1. Introduction
While cancer immunotherapy has a rich and interesting history [
1], recent advances in our understanding of the complex interactions between the tumor and immune cells have provided the opportunity to exploit the power of the immune system for the benefit of patients. A range of different approaches have been explored in the past including the use of cytokines, anti-tumor antibodies, adoptive cell transfer, and cancer vaccines, with the use immune checkpoint blockade agents coming to prominence over the last decade.
Immune checkpoint molecules include cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PDCD1, PD-1). Both molecules act to inhibit T lymphocytes—CTLA-4 acts to block T cell co-stimulatory signaling via preventing the interactions between CD28 and CD80/86, and PD-1 via T cell inhibitory signaling upon engagement with its ligands PD-L1 (CD274) and PD-L2 (PDCD1LG2). Strategies that block these T cell-inhibitory molecules have proved successful in unleashing the patient’s own T cell immunity against tumor antigens in cancer.
PDCD1 was first described in 1992 [
2] and analysis of knockout mouse models revealed the role it plays in suppressing T cell responses with the development of autoimmunity in its absence [
3,
4]. Expression of CD274 ligands on tumor cells was found to contribute to tumor immune evasion and, in mouse models, antibody-mediated blockade of CD274 caused suppression of transplanted melanoma tumor growth [
5]. Based on these observations nivolumab, a fully humanized anti-PDCD1 antibody, was developed and approved for treatment, initially for malignant melanoma. Since then, a variety of other agents have been developed including those targeting CD274 (atezolizumab, avelumab, and durvalumab) as well as PDCD1 (pembrolizumab and cemiplimab). The range of approved cancers has also grown to include small and non-small cell lung cancer, renal cell carcinoma, squamous cell head and neck carcinoma, non-Hodgkin lymphoma, gastric cancer, triple negative breast cancer, and cervical cancer, among others (reviewed in [
6]).
Recent meta-analyses of the efficacy of antibodies targeting PDCD1/CD274 as monotherapy [
7,
8] found that this treatment is associated with more tumor responses and increased overall survival (OS) compared to conventional therapy. Although there are some patients who show long-term complete responses [
9,
10], the average response rate is 20% with significant differences across different tumor types [
8]. It is of significant interest to identify the minority of patients who will benefit from immune checkpoint blockade to better direct this therapy and avoid the (often immune-mediated) adverse events [
11]. Higher objective response rates to PDCD1/CD274 monotherapy have been associated with a number of factors, including immunogenicity of the tumor [
8], male sex, age < 65 years, current and former smokers, a lack of central nervous system or liver metastasis, and a lack of EGFR mutations [
7]. Therapy responses are not limited to those cancers in which CD274 expression is detected but high CD274 is associated with a greater response [
8,
12,
13]. In contrast, high levels of serum [
14] or exosomal [
15] CD274 have been associated with non-responders.
One approach to increase the pool of patients who benefit from checkpoint blockade therapy is to explore combination therapy. A recent analysis of clinical trials found that PDCD1/CD274 monotherapy studies are declining from a peak in 2017, while combination therapies show consistent increases over time and represent a large majority of recent trials [
16]. A range of different agents have been explored in combination, including chemotherapy and interventions targeting CTLA-4 and VEGF [
16]. An early study in melanoma found that the combination of the anti-CTLA-4 antibody ipilimumab plus nivolumab (anti-PD-1) resulted in responses greater than those seen in monotherapy trials [
17]. At maximum acceptable doses, objective responses were observed in 53% of patients, with responding patients showing tumor reduction of at least 80% [
17]. Identification of appropriate adjuvant therapies is of considerable interest, with the repositioning of previously approved drugs potentially minimizing the cost and time needed for clinical use approval [
18].
This exploratory study uses a bioinformatic approach to examine the impact of mRNA levels of PDCD1, CD274, and genes showing correlated expression levels on the survival outcome of a range of cancers. Based on a panel of genes predicted to impact patient outcomes, potential therapeutic strategies were explored including the prediction of microRNAs and identification of approved drugs capable of targeting the relevant proteins. In this way, additional therapeutic targets, likely to complement direct PDCD1/CD274 antibody targeting, are identified.
3. Discussion
This study utilized a bioinformatic approach to explore potential factors contributing to the PDCD1/CD274 network on a pan-cancer basis. Through this approach, several genes commonly co-expressed with PDCD1/CD274 were identified and their clinical importance ascertained through Kaplan–Meier plots. Further molecular insights were generated by assessing potential links to microRNAs, which were in turn assessed for clinical relevance. Finally, potential repurposed drugs were identified which target the genes that commonly co-express with PDCD1/CD274.
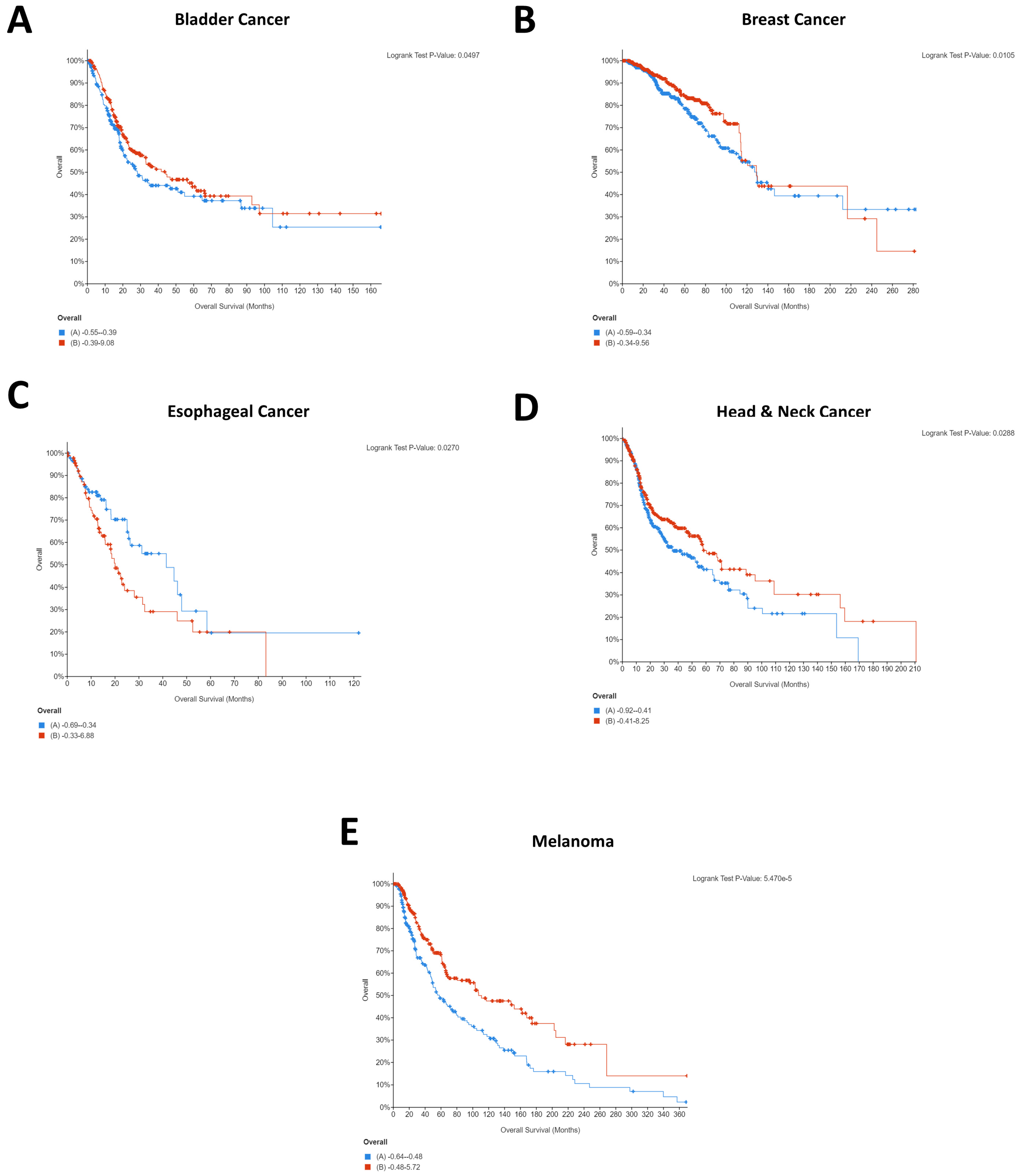
It is interesting to note that for breast cancer, renal cancer, and melanoma, high CD274 expression was statistically significantly beneficial for patients. Whilst this was initially surprising, as it is logical that CD274 expression would result in immune evasion and thereby cancer survival, a recent pooled analysis from 1062 melanoma patients showed that CD274 expression had no significant relationship with overall survival but that high CD274 expression was significantly associated with lymph node metastases [
27]. As such, it is highly probable that indications of survival will be cohort specific. A similar pattern was seen for PDCD1 expression, with four of five cancers demonstrating that high PDCD1 expression was statistically significantly beneficial. Comparatively, esophageal cancer showed the opposite, with low expression being beneficial for patients (
Figure 2C).
This difference could explain the starkly different Kaplan–Meier plots that were identified for the PDCD1/CD274-correlated genes, with the majority of cancers showing the same pattern for the co-expressed genes whilst esophageal and renal were notably different. Importantly, these differences could provide insight into the different underlying networks supporting the immunotherapy response and provide an opportunity for the identification of cancer type-specific adjuvants to immunotherapeutic agents.
Subsequent to the gene-level analysis was the microRNA analysis. Although 49 microRNAs were identified as linked to the CD274/PDCD1 network and many showed survival relationships (with 130 significant survival relationships being identified across fourteen cancer types), it is beyond the scope of this article to validate each of them. However, literature validation of a subset shows good consistency with previous research. For instance, a 2017 article demonstrated that hsa-miR-142-5p promotes cell growth and migration in renal cell carcinoma; therefore, low hsa-miR-142-5p expression would be beneficial for patients [
28]. This is consistent with the data shown in
Figure 3, where low expression of hsa-miR-142-5p was significantly beneficial for patients.
A similar outcome was that hsa-miR-337-3p was identified as potentially capable of targeting JAK2 and
Table 2 demonstrates that high expression of the JAK2 gene was beneficial in several cancers, including renal and bladder cancer. In concordance with this, high expression of hsa-miR-337-3p was associated with poor outcomes in these cancer types (
Figure 3). There is also experimental evidence to support the ability of this microRNA to suppress expression of JAK2 [
29,
30] via decreased luciferase activity upon co-transfection of the JAK2 3′UTR luciferase reporter and hsa-miR-337-3p, and decreased JAK2 protein levels upon overexpression of the microRNA in liver cancer cell lines. It should be noted, however, that the study by Zuo and colleagues [
29] was conducted in hepatocellular carcinoma cells and hsa-miR-337-3p acted as a tumor suppressor and suppressed proliferation and invasion. Although
Figure 3 does not demonstrate a significant survival effect for hsa-miR-337-3p in liver cancer, this points to patient cohort-specific differences and the possibility of differential survival relevance between different cancer types.
Figure 3 also shows high expression of hsa-miR-146a-5p to be beneficial in several cancers (cervical, ovarian, breast, head and neck, and gastric). The tumor-suppressive role of this microRNA is well established [
31], but in other cancer types, this microRNA can act to promote cancer, working as an oncomiR [
31]. Within ovarian cancer, the protective role of hsa-miR-146a-5p may be explained in part by its effect on superoxide dismutase 2 (SOD2), which leads to increased levels of reactive oxygen species (ROS), decreased proliferation, increased apoptosis, and enhanced sensitivity to chemotherapy [
32].
From the panel of identified microRNAs, the one showing a significant survival association with the greatest number of cancers was hsa-miR-125-5p. The pattern was primarily for high expression of hsa-miR-125-5p to be good for patient outcomes (in pancreatic cancer, lung cancer, renal cancer, bladder cancer, and mesothelioma) which is in agreement with a recent metanalysis of patient data [
33]. The mechanistic understanding of this effect is supported by a body of evidence that shows that hsa-miR-125-5p acts as a tumor suppressor in a variety of cancers. In non-small cell lung cancer [
34,
35] and lung adenocarcinoma [
36], expression of hsa-miR-125-5p was associated with reduced cell growth, increased apoptosis, and increased differentiation. In vitro models have also identified a role for this microRNA in inhibiting invasion and migration of lung cancer cells [
37]. Identified in this study as a potential modulator of DUS1L, this gene has not been experimentally verified to be the target of hsa-miR-125-5p. However, a range of identified targets are involved in the KRAS and NF-kappaB pathways, for example, SOS1, GRB2, IQGAP1, RALA, RAF-1, IKKβ, AKT2, ERK2 and KRAS [
38], STAT3 [
39], and antiapoptotic proteins such as BCL2, BCL2L12, MCL1 [
40], BAP1 [
41], and TMPRSS4 [
36]. Contrary to the general trend, however, our data showed that high expression of hsa-miR-125-5p was detrimental in both gastric cancer and melanoma (
Figure 3). Despite this, there is evidence in the literature that tumor expression of hsa-miR-125-5p can suppress proliferation in gastric cancer [
42] and melanoma [
43], together with increased senescence in melanoma [
44]. This discrepancy may be explained at least in part by effects of microRNA on the tumor microenvironment. In melanoma, exosomal hsa-miR-125-5p was found to influence tumor-associated macrophages [
45] and, potentially via targeting of lysosomal acid lipase A (LIPA), helps in macrophage polarization to a tumor-promoting phenotype.
A 2016 study compared microRNA expression in short-surviving and long-surviving mesothelioma patients [
46] and identified that hsa-miR-17-5p, hsa-miR-22-3p, hsa-miR-27b-3p, and hsa-miR-93-5p were all expressed at a higher level in short-surviving patients versus long-surviving patients. This is partially consistent with the data presented in
Figure 3, where low expression of hsa-miR-17-5p and hsa-miR-93-5p was significantly beneficial for patient survival. However,
Figure 3 also shows that high expression of hsa-miR-22-3p and hsa-miR-27b-3p was beneficial for patients. Notably, the 2016 study [
46] did not identify hsa-miR-1323, hsa-miR-216a-5p, hsa-miR-380-3p, hsa-miR-497-5p, hsa-miR-580-3p, hsa-miR-125b-5p, and hsa-miR-125a-5p to be important for patient survival. Differences could arise due to patient cohort-specific differences and analytical methods, as well as the number of patients included (
n = 16 for the 2016 study [
46] and
n = 86 for the mesothelioma study from OncomiR [
24]).
This study has ultimately identified a significant number of microRNAs which have prognostic importance and may be related to the molecular network of immune checkpoint blockade. Similarly, the cancer-specific differences of the co-expressed genes highlighted in
Table 2 and
Table 3 also warrant further investigation, particularly in the case of renal cancer which was surprisingly oppositional to the other cancer types included.
The purpose of the DRUGSURV analysis was to identify repositionable drugs that could be used to target the proteins that correlated with PDCD1/CD274. In total, 23 drugs were identified targeting various members of the network. In theory, as the genes correlate with PDCD1/CD274, targeting them in conjunction with immunotherapy could boost the response and improve outcomes, or perhaps allow for lower dosages to be used whilst maintaining therapeutic effects. Although in vitro or in vivo validation has not been performed in this study, there is literature supporting this. For example, disulfiram, targeting CCR5 and CXCR6 (
Table 5), has been shown recently to have a synergistic effect with an anti-PD-1 antibody (clone J43, BioXcell ) [
47]. Disulfiram, clinically approved for alcoholism, increased the numbers of cytotoxic CD8+ T cells in the tumor when combined with the anti-PD-1 antibody and ultimately inhibited tumor growth and metastasis [
47]. The fact that disulfiram has been identified herein (
Table 5) again provides validation to the approach utilized, and indicates that the thirteen other drugs that currently do not have a cancer indication (
Table 5) could be of potential clinical utility, possibly as immunotherapeutic adjuvants.
A limitation of the present study is that only mRNA expression has been examined, and hence it is largely single-omics analyses other than the use of microRNA data in concurrence with mRNA expression. Recent reports highlight the importance of multi-omics investigation, as it allows for the flow of information at multiple biological levels to be analyzed and the data to be examined holistically [
48,
49,
50]. That said, several promising avenues have been identified in the current study that warrant further investigation at the in vitro or in vivo level. Immunotherapy in general holds significant promise, but any clinical trial pertaining to it should interpret findings with caution, as research has shown that surrogate clinical trial endpoints such as progression-free survival are not fully reflective of overall survival and therefore caution should be used when interpreting data that do not include overall survival [
51]. Ultimately, this research has identified multiple candidate genes, microRNAs, and drugs to be further investigated at the in vitro or in vivo level that could have impact in the immune checkpoint arena.