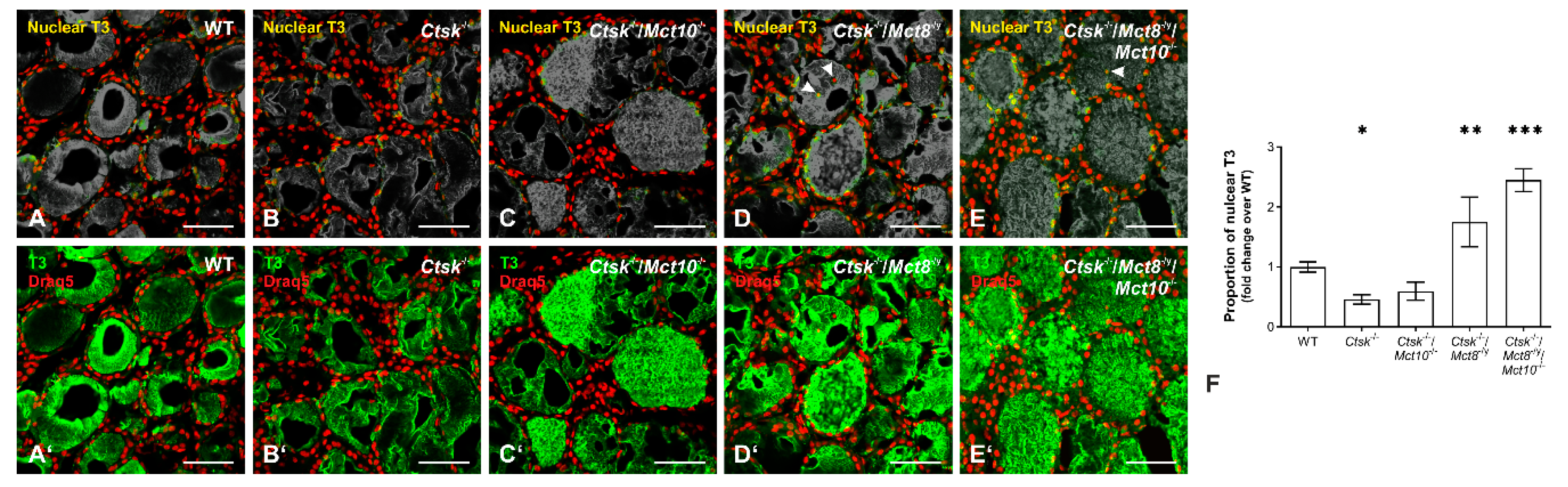
2.9. Accumulation of T3 in the Nuclei of Ctsk-/-/Mct8-/y and Ctsk-/-/Mct8-/y/Mct10-/- Thyrocytes
3. Discussion
3.1. Cathepsin K Deficient Mice Are a Suitable Model to Explore Auto-Regulatory Mechanisms of Thyroid Gland Function
3.2. Thyroid Gland Functionality Is Assessed Regarding TH Synthesis, TH Liberation and TH Release from Thyroid Follicles
3.3. Lysosomal Biogenesis via the Induction of Autophagy Is the Cause for the Unusual Intrathyroidal Hyperthyroid States Observed upon Combined Cathepsin K and Mct8 Deficiency
3.4. Perspectives in Light of Fast, Non-Genomic TH Actions
4. Materials and Methods
4.1. Animals
4.2. Tissue Sampling and Cryosectioning
4.3. Indirect Immunofluorescence
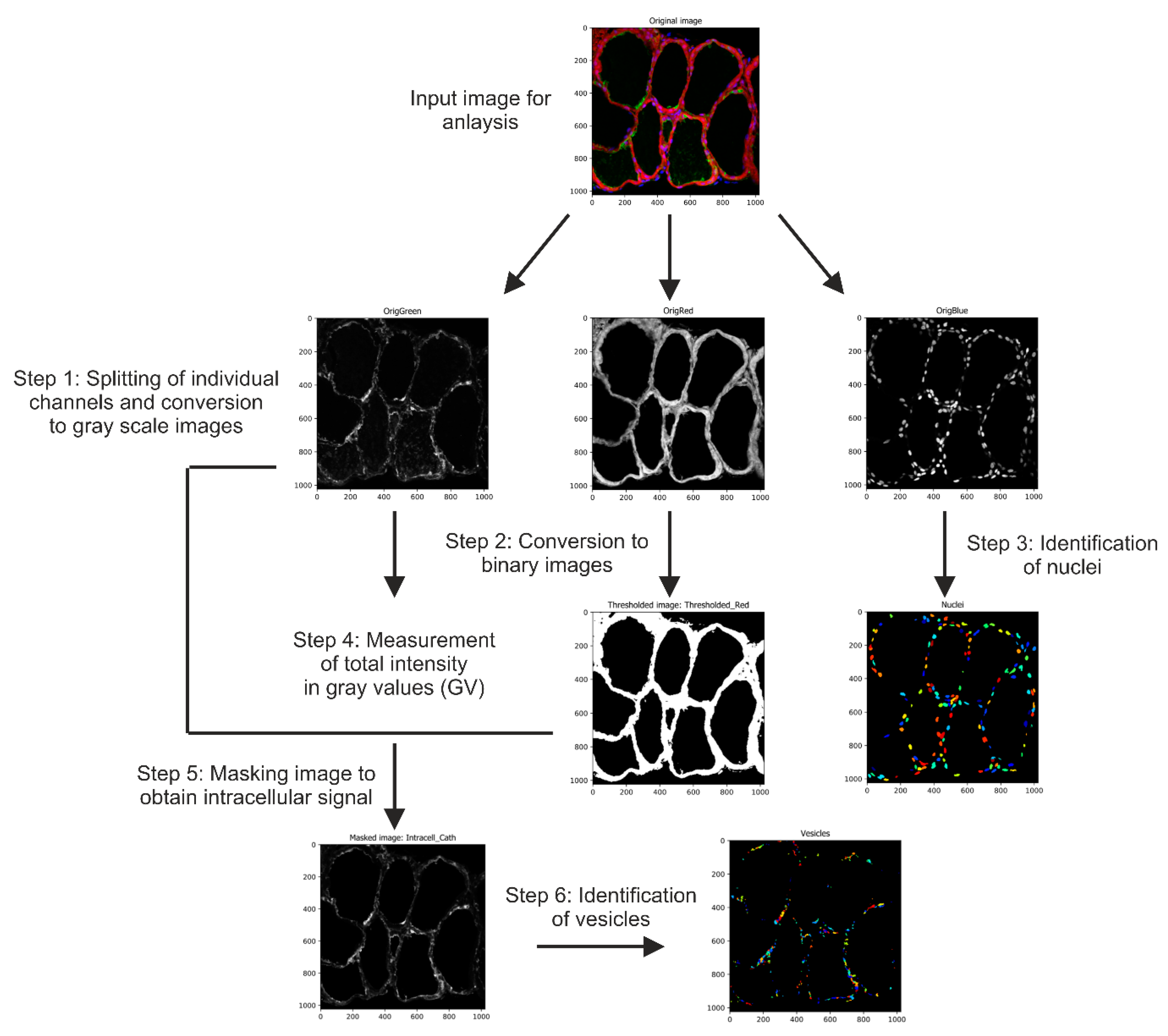
4.4. Image Acquisition and Automated Image Analysis
4.5. Tissue Lysate Preparation, SDS-PAGE and Immunoblotting
4.6. Iodine Quantification in Thyroid Tissue Lysates
2 Ce4+ + 2 I− → 2 Ce3+ + I2
4.7. Omics Analyses
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFU | Angio-follicular units |
| AMPK | AMP-activated protein kinase |
| BSA | bovine serum albumin |
| CLEAR | Coordinated Lysosomal Expression and Regulation |
| CMF-PBS | calcium- and magnesium-free PBS |
| DDA | data dependent acquisition |
| DUOX | dual oxidase |
| DUOXA | dual oxidase activation maturation factor |
| ER | Endoplasmic reticulum |
| HCD | higher energy collisional dissociation |
| HPT | hypothalamus pituitary thyroid |
| LC3 | microtubule-associated protein 1A/1B-light chain 3 |
| LFQ | label-free quantification |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NIS | sodium-iodide symporter |
| PBS-T | PBS containing 0.3% Tween-20 |
| ROS | reactive oxygen species |
| SDS | sodium dodecyl sulfate |
| SK | Sandell-Kolthoff |
| T3 | 3′,3,5 triiodothyronine |
| T4 | 3,3′,5,5′ tetraiodo-L-thyronine |
| TFEB | transcription factor EB |
| Tg | thyroglobulin |
| TH | thyroid hormones |
| TPO | thyroid peroxidase |
| TRH | thyrotropin-releasing hormone |
| TSH | thyroid stimulating hormone |
| ULK1 | Unc-51 related kinase |
| WT | wild-type |
References
- Fujita, H. Functional Morphology of the Thyroid. In International Review of Cytology; Jeon, K.W., Friedlander, M., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 113, pp. 145–185. [Google Scholar]
- Nilsson, M.; Fagman, H. Mechanisms of thyroid development and dysgenesis: An analysis based on developmental stages and concurrent embryonic anatomy. Curr. Top. Dev. Biol. 2013, 106, 123–170. [Google Scholar] [CrossRef]
- Brix, K.; Qatato, M.; Szumska, J.; Venugopalan, V.; Rehders, M. Thyroglobulin Storage, Processing and Degradation for Thyroid Hormone Liberation. In The Thyroid and Its Diseases; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–48. [Google Scholar] [CrossRef]
- Colin, I.M.; Denef, J.F.; Lengelé, B.; Many, M.C.; Gérard, A.C. Recent insights into the cell biology of thyroid angiofollicular units. Endocr. Rev. 2013, 34, 209–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fliers, E.; Kalsbeek, A.; Boelen, A. Beyond the fixed setpoint of the hypothalamus-pituitary-thyroid axis. Eur. J. Endocrinol. 2014, 171, R197–R208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Linke, M.; Tepel, C.; Herzog, V. Cysteine proteinases mediate extracellular prohormone processing in the thyroid. Biol. Chem. 2001, 382, 717–725. [Google Scholar] [CrossRef]
- Linke, M.; Jordans, S.; Mach, L.; Herzog, V.; Brix, K. Thyroid stimulating hormone upregulates secretion of cathepsin B from thyroid epithelial cells. Biol. Chem. 2002, 383, 773–784. [Google Scholar] [CrossRef]
- Brix, K.; Szumska, J.; Weber, J.; Qatato, M.; Venugopalan, V.; Al-Hashimi, A.; Rehders, M. Auto-Regulation of the Thyroid Gland Beyond Classical Pathways. Exp. Clin. Endocrinol. Diabetes Off. J. German Soc. Endocrinol. German Diabetes Assoc. 2020, 128, 437–445. [Google Scholar] [CrossRef]
- Friesema, E.C.; Docter, R.; Moerings, E.P.; Stieger, B.; Hagenbuch, B.; Meier, P.J.; Krenning, E.P.; Hennemann, G.; Visser, T.J. Identification of thyroid hormone transporters. Biochem. Biophys. Res. Commun. 1999, 254, 497–501. [Google Scholar] [CrossRef]
- Di Cosmo, C.; Liao, X.H.; Dumitrescu, A.M.; Philp, N.J.; Weiss, R.E.; Refetoff, S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J. Clin. Investig. 2010, 120, 3377–3388. [Google Scholar] [CrossRef] [Green Version]
- Mariotta, L.; Ramadan, T.; Singer, D.; Guetg, A.; Herzog, B.; Stoeger, C.; Palacín, M.; Lahoutte, T.; Camargo, S.M.; Verrey, F. T-type amino acid transporter TAT1 (Slc16a10) is essential for extracellular aromatic amino acid homeostasis control. J. Physiol. 2012, 590, 6413–6424. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Mayerl, S.; Visser, T.J.; Darras, V.M.; Boelen, A.; Frappart, L.; Mariotta, L.; Verrey, F.; Heuer, H. Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology 2014, 155, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; van Geest, F.S.; Peeters, R.P.; Heuer, H.; Visser, W.E. Thyroid Hormone Transporters. Endocr. Rev. 2020, 41, 146–201. [Google Scholar] [CrossRef] [PubMed]
- Führer, D.; Brix, K.; Biebermann, H. Understanding the Healthy Thyroid State in 2015. Eur. Thyroid J. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Tseleni-Balafouta, S. Classification of Thyroid Diseases. In The Thyroid and Its Diseases; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 87–99. [Google Scholar] [CrossRef]
- Weber, J.; McInnes, J.; Kizilirmak, C.; Rehders, M.; Qatato, M.; Wirth, E.K.; Schweizer, U.; Verrey, F.; Heuer, H.; Brix, K. Interdependence of thyroglobulin processing and thyroid hormone export in the mouse thyroid gland. Eur. J. Cell Biol. 2017, 96, 440–456. [Google Scholar] [CrossRef] [Green Version]
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Dauth, S.; Rakov, H.; Sîrbulescu, R.F.; Ilieş, I.; Weber, J.; Batbajar Dugershaw, B.; Braun, D.; Rehders, M.; Wirth, E.K.; Führer, D.; et al. Function of Cathepsin K in the Central Nervous System of Male Mice is Independent of Its Role in the Thyroid Gland. Cell. Mol. Neurobiol. 2020, 40, 695–710. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [Green Version]
- Visser, W.E.; Friesema, E.C.; Visser, T.J. Minireview: Thyroid hormone transporters: The knowns and the unknowns. Mol. Endocrinol. 2011, 25, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rakov, H.; Engels, K.; Hönes, G.S.; Brix, K.; Köhrle, J.; Moeller, L.C.; Zwanziger, D.; Führer, D. Sex-specific phenotypes of hyperthyroidism and hypothyroidism in aged mice. Biol. Sex Differ. 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Alavez, M.; Alboni, S.; Conti, B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age 2011, 33, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salveridou, E.; Mayerl, S.; Sundaram, S.M.; Markova, B.; Heuer, H. Tissue-Specific Function of Thyroid Hormone Transporters: New Insights from Mouse Models. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2020, 128, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Herzog, V.; Berndorfer, U.; Saber, Y. Isolation of insoluble secretory product from bovine thyroid: Extracellular storage of thyroglobulin in covalently cross-linked form. J. Cell Biol. 1992, 118, 1071–1083. [Google Scholar] [CrossRef]
- Berndorfer, U.; Wilms, H.; Herzog, V. Multimerization of thyroglobulin (TG) during extracellular storage: Isolation of highly cross-linked TG from human thyroids. J. Clin. Endocrinol. Metab. 1996, 81, 1918–1926. [Google Scholar] [CrossRef] [Green Version]
- Saber-Lichtenberg, Y.; Brix, K.; Schmitz, A.; Heuser, J.E.; Wilson, J.H.; Lorand, L.; Herzog, V. Covalent cross-linking of secreted bovine thyroglobulin by transglutaminase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 1005–1014. [Google Scholar] [CrossRef]
- Desruisseau, S.; Franc, J.L.; Gruffat, D.; Chabaud, O. Glycosylation of thyroglobulin secreted by porcine cells cultured in chamber system: Thyrotropin controls the number of oligosaccharides and their anionic residues. Endocrinology 1994, 134, 1676–1684. [Google Scholar] [CrossRef]
- Yang, S.X.; Pollock, H.G.; Rawitch, A.B. Glycosylation in human thyroglobulin: Location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin. Arch. Biochem. Biophys. 1996, 327, 61–70. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Reichert, C.M.; Misaki, A. Interaction of concanavalin A with model substrates. Ann. N. Y. Acad. Sci. 1974, 234, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Qatato, M.; Szumska, J.; Skripnik, V.; Rijntjes, E.; Köhrle, J.; Brix, K. Canonical TSH Regulation of Cathepsin-Mediated Thyroglobulin Processing in the Thyroid Gland of Male Mice Requires Taar1 Expression. Front. Pharmacol. 2018, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; McInnes, J.; Al-Hashimi, A.; Rehders, M.; Tamhane, T.; Haugen, M.H. Proteolysis mediated by cysteine cathepsins and legumain-recent advances and cell biological challenges. Protoplasma 2015, 252, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saftig, P. Lysosomal Membrane Proteins. In Lysosomes; Saftig, P., Ed.; Springer: Boston, MA, USA, 2005; pp. 37–49. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, W.W.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, E.K.; Sheu, S.Y.; Chiu-Ugalde, J.; Sapin, R.; Klein, M.O.; Mossbrugger, I.; Quintanilla-Martinez, L.; de Angelis, M.H.; Krude, H.; Riebel, T.; et al. Monocarboxylate transporter 8 deficiency: Altered thyroid morphology and persistent high triiodothyronine/thyroxine ratio after thyroidectomy. Eur. J. Endocrinol. 2011, 165, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Dumitrescu, A.M.; Liao, X.H.; Weiss, R.E.; Millen, K.; Refetoff, S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 2006, 147, 4036–4043. [Google Scholar] [CrossRef] [Green Version]
- Trajkovic, M.; Visser, T.J.; Mittag, J.; Horn, S.; Lukas, J.; Darras, V.M.; Raivich, G.; Bauer, K.; Heuer, H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Investig. 2007, 117, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Heuer, H.; Visser, T.J. The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models. Biochim. Biophys. Acta 2013, 1830, 3974–3978. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Families of cysteine peptidases. Methods Enzymol. 1994, 244, 461–486. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Riese, R.J.; Shi, G.P. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 1997, 59, 63–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.D.; Crutchfield, H.E.; Dunn, J.T. Thyroglobulin processing by thyroidal proteases. Major sites of cleavage by cathepsins B, D, and L. J. Biol. Chem. 1991, 266, 20198–20204. [Google Scholar] [PubMed]
- Brix, K.; Lemansky, P.; Herzog, V. Evidence for extracellularly acting cathepsins mediating thyroid hormone liberation in thyroid epithelial cells. Endocrinology 1996, 137, 1963–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepel, C.; Brömme, D.; Herzog, V.; Brix, K. Cathepsin K in thyroid epithelial cells: Sequence, localization and possible function in extracellular proteolysis of thyroglobulin. J. Cell Sci. 2000, 113 Pt 24, 4487–4498. [Google Scholar]
- Jordans, S.; Jenko-Kokalj, S.; Kühl, N.M.; Tedelind, S.; Sendt, W.; Brömme, D.; Turk, D.; Brix, K. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 2009, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Fekete, C.; Lechan, R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, M.E.G.; Liebscher, G.; Hess, M.W.; Huber, L.A. Lysosomal size matters. Traffic 2020, 21, 60–75. [Google Scholar] [CrossRef] [Green Version]
- Phillips, I.D.; Black, E.G.; Sheppard, M.C.; Docherty, K. Thyrotrophin, forskolin and ionomycin increase cathepsin B mRNA concentrations in rat thyroid cells in culture. J. Mol. Endocrinol. 1989, 2, 207–212. [Google Scholar] [CrossRef]
- Petanceska, S.; Devi, L. Sequence analysis, tissue distribution, and expression of rat cathepsin S. J. Biol. Chem. 1992, 267, 26038–26043. [Google Scholar] [CrossRef]
- Coscia, F.; Taler-Verčič, A.; Chang, V.T.; Sinn, L.; O’Reilly, F.J.; Izoré, T.; Renko, M.; Berger, I.; Rappsilber, J.; Turk, D.; et al. The structure of human thyroglobulin. Nature 2020, 578, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M. Iodide handling by the thyroid epithelial cell. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2001, 109, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Spitzweg, C.; Morris, J.C. Genetics and phenomics of hypothyroidism and goiter due to NIS mutations. Mol. Cell. Endocrinol. 2010, 322, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Kopp, P.; Pesce, L.; Solis, S.J. Pendred syndrome and iodide transport in the thyroid. Trends Endocrinol. Metab. TEM 2008, 19, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.C.; Kopp, P.A. Pendrin and anoctamin as mediators of apical iodide efflux in thyroid cells. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Suzuki, A.; Kimura, T.; Tateyama, M.; Tanaka, T.; Yazawa, T.; Arimasu, Y.; Chen, I.S.; Aoyama, K.; Kubo, Y.; et al. Congenital goitrous hypothyroidism is caused by dysfunction of the iodide transporter SLC26A7. Commun. Biol. 2019, 2, 270. [Google Scholar] [CrossRef] [Green Version]
- Ohtaki, S.; Nakagawa, H.; Nakamura, M.; Kotani, T. Thyroid peroxidase: Experimental and clinical integration. Endocr. J. 1996, 43, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ruf, J.; Carayon, P. Structural and functional aspects of thyroid peroxidase. Arch. Biochem. Biophys. 2006, 445, 269–277. [Google Scholar] [CrossRef]
- Morand, S.; Ueyama, T.; Tsujibe, S.; Saito, N.; Korzeniowska, A.; Leto, T.L. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1205–1218. [Google Scholar] [CrossRef] [Green Version]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A gene network regulating lysosomal biogenesis and function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell. Mol. Life Sci. CMLS 2006, 63, 414–434. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, R.A.; Singh, B.K.; Zhou, J.; Wu, Y.; Farah, B.L.; Ohba, K.; Lesmana, R.; Gooding, J.; Bay, B.H.; Yen, P.M. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 2015, 11, 1341–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesmana, R.; Sinha, R.A.; Singh, B.K.; Zhou, J.; Ohba, K.; Wu, Y.; Yau, W.W.; Bay, B.H.; Yen, P.M. Thyroid Hormone Stimulation of Autophagy Is Essential for Mitochondrial Biogenesis and Activity in Skeletal Muscle. Endocrinology 2016, 157, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.S.; Arvan, P. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: Disorders of protein trafficking and the role of ER molecular chaperones. Endocr. Rev. 1998, 19, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Di Jeso, B.; Arvan, P. Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr. Rev. 2016, 37, 2–36. [Google Scholar] [CrossRef] [Green Version]
- Hönes, G.S.; Rakov, H.; Logan, J.; Liao, X.H.; Werbenko, E.; Pollard, A.S.; Præstholm, S.M.; Siersbæk, M.S.; Rijntjes, E.; Gassen, J.; et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E11323–E11332. [Google Scholar] [CrossRef] [Green Version]
- Saelim, N.; John, L.M.; Wu, J.; Park, J.S.; Bai, Y.; Camacho, P.; Lechleiter, J.D. Nontranscriptional modulation of intracellular Ca2+ signaling by ligand stimulated thyroid hormone receptor. J. Cell Biol. 2004, 167, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, M.; Kambe, F.; Cao, X.; Lu, X.; Kozaki, Y.; Oiso, Y.; Seo, H. Thyroid hormone activates adenosine 5’-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-beta. Mol. Endocrinol. 2008, 22, 893–903. [Google Scholar] [CrossRef] [Green Version]
- D’Arezzo, S.; Incerpi, S.; Davis, F.B.; Acconcia, F.; Marino, M.; Farias, R.N.; Davis, P.J. Rapid nongenomic effects of 3,5,3’-triiodo-L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology 2004, 145, 5694–5703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Xu, X.; Wang, X.; Zhang, B. Regulation of autophagy by Ca(2). Tumour Biol. J. Int. Soc.Oncodev. Biol. Med. 2016, 37, 15467–15476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- East, D.A.; Campanella, M. Ca2+ in quality control: An unresolved riddle critical to autophagy and mitophagy. Autophagy 2013, 9, 1710–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, E.K.; Roth, S.; Blechschmidt, C.; Hölter, S.M.; Becker, L.; Racz, I.; Zimmer, A.; Klopstock, T.; Gailus-Durner, V.; Fuchs, H.; et al. Neuronal 3’,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9439–9449. [Google Scholar] [CrossRef]
- Brix, K.; Summa, W.; Lottspeich, F.; Herzog, V. Extracellularly occurring histone H1 mediates the binding of thyroglobulin to the cell surface of mouse macrophages. J. Clin. Investig. 1998, 102, 283–293. [Google Scholar] [CrossRef]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [Green Version]
- Greenbaum, D.; Medzihradszky, K.F.; Burlingame, A.; Bogyo, M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem. Biol. 2000, 7, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Neuhoff, V.; Philipp, K.; Zimmer, H.G.; Mesecke, S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe-Seyler’s Zeitschrift fur physiologische Chemie 1979, 360, 1657–1670. [Google Scholar] [CrossRef]
- Sandell, E.B.; Kolthoff, I.M. Micro determination of iodine by a catalytic method. Microchim. Acta 1937, 1, 9–25. [Google Scholar] [CrossRef]
- Jayarama-Naidu, R.; Johannes, J.; Meyer, F.; Wirth, E.K.; Schomburg, L.; Köhrle, J.; Renko, K. A Nonradioactive Uptake Assay for Rapid Analysis of Thyroid Hormone Transporter Function. Endocrinology 2015, 156, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Lietzow, J.; Golchert, J.; Homuth, G.; Völker, U.; Jonas, W.; Köhrle, J. 3,5-T2 alters murine genes relevant for xenobiotic, steroid, and thyroid hormone metabolism. J. Mol. Endocrinol. 2016, 56, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Antigen | Biological Source | Species Reactivity | Company/ Provider | Catalog No. | Dilution in IIF 1 | Dilution in IB 1 |
|---|---|---|---|---|---|---|
| Cathepsin B | goat | mouse | Neuromics | GT15057 | 1:100 | 1:1000 |
| Cathepsin D | rabbit | human | Calibochem | IM-16 | 1:10 | 1:80 |
| Cathepsin L | goat | mouse | Neuromics | GT15049 | 1:100 | 1:1000 |
| Cystatin C | rabbit | mouse | Dr. Magnus Abrahamson | 1:50 | - | |
| Cystatin D | rabbit | mouse | Dr. Magnus Abrahamson | 1:50 | - | |
| Lamp1 | rabbit | mouse, human, rat | Sigma Aldrich | L1418 | 1:100 | - |
| LC3 | rabbit | mouse, human, rat | Novus Biologicals | NB100-2331 | 1:100 | 1:500 |
| NIS | mouse | human, rat | Abcam | ab17795 | - | 1:500 |
| p62 | mouse | human | Abcam | ab56146 | - | 1:500 |
| Thyroglobulin | rabbit | bovine | [80] | 1:30 | 1:5000 | |
| T3 | mouse | mouse, human, rat | ICN Biochemicals | 63030 | 1:50 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopalan, V.; Al-Hashimi, A.; Rehders, M.; Golchert, J.; Reinecke, V.; Homuth, G.; Völker, U.; Manirajah, M.; Touzani, A.; Weber, J.; et al. The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy. Int. J. Mol. Sci. 2021, 22, 462. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010462
Venugopalan V, Al-Hashimi A, Rehders M, Golchert J, Reinecke V, Homuth G, Völker U, Manirajah M, Touzani A, Weber J, et al. The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy. International Journal of Molecular Sciences. 2021; 22(1):462. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010462
Chicago/Turabian StyleVenugopalan, Vaishnavi, Alaa Al-Hashimi, Maren Rehders, Janine Golchert, Vivien Reinecke, Georg Homuth, Uwe Völker, Mythili Manirajah, Adam Touzani, Jonas Weber, and et al. 2021. "The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy" International Journal of Molecular Sciences 22, no. 1: 462. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010462