Effects of Virgin Olive Oil on Blood Pressure and Renal Aminopeptidase Activities in Male Wistar Rats
Abstract
:1. Introduction
2. Results
2.1. Systolic Blood Pressure, Water Intake and Diuresis
2.2. Angiotensinase Activities
2.3. Tyrosine Aminopeptidase Activity
2.4. Pyroglutamyl Aminopeptidase Activity
2.5. Determination of Renal Pathological Changes Using Urine Test Strip
3. Discussion
4. Materials and Methods
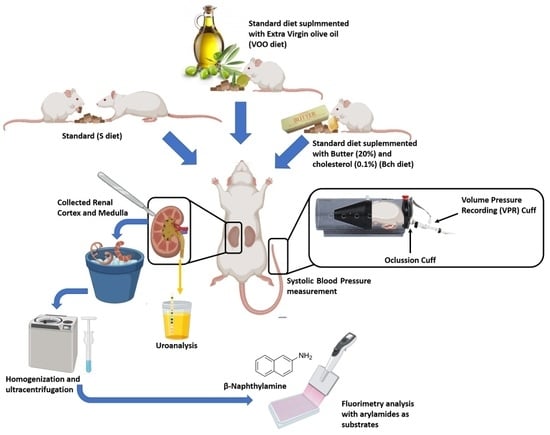
4.1. Animals and Diets
4.2. Systolic Blood Pressure, Water Intake and Diuresis Quantification
4.3. Aminopeptidases Activities Assay
4.4. Protein Measurement
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pi-Sunyer, F.X. The Obesity Epidemic: Pathophysiology and Consequences of Obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Elzorba, H.Y.; Kamel, G.M. Effect of α-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol. Res. 2004, 50, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Domínguez-Vías, G.; Segarra, A.B.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Influence of a virgin olive oil versus butter plus cholesterol-enriched diet on testicular enzymatic activities in adult male rats. Int. J. Mol. Sci. 2017, 18, 1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. The role of high fat diets and liver peptidase activity in the development of obesity and insulin resistance in wistar rats. Nutrients 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valensi, P. Hypertension, single sugars and fatty acids. J. Hum. Hypertens. 2005, 19, S5–S9. [Google Scholar] [CrossRef] [Green Version]
- Stupin, M.; Kibel, A.; Stupin, A.; Selthofer-Relatić, K.; Matić, A.; Mihalj, M.; Mihaljević, Z.; Jukić, I.; Drenjančević, I. The physiological effect of n-3 polyunsaturated fatty acids (N-3 pufas) intake and exercise on hemorheology, microvascular function, and physical performance in health and cardiovascular diseases; is there an interaction of exercise and dietary n-3 pufa intake? Front. Physiol. 2019, 10, 1129. [Google Scholar] [CrossRef] [Green Version]
- Van Wagoner, D.R.; Rennison, J.H. Impact of dietary fatty acids on cardiac arrhythmogenesis. Circ. Arrhythm. Electrophysiol. 2009, 2, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Harris, W.S.; West, S.G. Effects of marine-derived omega-3 fatty acids on systemic hemodynamics at rest and during stress: A dose-response study. Ann. Behav. Med. 2012, 44, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.Z.; Lu, W.; Zong, X.F.; Ruan, H.Y.; Liu, Y. Obesity and hypertension. Exp. Ther. Med. 2016, 12, 2395–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilopoulou, D.; Markey, O.; Kliem, K.E.; Fagan, C.C.; Grandison, A.S.; Humphries, D.J.; Todd, S.; Jackson, K.G.; Givens, D.I.; Lovegrove, J.A. Reformulation initiative for partial replacement of saturated with unsaturated fats in dairy foods attenuates the increase in LDL cholesterol and improves flow-mediated dilatation compared with conventional dairy: The randomized, controlled REplacement of SaturatEd fat in dairy on Total cholesterol (RESET) study. Am. J. Clin. Nutr. 2020, 111, 739–748. [Google Scholar] [CrossRef]
- Schwartz, J.H.; Young, J.B.; Landsberg, L. Effect of dietary fat on sympathetic nervous system activity in the rat. J. Clin. Investig. 1983, 72, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; Dehghan, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Li, W.; Chen, H.; Yi, S.; et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 2017, 5, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Segarra, A.B.; Ramirez, M.; Banegas, I.; Alba, F.; Vives, F.; Gasparo, M.D.; Ortega, E.; Ruiz, E.; Prieto, I. Dietary Fat Influences Testosterone, Cholesterol, Aminopeptidase A, and Blood Pressure in Male Rats. Horm. Metab. Res. 2008, 40, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.; Ramírez-Sánchez, M.; Segarra, A.B.; Martínez-Cañamero, M.; Prieto, I. Influence of Extra Virgin Olive Oil on Blood Pressure and Kidney Angiotensinase Activities in Spontaneously Hypertensive Rats. Planta Med. 2015, 81, 664–669. [Google Scholar] [CrossRef]
- Prieto, I.; Hermoso, F.; Gasparo, M.D.; Vargas, F.; Alba, F.; Segarra, A.B.; Banegas, I.; Ramírez, M. Angiotensinase activities in the kidney of renovascular hypertensive rats. Peptides 2003, 24, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Kobori, H.; Nangaku, M.; Navar, L.G.; Nishiyama, A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007, 59, 251–287. [Google Scholar] [CrossRef]
- Navar, L.G.; Prieto, M.C.; Satou, R.; Kobori, H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr. Opin. Pharmacol. 2011, 11, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Barrett, A.J.; Rawlings, N.D.; Woessner, J.F. Handbook of Proteolytic Enzymes; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Banegas, I.; Wangensteen, R.; Martinez-Cañamero, M.; Gasparo, M.D.; Vives, F.; Ramírez-Sánchez, M. Brain, heart and kidney correlate for the control of blood pressure and water balance: Role of angiotensinases. Neuroendocrinology 2014, 100, 198–208. [Google Scholar] [CrossRef]
- Gasparo, M.D.; Whitebread, S.; Bottari, S.P.; Levens, N.R. Heterogeneity of Angiotensin Receptor Subtypes. In Medicinal Chemistry of the Renin-Angiotensin System; Timmermans, P.B.M.W.M., Wexler, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 269–294. [Google Scholar]
- García-Sáinz, J.A.; Martínez-Alfaro, M.; Romero-Avila, M.T.; González-Espinosa, C. Characterization of the AT1 angiotensin II receptor expressed in guinea pig liver. J. Endocrinol. 1997, 154, 133–138. [Google Scholar] [CrossRef]
- Chai, S.Y.; Fernando, R.; Peck, G.; Ye, S.Y.; Mendelsohn, F.A.; Jenkins, T.A.; Albiston, A.L. What’s new in the renin-angiotensin system? The angiotensin IV/AT4 receptor. Cell. Mol. Life Sci. 2004, 61, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.K.; Krebs, L.T.; Hamilton, T.A.; Ong, B.; Lawrence, K.A.; Sardinia, M.F.; Harding, J.W.; Wright, J.W. Autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in rats. Peptides 1998, 19, 269–277. [Google Scholar] [CrossRef]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Wangensteen, R.; Banegas, I.; Gasparo, M.D.; Vanderheyden, P.; Zorad, S.; Vives, F.; Ramírez-Sánchez, M. Tissue distribution of CysAP activity and its relationship to blood pressure and water balance. Life Sci. 2015, 134, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; Ramírez-Sánchez, M.; Gasparo, M.D.; Banegas, I.; Martínez-Cañamero, M.; Vives, F.; Prieto, I. The Profile of Fatty Acids in Frontal Cortex of Rats Depends on the Type of Fat Used in the Diet and Correlates with Neuropeptidase Activities. Horm. Metab. Res. 2011, 43, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Segarra, A.B.; Montoro-Molina, S.; Gracia, M.D.D.; Osuna, A.; O’Valle, F.; Gómez-Guzmán, M.; Vargas, F.; Wangensteen, R. Glutamyl aminopeptidase in microvesicular and exosomal fractions of urine is related with renal dysfunction in cisplatin-treated rats. PLoS ONE 2017, 12, e0175462. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Wangesteen, R.; Rodríguez-Gómez, I.; García-Estañ, J. Aminopeptidases in cardiovascular and renal function. Role as predictive renal injury biomarkers. Int. J. Mol. Sci. 2020, 21, 5615. [Google Scholar] [CrossRef]
- Vauquelin, G.; Michotte, Y.; Smolders, I.; Sarre, S.; Ebinger, G.; Dupont, A.; Vanderheyden, P. Cellular targets for angiotensin II fragments: Pharmacological and molecular evidence. J. Renin Angiotensin Aldosterone Syst. 2002, 3, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Wallis, M.G.; Lankford, M.F.; Keller, S.R. Vasopressin is a physiological substrate for the insulin-regulated aminopeptidase IRAP. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1092–E1102. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, M.; Prieto, I.; Alba, F.; Vives, F.; Banegas, I.; Gasparo, M.D. Role of central and peripheral aminopeptidase activities in the control of blood pressure: A working hypothesis. Heart Fail. Rev. 2008, 13, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sanchez, M.; Prieto, I.; Wangensteen, R.; Banegas, I.; Segarra, A.B.; Villarejo, A.B.; Vives, F.; Cobo, J.; Gasparo, M.D. The Renin-Angiotensin System: New Insight into Old Therapies. Curr. Med. Chem. 2013, 20, 1313–1322. [Google Scholar] [CrossRef]
- Arechaga, G.; Prieto, I.; Segarra, A.B.; Alba, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I.; Gasparo, M.D.; Ramírez, M. Dietary fatty acid composition affects aminopeptidase activities in the testes of mice. Int. J. Androl. 2002, 25, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, Y.; Luo, R.; Chen, S.; Wang, F.; Zheng, P.; Levi, M.; Yang, T.; Wang, W. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am. J. Physiol. Renal Physiol. 2016, 310, F351–F363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kincaid-Smith, P. Hypothesis: Obesity and the insulin resistance syndrome play a major role in end-stage renal failure attributed to hypertension and labelled “hypertensive nephrosclerosis”. J. Hypertens. 2004, 22, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Romero, R.; Lopez, D.; Navarro, M.; Esteve, A.; Perez, N.; Alastrue, A.; Ariza, A. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008, 73, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.A.; Davis, J.O.; Witty, R.T. Effects of Catecholamines and Renal Nerve Stimulation on Renin Release in the Nonfilterlng Kidney. Circ. Res. 1971, 29, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Z.; Yao, B.; Fang, X.; Wang, S.; Smith, J.P.; Harris, R.C. Intrarenal dopaminergic system regulates renin expression. Hypertension 2009, 53, 564–570. [Google Scholar] [CrossRef]
- Armando, I.; Konkalmatt, P.; Felder, R.A.; Jose, P.A. The renal dopaminergic system: Novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl. Res. 2015, 165, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Jackson, K.L.; Marques, F.Z.; Watson, A.M.D.; Palma-Rigo, K.; Nguyen-Huu, T.P.; Morris, B.J.; Charchar, F.J.; Davern, P.J.; Head, G.A. A novel interaction between sympathetic overactivity and aberrant regulation of renin by miR-181a in BPH/2J genetically hypertensive mice. Hypertension 2013, 62, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Watson, A.M.D.; Gould, E.A.M.; Penfold, S.A.; Lambert, G.W.; Pratama, P.R.; Dai, A.; Gray, S.P.; Head, G.A.; Jandeleit-Dahm, K.A. Diabetes and hypertension differentially affect renal catecholamines and renal reactive oxygen species. Front. Physiol. 2019, 10, 309. [Google Scholar] [CrossRef]
- Visser, T.J.; Klootwijk, W.; Docter, R.; Hennemann, G. Degradation of Thyrotropin Releasing Hormone and a Related Compound by Rat Liver and Kidney Homogenate. Neuroendocrinology 1976, 21, 204–213. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. The forgotten effects of thyrotropin-releasing hormone: Metabolic functions and medical applications. Front. Neuroendocrinol. 2019, 52, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug. Targets. 2018, 18, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Pinya, S.; Mar Bibiloni, M.D.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive Oil-related Anti-inflammatory Effects on Atherosclerosis: Potential Clinical Implications. Endocr. Metab. Immune Disord Drug Targets 2018, 18, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Incerti, J.; Zelmanovitz, T.; Camargo, J.L.; Gross, J.L.; Azevedo, M.J.D. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol. Dial. Transplant. 2005, 20, 2402–2407. [Google Scholar] [CrossRef] [Green Version]
- Bottari, S.P.; Gasparo, M.D.; Steckelings, U.M.; Levens, N.R. Angiotensin II receptor subtypes: Characterization, signalling mechanisms, and possible physiological implications. Front. Neuroendocrinol. 1993, 14, 123–171. [Google Scholar] [CrossRef]
- Imig, J.D. Eicosanoids and renal damage in cardiometabolic syndrome. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Rangel-Silvares, R.; Nunes-Goulart, D.S.P.E.; Ilaquita-Flores, E.E.; Lino-Rodrigues, K.; Ribeiro-Silva, A.; Gonçalves-de-Albuquerque, C.F.; Daliry, A. High-fat diet-induced kidney alterations in rats with metabolic syndrome: Endothelial dysfunction and decreased antioxidant defense. Diabetes Metab. Syndr. Obes. 2019, 12, 1773–1781. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Inoue, K.; Sodhi, K.; Puri, N.; Peterson, S.J.; Rezzani, R.; Abraham, N.G. High-fat diet exacerbates renal dysfunction in SHR: Reversal by induction of HO-1-adiponectin axis. Obesity 2012, 20, 945–953. [Google Scholar] [CrossRef] [Green Version]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Wu, H.; Yang, X.; Li, Y.; Zhang, Z.; Chen, F.; Zhao, L.; Zhang, C. Lactobacillus Mucosae Strain Promoted by a High-Fiber Diet in Genetic Obese Child Alleviates Lipid Metabolism and Modifies Gut Microbiota in ApoE-/- Mice on a Western Diet. Microorganisms 2020, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Wu, C.; Li, H.; Du, H.; Huang, C.; Chen, Y.; Wang, W.; Zhu, Q.; Wang, L. Enterococcus faecalis contributes to hypertension and renal injury in Sprague-Dawley rats by disturbing lipid metabolism. J. Hypertens. 2021, 39, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Karaduta, O.; Glazko, G.; Dvanajscak, Z.; Arthur, J.; Mackintosh, S.; Orr, L.; Rahmatallah, Y.; Yeruva, L.; Tackett, A.; Zybailov, B. Resistant starch slows the progression of CKD in the 5/6 nephrectomy mouse model. Physiol. Rep. 2020, 8, e14610. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, E.; Chung, H.J.; Hong, J.; Tang, W.H.W.; Lee, S.I.; Hong, S.T.; Lee, S. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC Microbiol. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Imig, J.D. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin. Sci. 2010, 118, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Huang, Y.; Frankel, J.; Addis, C.; Jaswani, L.; Wehner, P.S.; Mangiarua, E.I.; McCumbee, W.D. The short-term consumption of a moderately high-fat diet alters nitric oxide bioavailability in lean female Zucker rats. Can. J. Physiol. Pharmacol. 2011, 89, 245–257. [Google Scholar] [CrossRef]
- Rinaldi, A.E.M.; Gabriel, G.F.C.P.; Moreto, F.; Corrente, J.E.; McLellan, K.C.P.; Burini, R.C. Dietary factors associated with metabolic syndrome and its components in overweight and obese Brazilian schoolchildren: A cross-sectional study. Diabetol. Metab. Syndr. 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of metabolic syndrome and dietary intervention. Int. J. Mol. Sci. 2019, 20, 128. [Google Scholar] [CrossRef] [Green Version]
- Jahandideh, F.; Wu, J. Perspectives on the potential benefits of antihypertensive peptides towards metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2192. [Google Scholar] [CrossRef] [Green Version]
- Segarra, A.B.; Domínguez-Vías, G.; Redondo, J.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Hypothalamic renin–angiotensin system and lipid metabolism: Effects of virgin olive oil versus butter in the diet. Nutrients 2021, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; Bruss, Z.S.; Sehdev, J.S. Physiology, Renal, Blood Flow and Filtration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Van Beusecum, J.; Inscho, E.W. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr. Opin. Pharmacol. 2015, 21, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, M.; Prieto, I.; Martinez, J.M.; Vargas, F.; Alba, F. Renal aminopeptidase activities in animal models of hypertension. Regul. Pept. 1997, 72, 155–159. [Google Scholar] [CrossRef]
- Prieto, I.; Martinez, A.; Martinez, J.M.; Ramírez, M.J.; Vargas, F.; Alba, F.; Ramírez, M. Activities of aminopeptidases in a rat saline model of volume hypertension. Horm. Metab. Res. 1998, 30, 246–248. [Google Scholar] [CrossRef]
- Prieto, I.; Martínez, J.M.; Hermoso, F.; Ramírez, M.J.; Gasparo, M.D.; Vargas, F.; Alba, F.; Ramírez, M. Effect of valsartan on angiotensin II- and vasopressin-degrading activities in the kidney of normotensive and hypertensive rats. Horm. Metab. Res. 2001, 33, 559–563. [Google Scholar] [CrossRef]
- Prieto, I.; Hermoso, F.; Gasparo, M.D.; Vargas, F.; Alba, F.; Segarra, A.B.; Banegas, I.; Ramírez, M. Aminopeptidase activity in renovascular hypertension. Med. Sci. Monit. 2003, 9, 31–36. [Google Scholar] [PubMed]
- Villarejo, A.B.; Segarra, A.B.; Ramírez, M.; Banegas, I.; Wangensteen, R.; Gasparo, M.D.; Cobo, J.; Alba, F.; Vives, F.; Prieto, I. Angiotensinase and vasopressinase activities in hypothalamus, plasma, and kidney after inhibition of angiotensin-converting enzyme: Basis for a new working hypothesis. Horm. Metab. Res. 2012, 44, 152–154. [Google Scholar] [CrossRef]
- Grobe, N.; Elased, K.M.; Cool, D.R.; Morris, M. Mass spectrometry for the molecular imaging of angiotensin metabolism in kidney. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Kemp, B.A.; Bell, J.F.; Rottkamp, D.M.; Howell, N.L.; Shao, W.; Navar, L.G.; Padia, S.H.; Carey, R.M. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension 2012, 60, 387–395. [Google Scholar] [CrossRef]
- Chung, S.; Park, C.W.; Shin, S.J.; Lim, J.H.; Chung, H.W.; Youn, D.Y.; Kim, H.W.; Kim, B.S.; Lee, J.H.; Kim, G.H.; et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol. Dial. Transplant. 2010, 25, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Reverte, V.; Gogulamudi, V.R.; Rosales, C.B.; Musial, D.C.; Gonsalez, S.R.; Parra-Vitela, A.J.; Galeas-Pena, M.; Sure, V.N.; Visniauskas, B.; Lindsey, S.H.; et al. Urinary angiotensinogen increases in the absence of overt renal injury in high fat diet-induced type 2 diabetic mice: uAGT in mice with HFD-induced T2DM. J. Diabetes Complicat. 2020, 34, 107448. [Google Scholar] [CrossRef]
- Albiston, A.L.; Yeatman, H.R.; Pham, V.; Fuller, S.J.; Diwakarla, S.; Fernando, R.N.; Chai, S.Y. Distinct distribution of GLUT4 and insulin regulated aminopeptidase in the mouse kidney. Regul. Pept. 2011, 166, 83–89. [Google Scholar] [CrossRef]
- Coatmellec-Taglioni, G.; Ribière, C. Factors that influence the risk of hypertension in obese individuals. Curr. Opin. Nephrol. Hypertens. 2003, 12, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Coatmellec-Taglioni, G.; Dausse, J.P.; Ribière, C.; Giudicelli, Y. Hypertension in cafeteria-fed rats: Alterations in renal α2-adrenoceptor subtypes. Am. J. Hypertens. 2000, 13, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Hernández, A.; Figuero-Pérez, L.; Cruz-Hernandez, J.J.; Sarmiento, R.G.; Usategui-Martin, R.; Miramontes-González, J.P. Dopamine receptors and the kidney: An overview of health-and pharmacological-targeted implications. Biomolecules 2021, 11, 254. [Google Scholar] [CrossRef]
- Kouyoumdzian, N.M.; Rukavina-Mikusic, N.L.; Robbesaul, G.D.; Gorzalczany, S.B.; Carranza, A.; Trida, V.; Fernández, B.E.; Choi, M.R. Acute infusion of angiotensin II regulates organic cation transporters function in the kidney: Its impact on the renal dopaminergic system and sodium excretion. Hypertens. Res. 2021, 44, 286–298. [Google Scholar] [CrossRef]
- Abe, K.; Fukuda, K.; Tokui, T. Marginal involvement of pyroglutamyl aminopeptidase I in metabolism of thyrotropin-releasing hormone in rat brain. Biol. Pharm. Bull. 2004, 27, 1197–11201. [Google Scholar] [CrossRef] [Green Version]
- Landa, M.S.; García, S.I.; Schuman, M.L.; Peres-Diaz, L.S.; Aisicovich, M.; Pirola, C.J. Cardiovascular and body weight regulation changes in transgenic mice overexpressing thyrotropin-releasing hormone (TRH). J. Physiol. Biochem. 2020, 76, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Arechaga, G.; Segarra, A.B.; Alba, F.; Gasparo, M.; Ramirez, M. Effects of dehydration on renal aminopeptidase activities in adult male and female rats. Regul. Pept. 2002, 106, 27–32. [Google Scholar] [CrossRef]
- Gasparello-Clemente, E.; Casis, L.; Varona, A.; Gil, J.; Irazusta, J.; Silveira, P.F. Aminopeptidases in visceral organs during alterations in body fluid volume and osmolality. Peptides 2003, 24, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Alcaide-Hidalgo, J.M.; Romero, M.; Duarte, J.; López-Huertas, E. Antihypertensive Effects of Virgin Olive Oil (Unfiltered) Low Molecular Weight Peptides with ACE Inhibitory Activity in Spontaneously Hypertensive Rats. Nutrients 2020, 12, 271. [Google Scholar] [CrossRef] [Green Version]
- Segarra, A.B.; Ramírez-Sánchez, M.; Banegas, I.; Hermoso, F.; Vargas, F.; Vives, F.; Alba, F.; de Gasparo, M.; Prieto, I. Influence of thyroid disorders on kidney angiotensinase activity. Horm. Metab. Res. 2006, 38, 48–52. [Google Scholar] [CrossRef]
- Banegas, I.; Prieto, I.; Vives, F.; Alba, F.; Gasparo, M.D.; Duran, R.; Luna, J.D.; Segarra, A.B.; Hermoso, F.; Ramírez, M. Asymmetrical response of aminopeptidase A and nitric oxide in plasma of normotensive and hypertensive rats with experimental hemiparkinsonism. Neuropharmacology 2009, 56, 573–579. [Google Scholar] [CrossRef]
- Greenberg, L.J. Fluorometric measurement of alkaline phosphatase and aminopeptidase activities in the order of 10−14 mole. Biochem. Biophys. Res. Commun. 1962, 9, 430–435. [Google Scholar] [CrossRef]
- Cheung, H.S.; Cushman, D.W. A soluble aspartate aminopeptidase from dog kidney. Biochim. Biophys. Acta 1971, 242, 190–193. [Google Scholar] [CrossRef]
- Tobe, H.; Kojima, F.; Aoyagi, T.; Umezawa, H. Purification by affinity chromatography using amastatin and properties of aminopeptidase A from pig kidney. Biochim. Biophys. Acta 1980, 613, 459–468. [Google Scholar] [CrossRef]
- Ramírez, M.; Prieto, I.; Banegas, I.; Segarra, A.B.; Alba, F. Neuropeptidases. Methods Mol. Biol. 2011, 789, 287–294. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]



| Median; (Interquartile Range) | S Diet | VOO Diet | Bch Diet | p Value |
|---|---|---|---|---|
| Nitrite (mg/dL) | 0; (0–0) | 0; (0–0) | 0; (0–1) | 0.038 |
| Protein (mg/dL) | 2; (1.5–2) | 1.5; (1–2) | 1.5; (1–2) | 0.438 |
| Bilirrubin (mg/dL) | 1; (0.5–1) | 0; (0–0) | 1; (0–1) | 0.004 S vs. VOO |
| Ketone (mmol/L) | 1; (0.5–1) | 0; (0–0.5) | 0; (0–0.5) | ≤0.001 S vs. VOO S vs. Bch |
| pH | 8; (7.75–8.5) | 6.5; (6.5–7.75) | 6.25; (6–7.25) | 0.001 S vs. VOO S vs. Bch |
| Density (SG) | 1002.5; (1000–1010) | 1027.5; (1010–1030) | 1030; (1017.5–1030) | 0.001 S vs. VOO S vs. Bch |
| Leukocytes (WBC/µL) | 0.5; (0.5–1) | 0; (0–0) | 0.5; (0–0.5) | 0.008 S vs. VOO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. Effects of Virgin Olive Oil on Blood Pressure and Renal Aminopeptidase Activities in Male Wistar Rats. Int. J. Mol. Sci. 2021, 22, 5388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105388
Domínguez-Vías G, Segarra AB, Ramírez-Sánchez M, Prieto I. Effects of Virgin Olive Oil on Blood Pressure and Renal Aminopeptidase Activities in Male Wistar Rats. International Journal of Molecular Sciences. 2021; 22(10):5388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105388
Chicago/Turabian StyleDomínguez-Vías, Germán, Ana Belén Segarra, Manuel Ramírez-Sánchez, and Isabel Prieto. 2021. "Effects of Virgin Olive Oil on Blood Pressure and Renal Aminopeptidase Activities in Male Wistar Rats" International Journal of Molecular Sciences 22, no. 10: 5388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105388