A Heterotypic Tridimensional Model to Study the Interaction of Macrophages and Glioblastoma In Vitro
Abstract
:1. Introduction
2. Results
2.1. Culture of Primary Monocytes with Conditioned Media of U87MG Cells Leads to an Increased Expression of M2 Phenotypic Markers
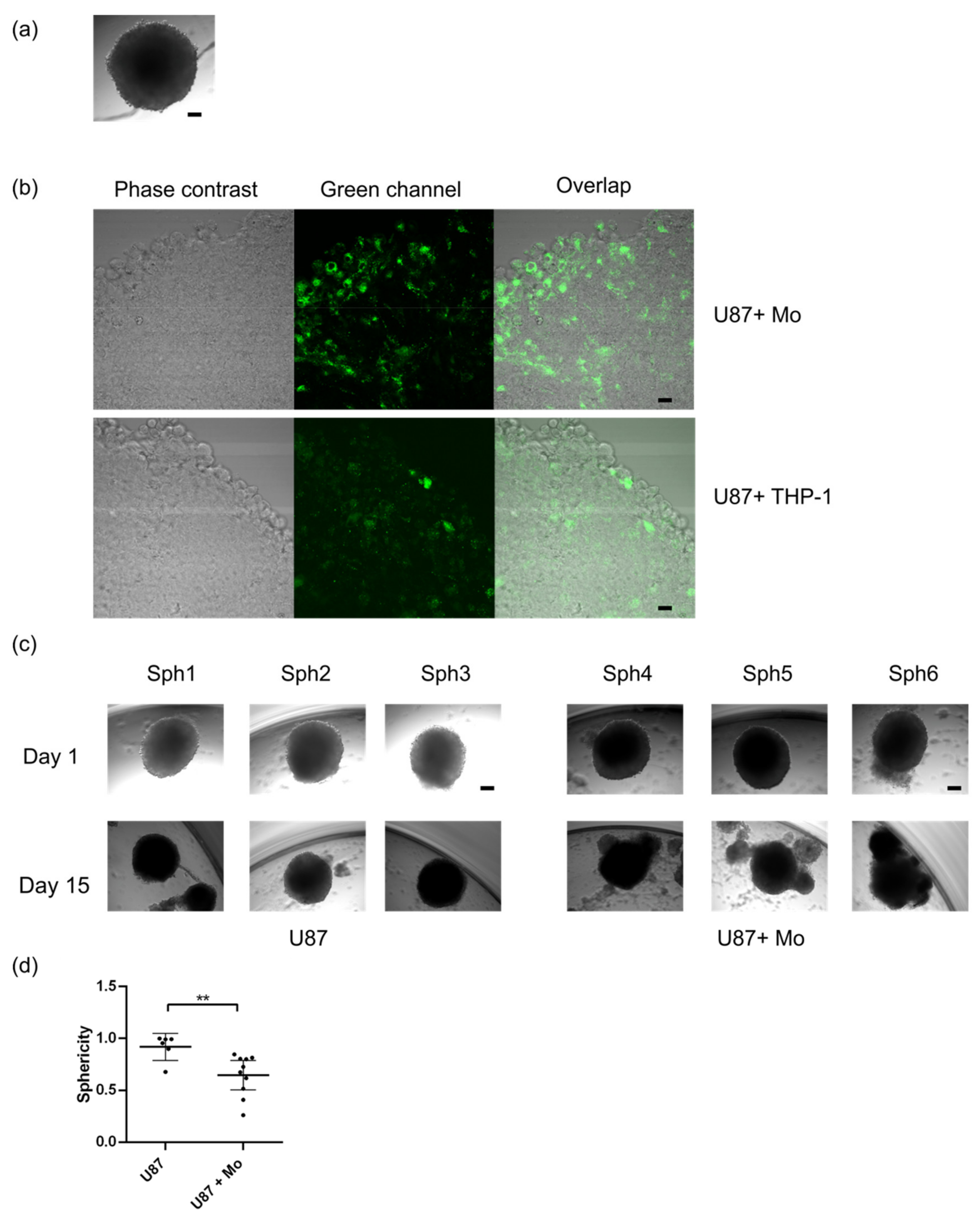
2.2. Primary Human Monocytes Infiltrate Human GBM Spheroids and Alter Their Growth and Sphericity
2.3. Infiltrated Monocytes Acquire an M2-Phenotype When They Invade the GBM Spheroids
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Blood Sample and Purification of Human CD14+ Monocytes
4.3. Collection of Conditioned Medium
4.4. Stimulation of CD14+ Cells and THP1 with Conditioned Medium
4.5. p32/gC1qR Staining
4.6. Tumor Spheroid Generation
4.7. Monocyte-Spheroid Co-Culture
4.8. Spheroid Dissociation
4.9. Surface and Intracellular Staining and Flow Cytometry Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahadur, S.; Sahu, A.K.; Baghel, P.; Saha, S. Current promising treatment strategy for glioblastoma multiform: A review. Oncol. Rev. 2019, 13, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, C.C.; Sarkar, S.; Yong, V.W.; Kelly, J.J.P. Glioblastoma-associated microglia and macrophages: Targets for therapies to improve prognosis. Brain 2017, 140, 1548–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronk, J.C.; Filiano, A.J.; Louveau, A.; Marin, I.; Marsh, R.; Ji, E.; Goldman, D.H.; Smirnov, I.; Geraci, N.; Acton, S.; et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 2018, 215, 1627–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Wouw, M.; Boehme, M.; Dinan, T.G.; Cryan, J.F. Monocyte mobilisation, microbiota & mental illness. Brain Behav. Immun. 2019, 81, 74–91. [Google Scholar] [CrossRef]
- Nowak, W.; Grendas, L.N.; Sanmarco, L.M.; Estecho, I.G.; Arena, A.R.; Eberhardt, N.; Rodante, D.E.; Aoki, M.P.; Daray, F.M.; Carrera Silva, E.A.; et al. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine 2019, 50, 290–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorger, M. Tumor microenvironment in the brain. Cancers 2012, 4, 218. [Google Scholar] [CrossRef]
- Kusne, Y.; Carrera-Silva, E.A.; Perry, A.S.; Rushing, E.J.; Mandell, E.K.; Dietrich, J.D.; Errasti, A.E.; Gibbs, D.; Berens, M.E.; Loftus, J.C.; et al. Targeting aPKC disables oncogenic signaling by both the EGFR and the proinflammatory cytokine TNFalpha in glioblastoma. Sci. Signal 2014, 7, ra75. [Google Scholar] [CrossRef] [Green Version]
- Carestia, A.; Mena, H.A.; Olexen, C.M.; Ortiz Wilczynski, J.M.; Negrotto, S.; Errasti, A.E.; Gomez, R.M.; Jenne, C.N.; Carrera Silva, E.A.; Schattner, M. Platelets Promote Macrophage Polarization toward Pro-inflammatory Phenotype and Increase Survival of Septic Mice. Cell Rep. 2019, 28, 896–908. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Markovic, D.S.; Vinnakota, K.; Chirasani, S.; Synowitz, M.; Raguet, H.; Stock, K.; Sliwa, M.; Lehmann, S.; Kalin, R.; van Rooijen, N.; et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 12530–12535. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Heppner, F.L.; Tsirka, S.E. Microglia/macrophages promote glioma progression. Glia 2011, 59, 472–485. [Google Scholar] [CrossRef] [Green Version]
- Ponten, J.; Macintyre, E.H. Long term culture of normal and neoplastic human glia. Acta Pathol. Microbiol. Scand. 1968, 74, 465–486. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Fogal, V.; Zhang, L.; Krajewski, S.; Ruoslahti, E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008, 68, 7210–7218. [Google Scholar] [CrossRef] [Green Version]
- Carrera Silva, E.A.; Nowak, W.; Tessone, L.; Olexen, C.M.; Ortiz Wilczynski, J.M.; Estecho, I.G.; Elena, G.; Errasti, A.E.; Rosso, D.A. CD207(+)CD1a(+) cells circulate in pediatric patients with active Langerhans cell histiocytosis. Blood 2017, 130, 1898–1902. [Google Scholar] [CrossRef] [Green Version]
- Manome, Y.; Mizuno, S.; Akiyama, N.; Fujioka, K.; Saito, H.; Hataba, Y.; Kobayashi, T.; Watanabe, M. Three-dimensional cell culture of glioma and morphological comparison of four different human cell lines. Anticancer. Res. 2010, 30, 383–389. [Google Scholar]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Yang, M.; McKay, D.; Pollard, J.W.; Lewis, C.E. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res. 2018, 78, 5492–5503. [Google Scholar] [CrossRef] [Green Version]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Landry, A.P.; Balas, M.; Alli, S.; Spears, J.; Zador, Z. Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma. Sci. Rep. 2020, 10, 19542. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A.; et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res. 2017, 77, 2266–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Caragher, S.; Chalmers, A.J.; Gomez-Roman, N. Glioblastoma’s Next Top Model: Novel Culture Systems for Brain Cancer Radiotherapy Research. Cancers 2019, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.A.; Bansal, R.; Lammers, T.; Zhang, Y.S.; Michel Schiffelers, R.; Prakash, J. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv. Mater. 2019, 31, e1806590. [Google Scholar] [CrossRef]
- Szatmari, T.; Lumniczky, K.; Desaknai, S.; Trajcevski, S.; Hidvegi, E.J.; Hamada, H.; Safrany, G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006, 97, 546–553. [Google Scholar] [CrossRef]
- Raschke, W.C.; Baird, S.; Ralph, P.; Nakoinz, I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 1978, 15, 261–267. [Google Scholar] [CrossRef]
- Leite, D.M.; Zvar Baskovic, B.; Civita, P.; Neto, C.; Gumbleton, M.; Pilkington, G.J. A human co-culture cell model incorporating microglia supports glioblastoma growth and migration, and confers resistance to cytotoxics. FASEB J. 2020, 34, 1710–1727. [Google Scholar] [CrossRef] [Green Version]
- Welch, W.C.; Morrison, R.S.; Gross, J.L.; Gollin, S.M.; Kitson, R.B.; Goldfarb, R.H.; Giuliano, K.A.; Bradley, M.K.; Kornblith, P.L. Morphologic, immunologic, biochemical, and cytogenetic characteristics of the human glioblastoma-derived cell line, SNB-19. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 610–616. [Google Scholar] [CrossRef]
- Janabi, N.; Peudenier, S.; Heron, B.; Ng, K.H.; Tardieu, M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci. Lett. 1995, 195, 105–108. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, J.; Fan, F.; Cao, H.; Dai, Z.Y.; Wang, Z.Y.; Feng, S.S. Identification and Analysis of Glioblastoma Biomarkers Based on Single Cell Sequencing. Front. Bioeng. Biotechnol. 2020, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.F.; Thomas, P.; Lopez Ortiz, A.O.; Errasti, A.E.; Charo, N.; Romanowski, V.; Gorgojo, J.; Rodriguez, M.E.; Carrera Silva, E.A.; Gomez, R.M. Junin Virus Triggers Macrophage Activation and Modulates Polarization According to Viral Strain Pathogenicity. Front. Immunol. 2019, 10, 2499. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, D.; Werbowetski, T.; Del Maestro, R.F. Spheroid preparation from hanging drops: Characterization of a model of brain tumor invasion. J. Neurooncol. 2004, 67, 295–303. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gattas, M.J.; Estecho, I.G.; Lago Huvelle, M.A.; Errasti, A.E.; Carrera Silva, E.A.; Simian, M. A Heterotypic Tridimensional Model to Study the Interaction of Macrophages and Glioblastoma In Vitro. Int. J. Mol. Sci. 2021, 22, 5105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105105
Gattas MJ, Estecho IG, Lago Huvelle MA, Errasti AE, Carrera Silva EA, Simian M. A Heterotypic Tridimensional Model to Study the Interaction of Macrophages and Glioblastoma In Vitro. International Journal of Molecular Sciences. 2021; 22(10):5105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105105
Chicago/Turabian StyleGattas, María José, Ivana Gisele Estecho, María Amparo Lago Huvelle, Andrea Emilse Errasti, Eugenio Antonio Carrera Silva, and Marina Simian. 2021. "A Heterotypic Tridimensional Model to Study the Interaction of Macrophages and Glioblastoma In Vitro" International Journal of Molecular Sciences 22, no. 10: 5105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105105





