Lack of Cannabinoid Receptor Type-1 Leads to Enhanced Age-Related Neuronal Loss in the Locus Coeruleus
Abstract
:1. Introduction
2. Results
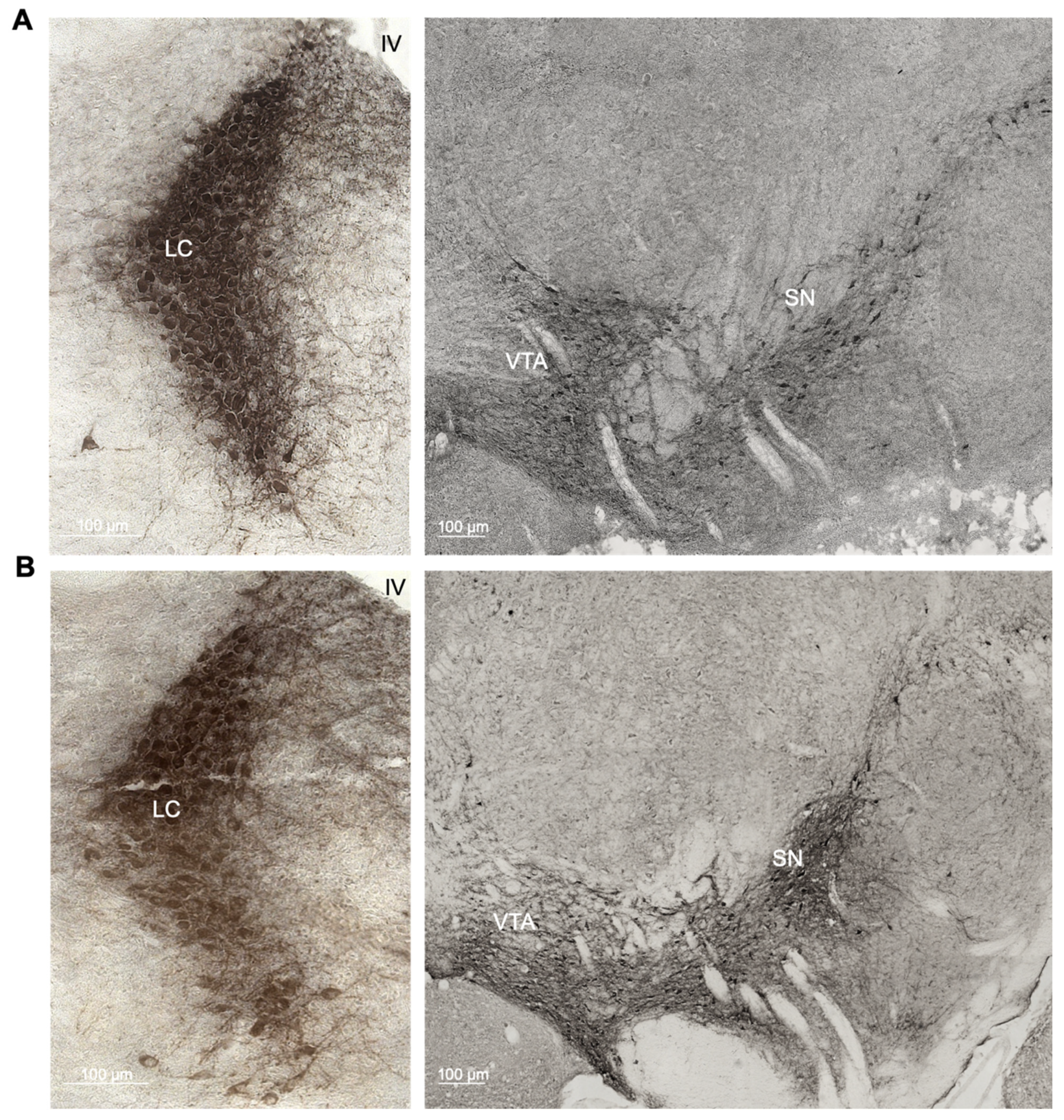
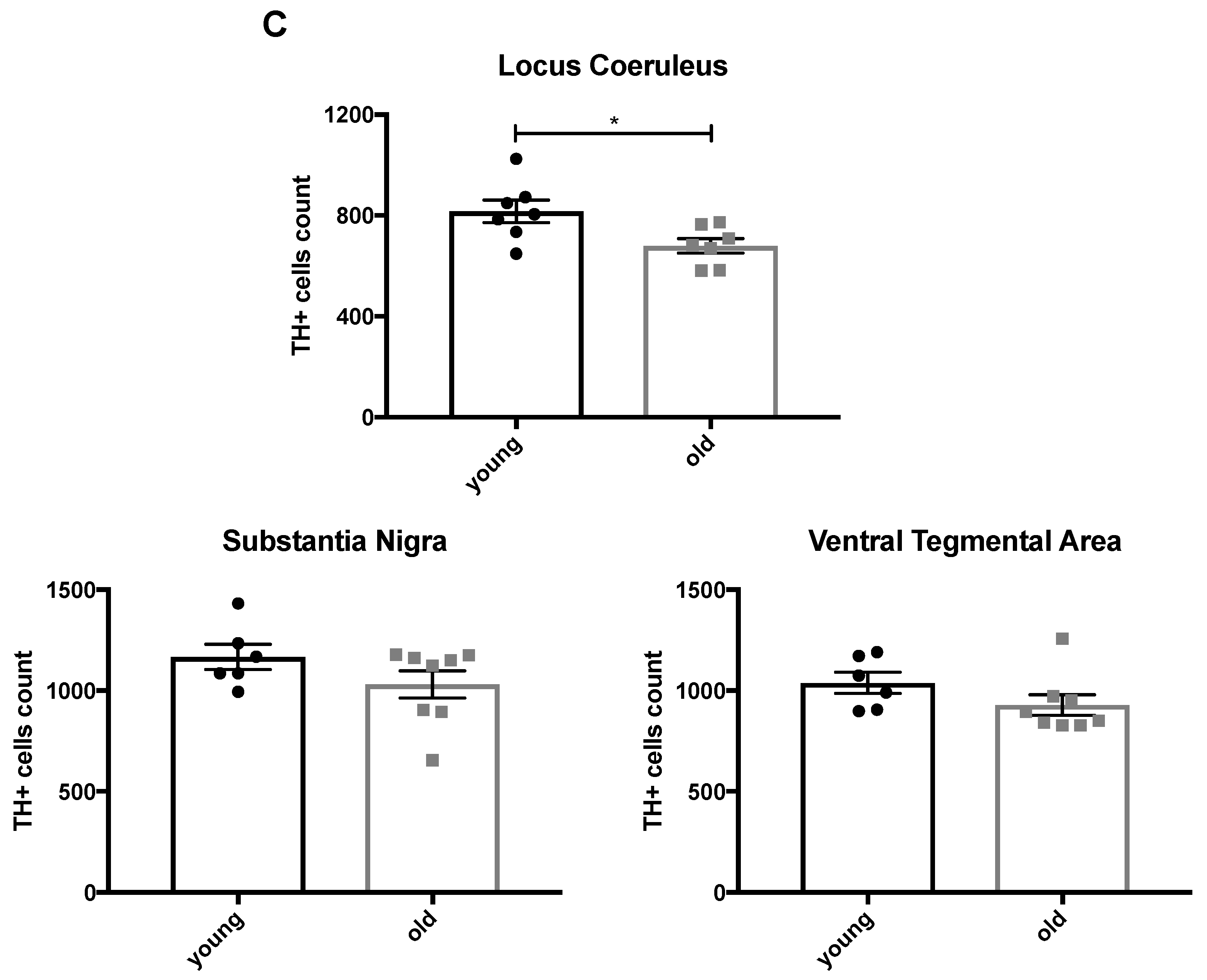
2.1. Enhanced Age-Related Neuronal Loss in the Locus Coeruleus in Cnr1−/− Mice
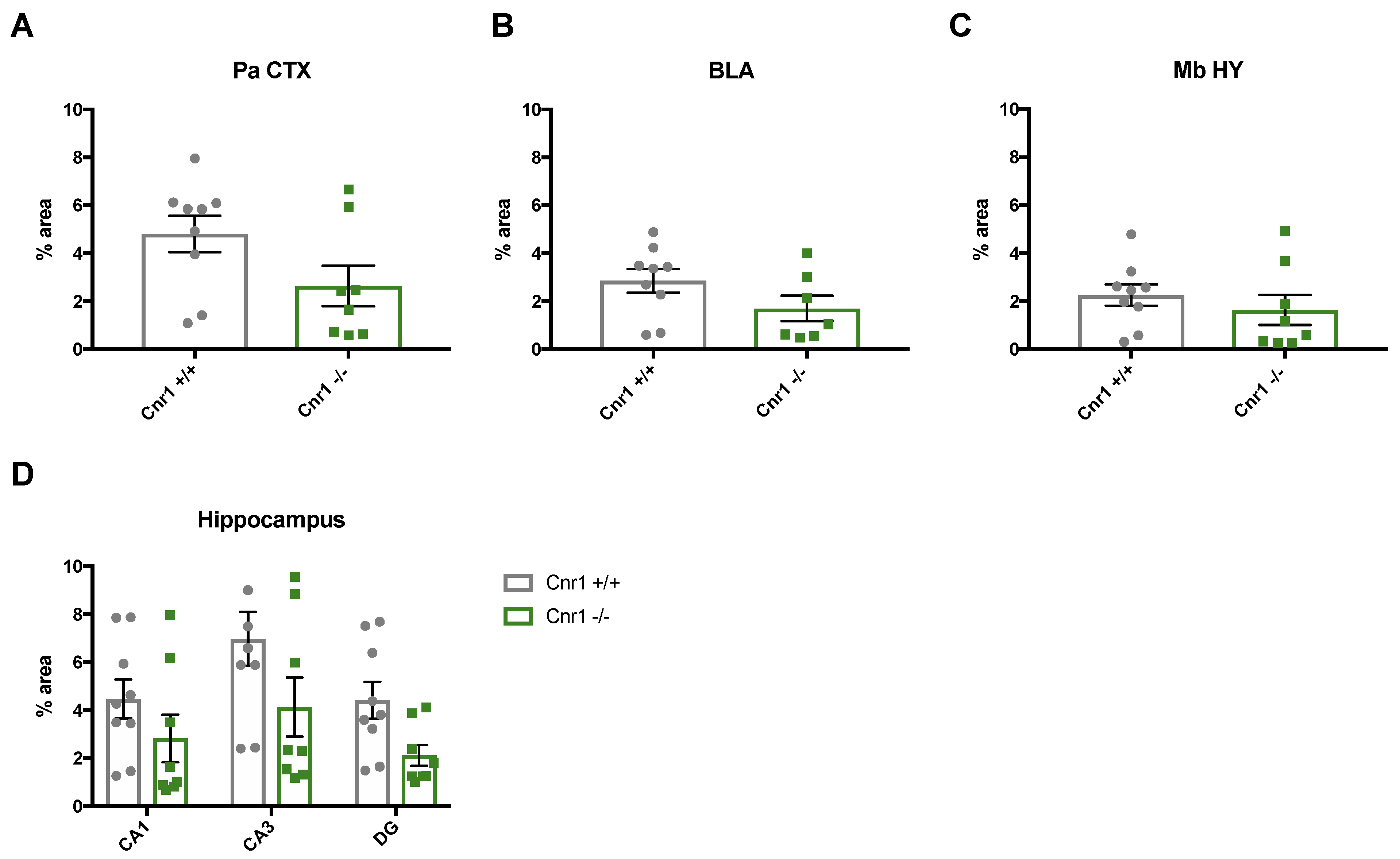
2.2. Reduced Density of Noradrenergic Terminals in Aged Cnr1−/− Mice
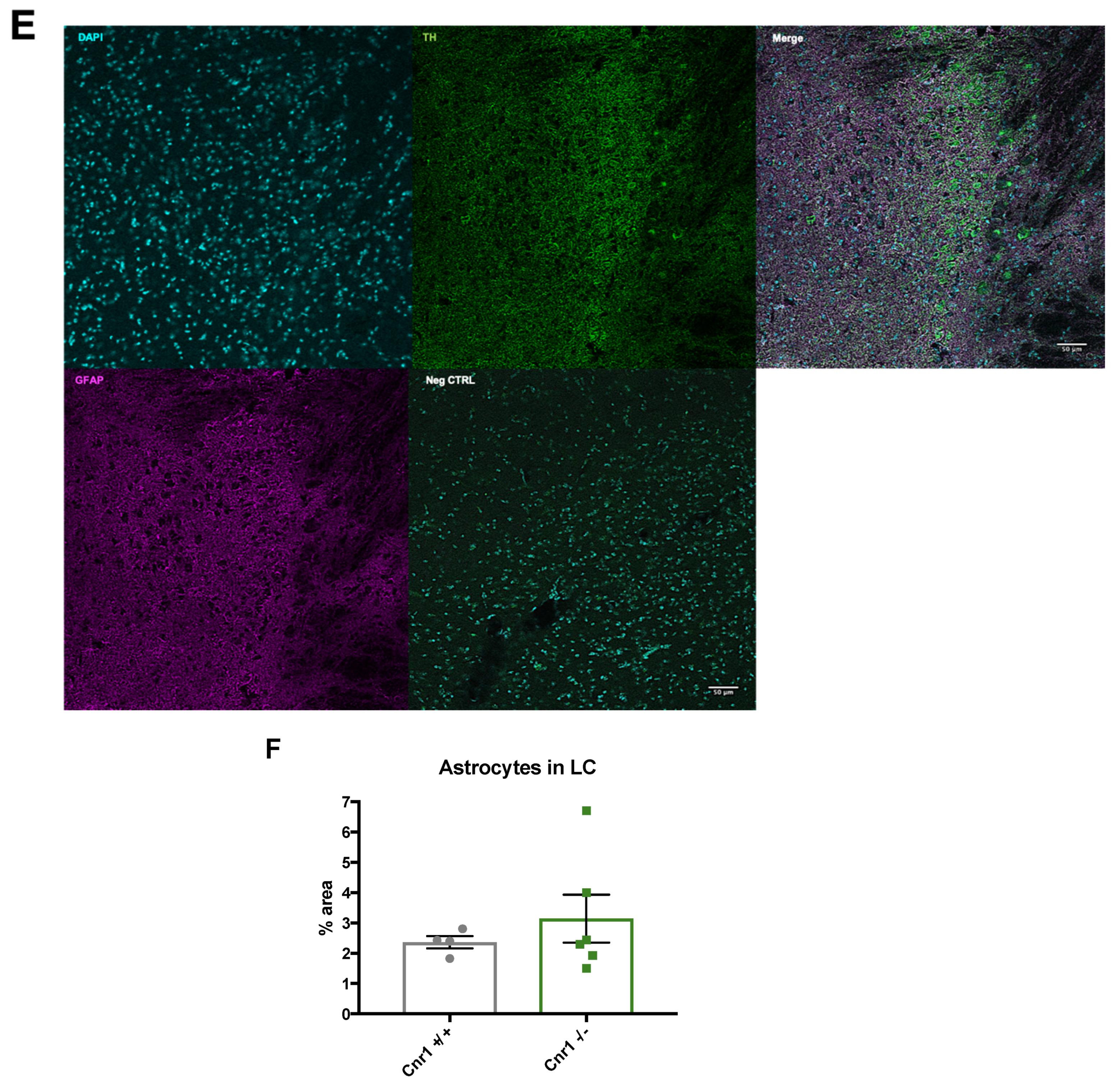
2.3. Enhanced Microglia Densities in the LC of Old Cnr1−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
4.3. Microscopy
4.4. Determination of Noradrenaline Levels
4.5. Statistics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CB1r | cannabinoid receptor type-1 |
| Cnr1−/− | cannabinoid 1 receptor knockout |
| GFAP | glial fibrillary acidic protein |
| Iba1 | ionized calcium-binding adapter molecule 1 |
| LC | locus coeruleus |
| NE | noradrenaline |
| NET | noradrenaline transporter |
| SN | substantia nigra |
| TH | tyrosine hydroxylase |
| TNFα | tumor necrosis factorα |
| VTA | ventral tegmental area |
| Pa CTX | parietal cortex |
| BLA | basolateral amygdala |
| Mb HY | mediobasal hypothalamus |
| HC | hippocampus |
| CA1 | cornu ammonis 1 |
| CA3 | cornu ammonis 3 |
| DG | dentate gyrus |
| mTOR | mechanistic target of rapamycin |
| AMPK | 5’ adenosine monophosphate-activated protein kinase |
| PBS | Phosphate buffered saline |
References
- Sharma, Y.; Xu, T.; Graf, W.M.; Fobbs, A.; Sherwood, C.C.; Hof, P.R.; Allman, J.M.; Manaye, K.F. Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J. Comp. Neurol. 2009, 518, 963–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturrock, R.R.; Rao, K.A. A Quantitative Histological Study of Neuronal Loss from the Locus Coeruleus of Ageing Mice. Neuropathol. Appl. Neurobiol. 1985, 11, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Berger, B.; Febvret, A.; Vigny, A.; Henry, J.P. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J. Comp. Neurol. 1989, 279, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Sadikot, A.; Parent, A. The monoaminergic innervation of the amygdala in the squirrel monkey: An immunohistochemical study. Neurosci. 1990, 36, 431–447. [Google Scholar] [CrossRef]
- Radley, J.J.; Williams, B.; Sawchenko, P.E. Noradrenergic Innervation of the Dorsal Medial Prefrontal Cortex Modulates Hypothalamo-Pituitary-Adrenal Responses to Acute Emotional Stress. J. Neurosci. 2008, 28, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Hof, P.R.; Young, W.G.; Morrison, J.H. Noradrenergic innervation of the hypothalamus of rhesus monkeys: Distribution of dopamine-?-hydroxylase immunoreactive fibers and quantitative analysis of varicosities in the paraventricular nucleus. J. Comp. Neurol. 1993, 327, 597–611. [Google Scholar] [CrossRef]
- Westlund, K.N.; Coulter, J.D. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: Axonal transport studies and dopamine-?-hydroxylase immunocytochemistry. Brain Res. Rev. 1980, 2, 235–264. [Google Scholar] [CrossRef]
- Arnsten, A.; Goldman-Rakic, P. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984, 306, 9–18. [Google Scholar] [CrossRef]
- Pammer, C.; Görcs, T.; Palkovits, M. Peptidergic innervation of the locus coeruleus cells in the human brain. Brain Res. 1990, 515, 247–255. [Google Scholar] [CrossRef]
- Westlund, K.N.; Craig, A.D. Association of spinal lamina I projections with brainstem catecholamine neurons in the monkey. Exp. Brain Res. 1996, 110, 151–162. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benarroch, E.E. Locus coeruleus. Cell Tissue Res. 2018, 373, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, A.; Tan, B.Z.; Johansen, J.P. Projection specificity in heterogeneous locus coeruleus cell populations: Implications for learning and memory. Learn. Mem. 2015, 22, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Lemon, N.; Aydin-Abidin, S.; Funke, K.; Manahan-Vaughan, D. Locus Coeruleus Activation Facilitates Memory Encoding and Induces Hippocampal LTD that Depends on -Adrenergic Receptor Activation. Cereb. Cortex 2009, 19, 2827–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, N.; Manahan-Vaughan, D. Locus Coeruleus Stimulation Facilitates Long-Term Depression in the Dentate Gyrus That Requires Activation of β-Adrenergic Receptors. Cereb. Cortex 2014, 25, 1889–1896. [Google Scholar] [CrossRef] [Green Version]
- Coradazzi, M.; Gulino, R.; Fieramosca, F.; Falzacappa, L.V.; Riggi, M.; Leanza, G. Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol. Aging 2016, 48, 93–102. [Google Scholar] [CrossRef]
- Alsene, K.M.; Bakshi, V.P. Pharmacological Stimulation of Locus Coeruleus Reveals a New Antipsychotic-Responsive Pathway for Deficient Sensorimotor Gating. Neuropsychopharmacology 2011, 36, 1656–1667. [Google Scholar] [CrossRef] [Green Version]
- Lapiz, M.D.S.; Bondi, C.O.; Morilak, D.A. Chronic Treatment with Desipramine Improves Cognitive Performance of Rats in an Attentional Set-Shifting Test. Neuropsychopharmacology 2006, 32, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Mather, M.; Harley, C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Ordureau, A.; Heo, J.-M.; Duda, D.M.; Paulo, J.A.; Olszewski, J.L.; Yanishevski, D.; Rinehart, J.; Schulman, B.A.; Harper, W. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc. Natl. Acad. Sci. USA 2015, 112, 6637–6642. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Fiesel, F.C.; Truban, D.; Casey, M.C.; Lin, W.-L.; Soto, A.I.; Tacik, P.; Rousseau, L.G.; Diehl, N.N.; Heckman, M.G.; et al. Age- and disease-dependent increase of the mitophagy marker phospho-ubiquitin in normal aging and Lewy body disease. Autophagy 2018, 14, 1404–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flood, D.G.; Coleman, P.D. Neuron numbers and sizes in aging brain: Comparisons of human, monkey, and rodent data. Neurobiol. Aging 1988, 9, 453–463. [Google Scholar] [CrossRef]
- Manaye, K.F.; McIntire, D.D.; Mann, D.M.A.; German, D.C. Locus coeruleus cell loss in the aging human brain: A non-random process. J. Comp. Neurol. 1995, 358, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mouton, P.R.; Pakkenberg, B.; Gundersen, H.J.G.; Price, D.L. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J. Chem. Neuroanat. 1994, 7, 185–190. [Google Scholar] [CrossRef]
- Ohm, T.; Busch, C.; Bohl, J. Unbiased Estimation of Neuronal Numbers in the Human Nucleus Coeruleus during Aging. Neurobiol. Aging 1997, 18, 393–399. [Google Scholar] [CrossRef]
- Liu, K.Y.; Acosta-Cabronero, J.; Cardenas-Blanco, A.; Loane, C.; Berry, A.J.; Betts, M.J.; Kievit, R.A.; Henson, R.N.; Düzel, E.; Howard, R.; et al. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol. Aging 2019, 74, 101–111. [Google Scholar] [CrossRef]
- Tatton, W.G.; Greenwood, C.E.; Verrier, M.C.; Holland, D.P.; Kwan, M.M.; Biddle, F.E. Different rates of age-related loss for four murine monoaminergic neuronal populations. Neurobiol. Aging 1991, 12, 543–556. [Google Scholar] [CrossRef]
- Leslie, F.; Loughlin, S.; Sternberg, D.B.; McGaugh, J.L.; Young, L.E.; Zornetzer, S.F. Noradrenergic changes and memory loss in aged mice. Brain Res. 1985, 359, 292–299. [Google Scholar] [CrossRef]
- Szot, P.; Van Dam, D.; White, S.S.; Franklin, A.; Staufenbiel, M.; De Deyn, P.P. Age-dependent changes in noradrenergic locus coeruleus system in wild-type and APP23 transgenic mice. Neurosci. Lett. 2009, 463, 93–97. [Google Scholar] [CrossRef]
- Scavone, J.L.; Mackie, K.; Bockstaele, E.J. Van Coeruleus: Relationship with Mu-Opioid Receptors. Brain Res. 2011, 1312, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Mendiguren, A.; Pineda, J. CB1 cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology 2007, 52, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, A.L.; Pillolla, G.; Melis, M.; Perra, S.; Gessa, G.L.; Pistis, M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2006, 23, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Wyrofsky, R.R.; Reyes, B.A.S.; Yu, D.; Kirby, L.G.; Bockstaele, E.J.V. Sex differences in the effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses. Eur. J. Neurosci. 2019, 48, 2118–2138. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Racz, I.; Valverde, O.; Otto, M.; Michel, K.; Sarstre, M.; Zimmer, A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 15670–15675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albayram, O.; Alferink, J.; Pitsch, J.; Piyanova, A.; Neitzert, K.; Poppensieker, K.; Mauer, D.; Michel, K.; Legler, A.; Becker, A.; et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA 2011, 108, 11256–11261. [Google Scholar] [CrossRef] [Green Version]
- Piyanova, A.; Albayram, Ö.; Rossi, C.A.; Farwanah, H.; Michel, K.; Nicotera, P.; Sandhoff, K.; Bilkeigorzo, A. Loss of CB1 receptors leads to decreased cathepsin D levels and accelerated lipofuscin accumulation in the hippocampus. Mech. Ageing Dev. 2013, 134, 391–399. [Google Scholar] [CrossRef]
- Pakkenberg, B.; Moller, A.; Gundersen, H.J.; Dam, A.M. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J. Neurol. Neurosurg. Psychiatry 1991, 54, 30–33. [Google Scholar] [CrossRef] [Green Version]
- Rudow, G.; O’Brien, R.; Savonenko, A.V.; Resnick, S.M.; Zonderman, A.B.; Pletnikova, O.; Marsh, L.; Dawson, T.M.; Crain, B.J.; West, M.J.; et al. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008, 115, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Arendt, T.; Brückner, M.K.; Morawski, M.; Jäger, C.; Gertz, H.-J. Early neurone loss in Alzheimer’s disease: Cortical or subcortical? Acta Neuropathol. Commun. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.-K.; et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063. [Google Scholar] [CrossRef] [Green Version]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.J.; Mather, M.; Düzel, S.; Bodammer, N.C.; Lindenberger, U.; Kühn, S.; Werkle-Bergner, M. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav. 2019, 3, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Szot, P.; White, S.S.; Greenup, J.L.; Leverenz, J.B.; Peskind, E.R.; Raskind, M.A. Compensatory Changes in the Noradrenergic Nervous System in the Locus Ceruleus and Hippocampus of Postmortem Subjects with Alzheimer’s Disease and Dementia with Lewy Bodies. J. Neurosci. 2006, 26, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Mather, M.; Clewett, D.; Sakaki, M.; Harley, C.W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 2016, 39, e200. [Google Scholar] [CrossRef] [Green Version]
- Twarkowski, H.; Manahan-Vaughan, D. Loss of Catecholaminergic Neuromodulation of Persistent Forms of Hippocampal Synaptic Plasticity with Increasing Age. Front. Synaptic Neurosci. 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Hagan, J. The effects of central catecholamine depletions on spatial learning in rats. Behav. Brain Res. 1983, 9, 83–104. [Google Scholar] [CrossRef]
- Chrobak, J.J.; DeHaven, D.L.; Walsh, T.J. Depletion of brain norepinephrine with DSP-4 does not alter acquisition or performance of a radial-arm maze task. Behav. Neural Biol. 1985, 44, 144–150. [Google Scholar] [CrossRef]
- De Carvalho, L.P.; Zornetzer, S.F. The involvement of the locus coeruleus in memory. Behav. Neural Biol. 1981, 31, 173–186. [Google Scholar] [CrossRef]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3326–3341. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Breuer, A.; Mechoulam, R. 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-α production in murine macrophages, and in mice. Eur. J. Pharmacol. 2000, 406, R5–R7. [Google Scholar] [CrossRef]
- Carracedo, A.; Geelen, M.J.H.; Diez, M.; Hanada, K.; Guzmán, M.; Velasco, G. Ceramide sensitizes astrocytes to oxidative stress: Protective role of cannabinoids. Biochem. J. 2004, 380, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, G.C.; Oprea, T.I.; Zhao, P.; Abood, M.E.; Brailoiu, E. Intracellular Cannabinoid Type 1 (CB1) Receptors Are Activated by Anandamide. J. Biol. Chem. 2011, 286, 29166–29174. [Google Scholar] [CrossRef] [Green Version]
- Noonan, J.; Tanveer, R.; Klompas, A.; Gowran, A.; McKiernan, J.; Campbell, V.A. Endocannabinoids Prevent β-Amyloid-mediated Lysosomal Destabilization in Cultured Neurons. J. Biol. Chem. 2010, 285, 38543–38554. [Google Scholar] [CrossRef] [Green Version]
- Puighermanal, E.; Marsicano, G.; Busquets-Garcia, A.; Lutz, B.; Maldonado, R.; Ozaita, A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat. Neurosci. 2009, 12, 1152–1158. [Google Scholar] [CrossRef]
- Blazquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; García-Rincón, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galveroperh, I.; et al. The CB1 cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Zottola, A.C.P.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Athanasiou, A.; Clarke, A.B.; Turner, A.E.; Mohana-Kumaran, N.; Vakilpour, S.; Smith, P.A.; Bagiokou, D.; Bradshaw, T.D.; Westwell, A.D.; Fang, L.; et al. Cannabinoid receptor agonists are mitochondrial inhibitors: A unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 2007, 364, 131–137. [Google Scholar] [CrossRef]
- Zaccagnino, P.; Corcelli, A.; Baronio, M.; Lorusso, M. Anandamide inhibits oxidative phosphorylation in isolated liver mitochondria. FEBS Lett. 2010, 585, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez-Pardo, C.; Jimenez-Del-Rio, M.; Lores-Arnaiz, S.; Bustamante, J. Protective Effects of the Synthetic Cannabinoids CP55,940 and JWH-015 on Rat Brain Mitochondria upon Paraquat Exposure. Neurochem. Res. 2010, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Hernández-Camacho, J.D.; Fernández-Ayala, D.J.M.; Navas, P. Mitochondrial dysfunction in metabolism and ageing: Shared mechanisms and outcomes? Biogerontology 2018, 19, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Kola, B.; Hubina, E.; Tucci, S.A.; Kirkham, T.C.; Garcia, E.A.; Mitchell, S.E.; Williams, L.M.; Hawley, S.A.; Hardie, D.G.; Grossman, A.B.; et al. Cannabinoids and Ghrelin Have Both Central and Peripheral Metabolic and Cardiac Effects via AMP-activated Protein Kinase. J. Biol. Chem. 2005, 280, 25196–25201. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, K.; Bilkei-Gorzo, A.; Nozaki, C.; Togo, A.; Nakamura, K.; Ohta, K.; Zimmer, A.; Asahi, T. Age-dependent Alteration in Mitochondrial Dynamics and Autophagy in Hippocampal Neuron of Cannabinoid CB1 Receptor-deficient Mice. Brain Res. Bull. 2020, 160, 40–49. [Google Scholar] [CrossRef]


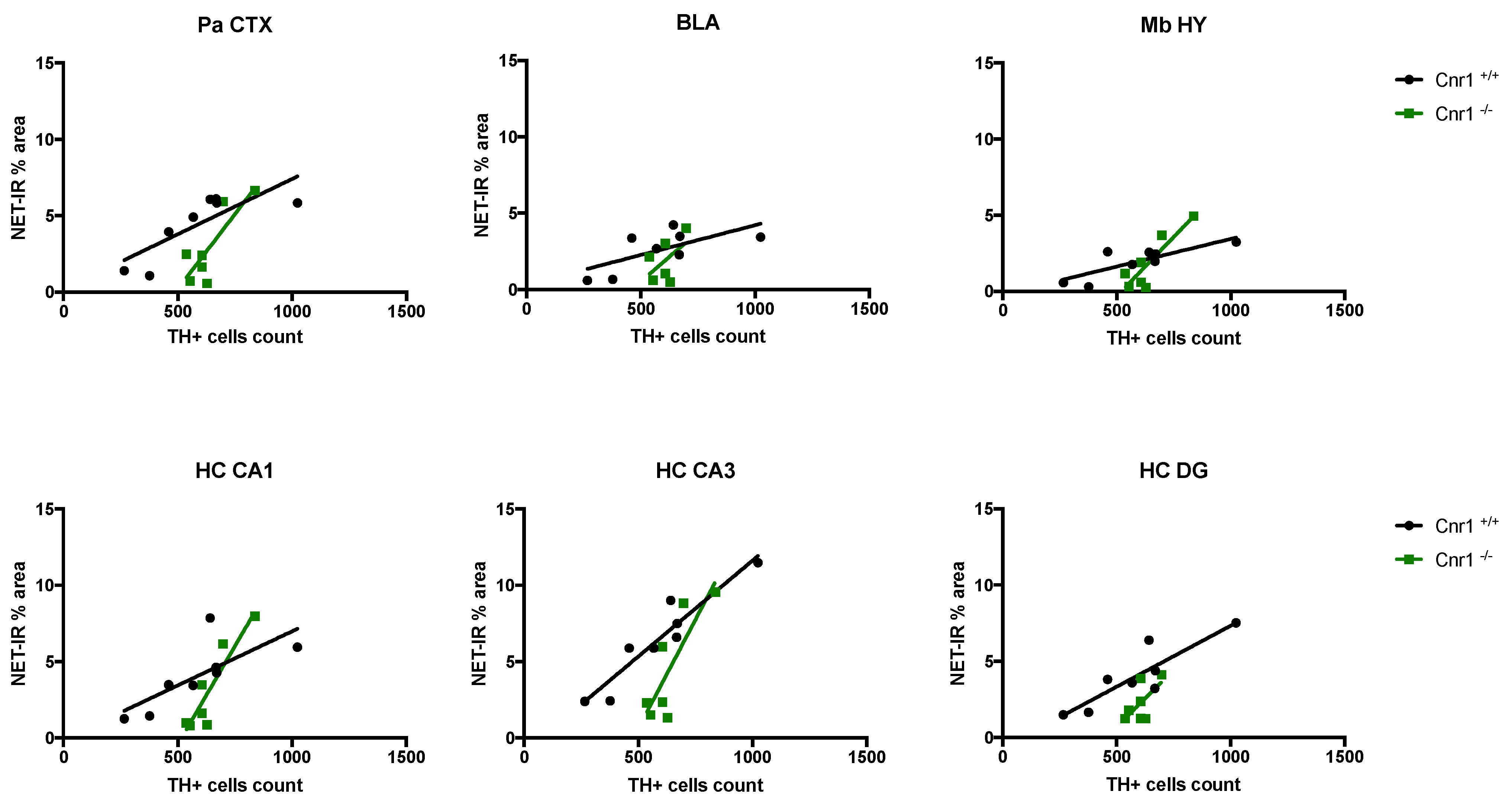

| Brain Area | Wild-Type | Cnr1−/− | ||
|---|---|---|---|---|
| Correlation (r, Spearman) | Significance | Correlation (r, Spearman) | Significance | |
| Pa CTX | 0.6399 | 0.0171 | 0.6857 | 0.0214 |
| BLA | 0.4502 | 0.0685 | 0.2713 | 0.2894 |
| Mb HY | 0.6643 | 0.0137 | 0.7682 | 0.0096 |
| HC, CA1 region | 0.5527 | 0.0345 | 0.8316 | 0.0042 |
| HC, CA3 region | 0.8863 | 0.0005 | 0.6826 | 0.022 |
| HC, DG region | 0.7701 | 0.0042 | 0.3857 | 0.1366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargano, A.; Beins, E.; Zimmer, A.; Bilkei-Gorzo, A. Lack of Cannabinoid Receptor Type-1 Leads to Enhanced Age-Related Neuronal Loss in the Locus Coeruleus. Int. J. Mol. Sci. 2021, 22, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010005
Gargano A, Beins E, Zimmer A, Bilkei-Gorzo A. Lack of Cannabinoid Receptor Type-1 Leads to Enhanced Age-Related Neuronal Loss in the Locus Coeruleus. International Journal of Molecular Sciences. 2021; 22(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010005
Chicago/Turabian StyleGargano, Alessandra, Eva Beins, Andreas Zimmer, and Andras Bilkei-Gorzo. 2021. "Lack of Cannabinoid Receptor Type-1 Leads to Enhanced Age-Related Neuronal Loss in the Locus Coeruleus" International Journal of Molecular Sciences 22, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010005




