Engineering of Janus-Like Dendrimers with Peptides Derived from Glycoproteins of Herpes Simplex Virus Type 1: Toward a Versatile and Novel Antiviral Platform
Abstract
:1. Introduction
2. Results and Discussion
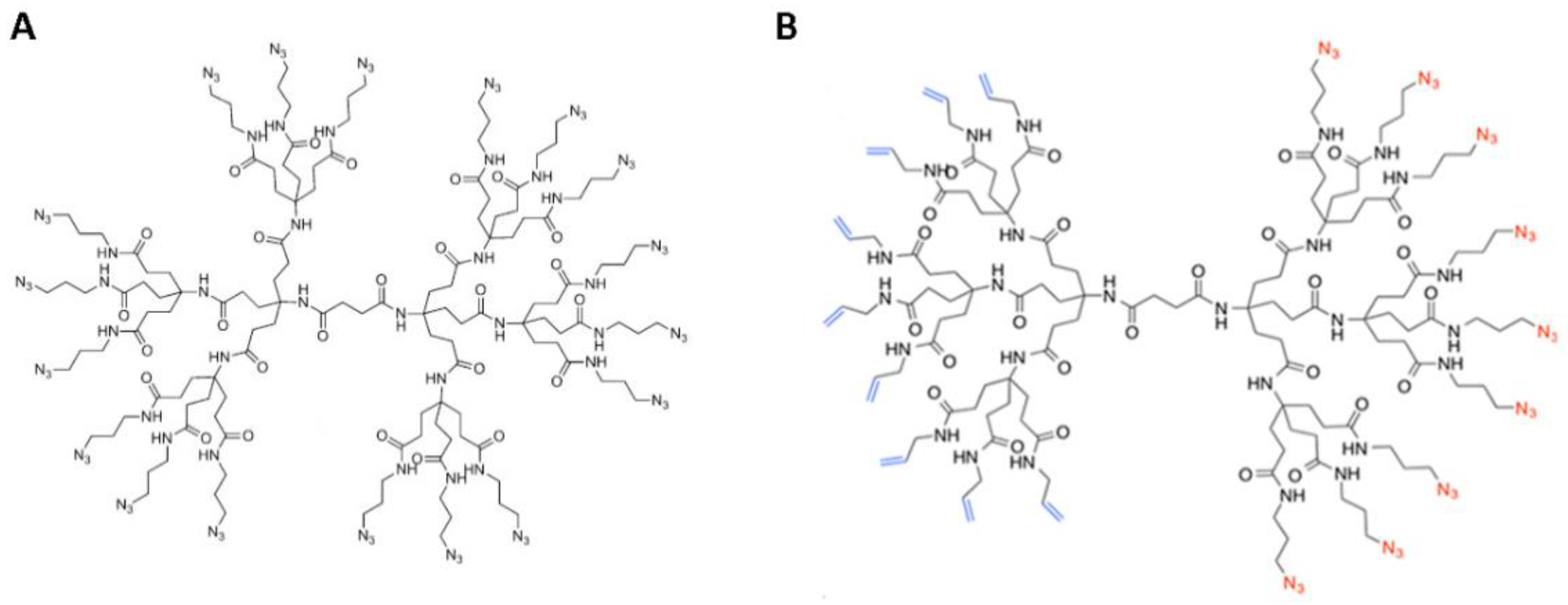
2.1. Synthesis of DendrimerA and DendrimerB
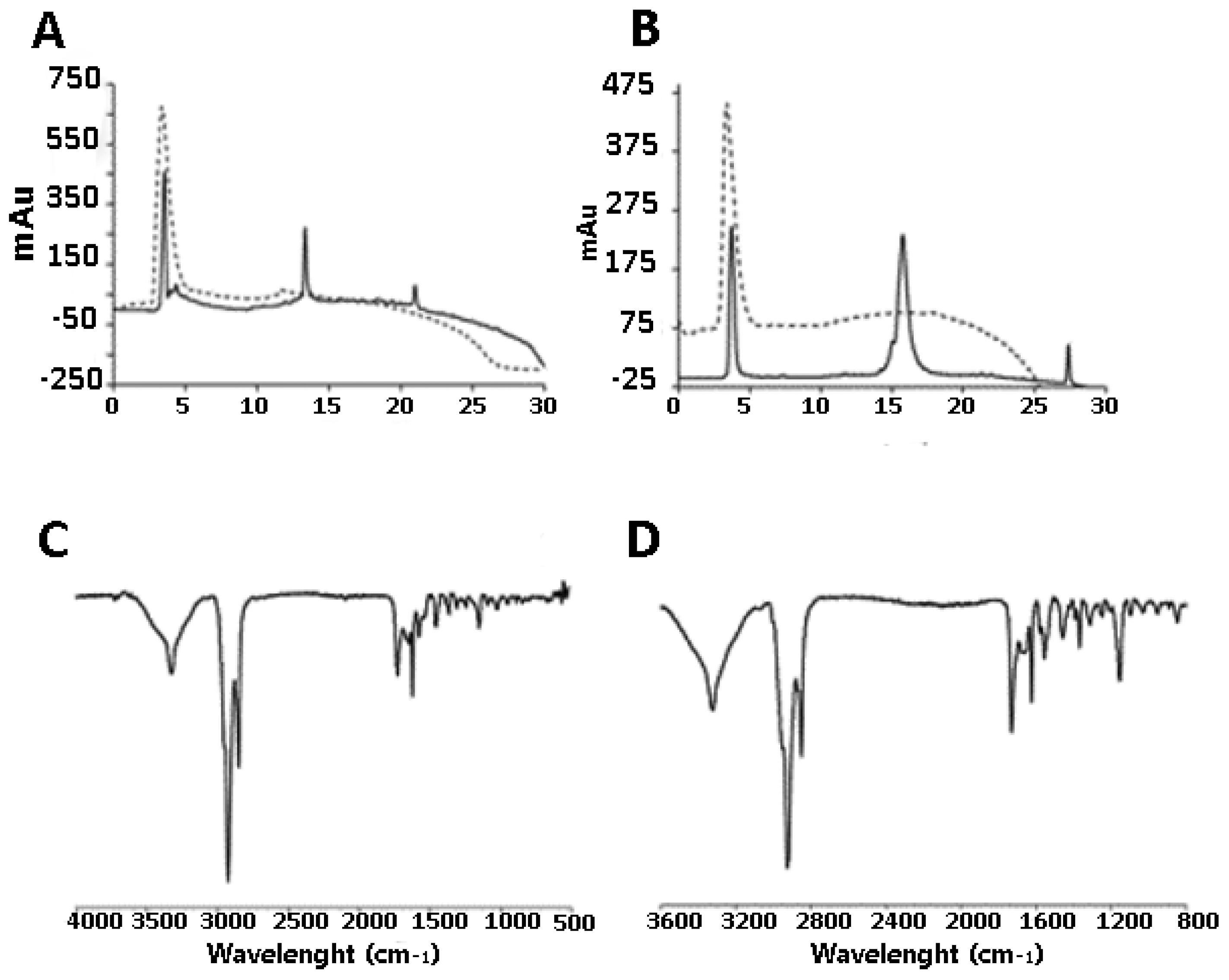
2.2. Peptide Functionalization of Monofunctional (DendrimerA) and Janus (DendrimerB) Dendrimers
2.3. Circular Dichroism
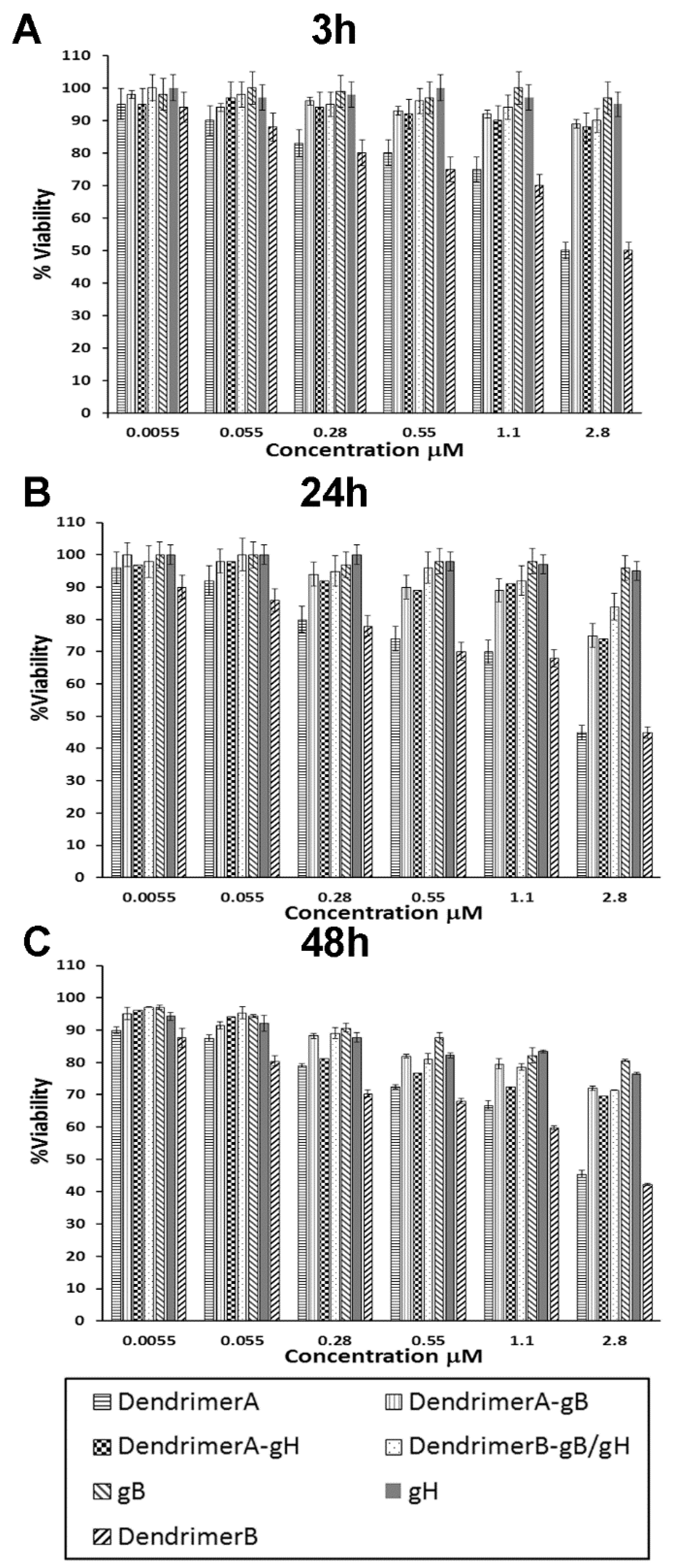
2.4. Cytotoxicity Studies
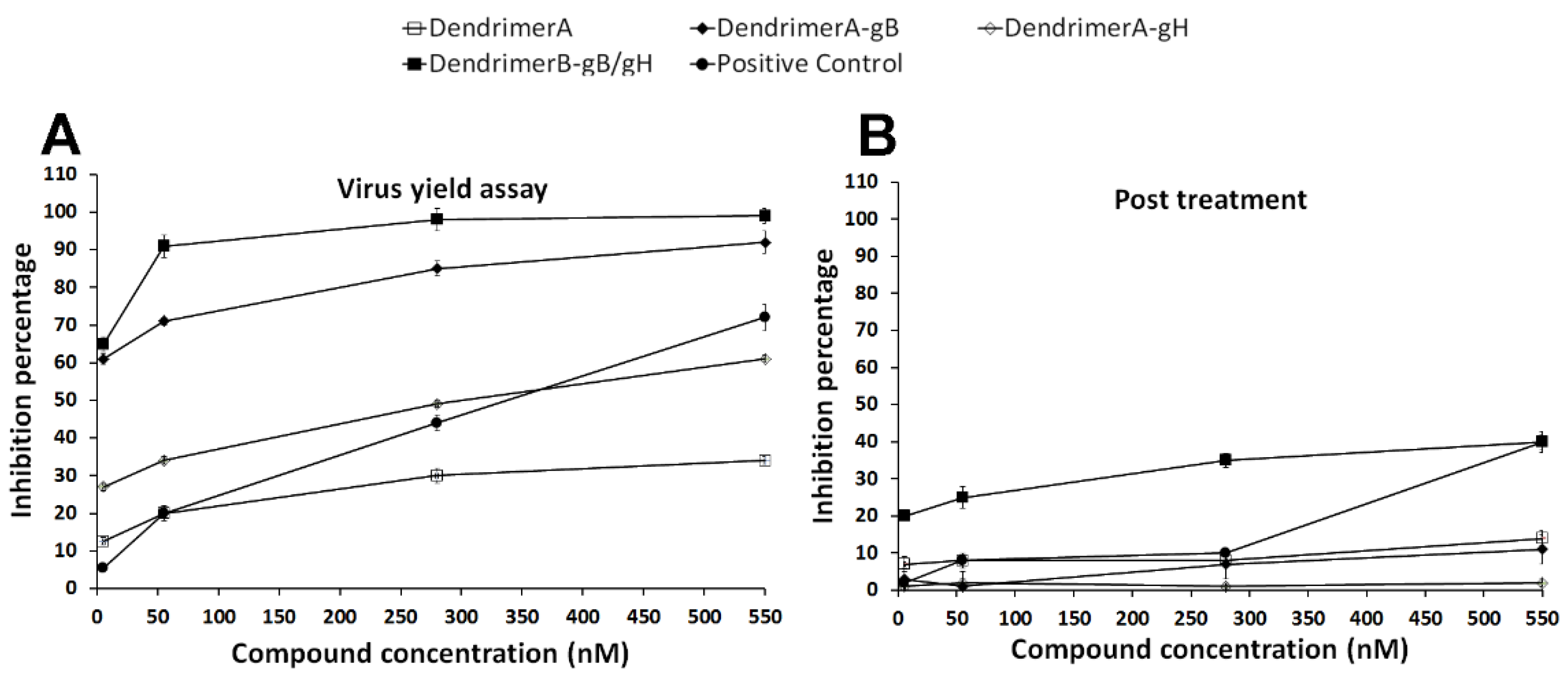
2.5. Antiviral Assays
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Monofunctional Dendrimer
4.3. Synthesis of the Bifunctional Dendrimer (Janus)
4.4. Synthesis of Peptides
4.5. Functionalization of Monofunctional Dendrimer
4.6. Functionalization of DendrimerB
4.7. IR Spectroscopy
4.8. ζ-Potential Measurement
4.9. Circular Dichroism
4.10. Cells and Viruses
4.11. Antiviral Assays
- Virus yield reduction assay. Confluent Vero cell monolayers (12-well plates) were washed with phosphate-buffered saline (PBS) and infected with HSV-1 at multiplicity of infection (MOI) of 1 for 1 h at 37 °C. Then, virus inocula were mixed with the antiviral compounds at the concentrations indicated above. Infected cells were washed with PBS, covered with fresh culture medium, and incubated for 48 h; then, they were scraped into culture medium and disrupted by sonication.
- Post-treatment assay. 5 × 105 Vero cells (12-well plates) were incubated firstly with virus (MOI 0.01) for 45 min at 37 °C and then the compounds were added to the cells followed by an additional incubation period of 30 min at 37 °C.
- Co-treatment. In co-exposure experiment, 5 × 105 cells were incubated with peptides and with the viral inoculum at MOI of 0.01 for 45 min at 37 °C.
- Cell pretreatment. In cell pre-exposure experiment, 5 × 105 Vero cells were incubated with compounds for 30 min at 37 °C and subsequently infected with HSV-1 at MOI of 0.01 for 45 min at 37 °C.
- Virus pre-treatment. In virus pre-exposure assay, HSV-1 at MOI of 0.1 was incubated with compounds for 45 min at 37 °C, and then the mixture was titrated on Vero cell monolayers.
4.12. Cytotoxicity
Author Contributions
Funding
Conflicts of Interest
References
- Baloch, S.; Baloch, M.A.; Zheng, T.; Pei, X. The coronavirus disease 2019 (covid-19) pandemic. Tohoku J. Exp. Med. 2020, 250, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.A.-O.; Lim, E.K.; Park, G.; Park, C.; Lim, J.W.; Lee, H.; Na, W.; Yeom, M.; Kim, J.; Song, D.; et al. Advanced nanomaterials for preparedness against (re-)emerging viral diseases. Adv. Mater. 2021. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, C.; Ding, X.; Engler, A.C.; Hedrick, J.L.; Yang, Y.Y. Broad-spectrum antimicrobial/antifouling soft material coatings using poly(ethylenimine) as a tailorable scaffold. Biomacromolecules 2015, 16, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, K.; Tew, G.N. Synthetic mimics of antimicrobial peptides—A versatile ring-opening metathesis polymerization based platform for the synthesis of selective antibacterial and cell-penetrating polymers. Chem. A Eur. J. 2009, 15, 11784–11800. [Google Scholar] [CrossRef]
- Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable polymers. Chem. Soc. Rev. 2013, 42, 7278–7288. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov, V.A.; Gusevskaya, K.V.; Litvinchuk, E.N.; Vlakh, E.G.; Tennikova, T.B. Enzyme-mediated ring-opening polymerization of pentadecalactone to obtain biodegradable polymer for fabrication of scaffolds for bone tissue engineering. Int. J. Polym. Sci. 2013, 2013, 476748. [Google Scholar] [CrossRef]
- Weichelt, F.; Frerich, B.; Lenz, S.; Tiede, S.; Buchmeiser, M.R. Ring-opening metathesis polymerization-based synthesis of caco3 nanoparticle-reinforced polymeric monoliths for tissue engineering. Macromol. Rapid Commun. 2010, 31, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Pan, Y.H.; Tzeng, J.J.; Wu, T.L.; Fong, T.H.; Feng, S.W.; Huang, H.M. Development and testing of x-ray imaging-enhanced poly-l-lactide bone screws. PLoS ONE 2015, 10, e0140354. [Google Scholar] [CrossRef] [Green Version]
- Goto, K.; Okuzu, Y.; So, K.; Kuroda, Y.; Matsuda, S. Clinical and radiographic evaluation of cemented socket fixation concomitant to acetabular bone grafting fixed with absorbable hydroxyapatite-poly-l-lactide composite screws. J. Orthop. Sci. 2016, 21, 57–62. [Google Scholar] [CrossRef]
- Undin, J.; Wistrand, A.F.; Albertsson, A.C. Amorphous and degradable polymers aimed for tissue engineering synthesized by radical ring-opening polymerization. Abstr. Pap. Am. Chem. Soc. 2012, 244. [Google Scholar]
- Dove, A.P. Organic catalysis for ring-opening polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Synthesis and characterizations of poly (lactic acid) by ring-opening polymerization for biomedical applications. Chem. Eng. Trans. 2014, 38, 331–336. [Google Scholar]
- Jung, H.; Carberry, T.P.; Weck, M. Synthesis of first- and second- generation poly(amide)-dendronized polymers via ring-opening metathesis polymerization. Macromolecules 2011, 44, 9075–9083. [Google Scholar] [CrossRef]
- Mackenzie, M.C.; Shrivats, A.R.; Konkolewicz, D.; Ayerick, S.E.; McDermott, M.C.; Hollinger, J.O.; Matyjaszewski, K. Synthesis of poly(meth)acrylates with thioether and tertiary sulfonium groups by arget atrp and their use as sirna delivery agents. Biomacromolecules 2015, 16, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.A.; Matyjaszewski, K. Expanding the atrp toolbox: Methacrylate polymerization with an elemental silver reducing agent. Macromolecules 2015, 48, 6457–6464. [Google Scholar] [CrossRef]
- Chmielarz, P.; Park, S.; Sobkowiak, A.; Matyjaszewski, K. Synthesis of beta-cyclodextrin-based star polymers via a simplified electrochemically mediated atrp. Polymer 2016, 88, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Iacono, M.; Heise, A. Stable poly(methacrylic acid) brush decorated silica nano-particles by arget atrp for bioconjugation. Polymers 2015, 7, 1427–1443. [Google Scholar] [CrossRef] [Green Version]
- Colzani, B.; Biagiotti, M.; Speranza, G.; Dorati, R.; Modena, T.; Conti, B.; Tomasi, C.; Genta, I. Smart biodegradable nanoparticulate materials: Poly-lactide-co-glycolide functionalization with selected peptides. Curr. Nanosci. 2016, 12, 347–356. [Google Scholar] [CrossRef]
- Kulkarni, A.; Lele, A.; Sivaram, S.; Rajamohanan, P.R.; Velankar, S.; Chatterji, A. Star telechelic poly(l-lactide) ionomers. Macromolecules 2015, 48, 6580–6588. [Google Scholar] [CrossRef]
- Noga, D.E.; Petrie, T.; Kumar, A.; Weck, M.; García, A.J.; Collard, D.M. Synthesis and modification of functional poly(lactide) copolymers: Towards biofunctional materials. Biomacromolecules 2008, 9, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Oquendo, L.E.; Schulze, M.W.; Lewis, R.M.; Gladfelter, W.L.; Hillmyer, M.A. Poly(cyclohexylethylene)-block-poly(lactide) oligomers for ultrasmall nanopatterning using atomic layer deposition. ACS Appl. Mater. Inter. 2016, 8, 7431–7439. [Google Scholar] [CrossRef] [PubMed]
- Isono, T.; Kondo, Y.; Ozawa, S.; Chen, Y.G.; Sakai, R.; Sato, S.; Tajima, K.; Kakuchi, T.; Satoh, T. Stereoblock-like brush copolymers consisting of poly(l-lactide) and poly(d-lactide) side chains along poly(norbornene) backbone: Synthesis, stereocomplex formation, and structure-property relationship. Macromolecules 2014, 47, 7118–7128. [Google Scholar] [CrossRef]
- Nystrom, A.; Malkoch, M.; Furo, I.; Nystrom, D.; Unal, K.; Antoni, P.; Vamvounis, G.; Hawker, C.J.; Wooley, K.; Malmstrom, E.; et al. Characterization of poly(norbornene) dendronized polymers prepared by ring-opening metathesis polymerization of dendron bearing monomers. Macromolecules 2006, 39, 7241–7249. [Google Scholar] [CrossRef]
- de Miguel, L.; Noiray, M.; Surpateanu, G.; Iorga, B.I.; Ponchel, G. Poly(gamma-benzyl-l-glutamate)-peg-alendronate multivalent nanoparticles for bone targeting. Int. J.Pharm. 2014, 460, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yong, X.; Xu, W.; Jin, X. Preparation and characterization of bimodal porous poly(gamma-benzyl-l-glutamate) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4587–4593. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Bhatt, A.N.; Mishra, A.K.; Dwarakanath, B.S.; Jain, S.; Schatz, C.; Le Meins, J.-F.; Farooque, A.; Chandraiah, G.; Jain, A.K.; et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(γ-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010, 31, 2882–2892. [Google Scholar] [CrossRef]
- Borchmann, D.E.; Tarallo, R.; Avendano, S.; Falanga, A.; Carberry, T.P.; Galdiero, S.; Weck, M. Membranotropic peptide-functionalized poly(lactide)-graft-poly(ethylene glycol) brush copolymers for intracellular delivery. Macromolecules 2015, 48, 942–949. [Google Scholar] [CrossRef]
- Lorenzo, M.M.; Decker, C.G.; Kahveci, M.U.; Paluck, S.J.; Maynard, H.D. Homodimeric protein-polymer conjugates via the tetrazine-trans-cyclooctene ligation. Macromolecules 2016, 49, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Punetha, V.D.; Bisht, G.; Bisht, S.S.; Sahoo, N.G.; Cho, J.W. Recent trends of polymer-protein conjugate application in biocatalysis: A review. Polym. Rev. 2015, 55, 163–198. [Google Scholar] [CrossRef]
- Borchmann, D.E.; Carberry, T.P.; Weck, M. “Bio”-macromolecules: Polymer-protein conjugates as emerging scaffolds for therapeutics. Macromol. Rapid Commun. 2014, 35, 27–43. [Google Scholar] [CrossRef]
- Heredia, K.L.; Tao, L.; Grover, G.N.; Maynard, H.D. Heterotelechelic polymers for capture and release of protein-polymer conjugates. Polym. Chem. UK 2010, 1, 168–170. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Klok, H.A. Peptide/protein-polymer conjugates: Synthetic strategies and design concepts. Chem. Commun. 2008, 2591–2611. [Google Scholar] [CrossRef]
- Shimoboji, T.; Ding, Z.L.; Stayton, P.S.; Hoffman, A.S. Photoswitching of ligand association with a photoresponsive polymer-protein conjugate. Bioconjug. Chem. 2002, 13, 915–919. [Google Scholar] [CrossRef]
- Liu, Q.M.; Chen, S.; Chen, J.; Du, J.Z. An asymmetrical polymer vesicle strategy for significantly improving t-1 mri sensitivity and cancer-targeted drug delivery. Macromolecules 2015, 48, 739–749. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Dong, C.H.; Wang, X.M.; Wang, H.J.; Li, W.; Tan, J.; Chang, J. Self-assembled biodegradable protein-polymer vesicle as a tumor-targeted nanocarrier. ACS Appl. Mater. Interfaces 2014, 6, 2393–2400. [Google Scholar] [CrossRef]
- Srinivas, G.; Mohan, R.V.; Kelkar, A.D. Polymer micelle assisted transport and delivery of model hydrophilic components inside a biological lipid vesicle: A coarse-grain simulation study. J. Phys. Chem. B 2013, 117, 12095–12104. [Google Scholar] [CrossRef]
- Zhu, H.S.; Geng, Q.R.; Chen, W.Q.; Zhu, Y.Q.; Chen, J.; Du, J.Z. Antibacterial high-genus polymer vesicle as an “armed” drug carrier. J. Mater. Chem. B 2013, 1, 5496–5504. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, A.; Uchegbu, I.F.; Schatzlein, A.G. Pei-based vesicle-polymer hybrid gene delivery system with improved biocompatibility. Int. J. Pharm. 2004, 274, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J. Drug-initiated synthesis of polymer prodrugs: Combining simplicity and efficacy in drug delivery. Chem. Mater. 2016, 28, 1591–1606. [Google Scholar] [CrossRef]
- Bai, Y.; Xing, H.; Vincil, G.A.; Lee, J.; Henderson, E.J.; Lu, Y.; Lemcoff, N.G.; Zimmerman, S.C. Practical synthesis of water-soluble organic nanoparticles with a single reactive group and a functional carrier scaffold. Chem. Sci. 2014, 5, 2862–2868. [Google Scholar] [CrossRef]
- Issberner, J.; Moors, R.; Vögtle, F. Dendrimers: From generations and functional groups to functions. Angew. Chem. Int. Ed. Engl. 1995, 33, 2413–2420. [Google Scholar] [CrossRef]
- Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Hawker, C.; Fréchet, J.M. A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc. Chem. Commun. 1990, 1010–1013. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Cheng, Y.; Man, N.; Xu, T.; Fu, R.; Wang, X.; Wang, X.; Wen, L. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (pamam) dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar]
- Fu, H.L.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Dendrimer/DNA complexes encapsulated functional biodegradable polymer for substrate-mediated gene delivery. J. Gene Med. 2008, 10, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Patel, P. Dendrimer applications—A review. Int. J. Pharm. Bio Sci. 2013, 4, 454–463. [Google Scholar]
- Ardestani, M.S.; Fordoei, A.S.; Abdoli, A.; Ahangari Cohan, R.; Bahramali, G.; Sadat, S.M.; Siadat, S.D.; Moloudian, H.; Nassiri Koopaei, N.; Bolhasani, A.; et al. Nanosilver based anionic linear globular dendrimer with a special significant antiretroviral activity. J. Mater. Sci. Mater. Med. 2015, 26, 179. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.V.; Schilly, K.M.; Schoenfisch, M.H. Anti-biofilm efficacy of dual-action nitric oxide-releasing alkyl chain modified poly(amidoamine) dendrimers. Mol. Pharm. 2015, 12, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G.; Hervet, H. Statistics of « starburst » polymers. J. Physique Lett. 1983, 44, 351–360. [Google Scholar] [CrossRef]
- Nourse, A.; Millar, D.B.; Minton, A.P. Physicochemical characterization of generation 5 polyamidoamine dendrimers. Biopolymers 2000, 53, 316–328. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; McLeod, V.M.; Porter, C.J.; Boyd, B.J. Association of chemotherapeutic drugs with dendrimer nanocarriers: An assessment of the merits of covalent conjugation compared to noncovalent encapsulation. Mol. Pharm. 2012, 9, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Whitley, R.J. Antiviral therapy of hsv-1 and -2. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Englund, J.A.; Zimmerman, M.E.; Swierkosz, E.M.; Goodman, J.L.; Scholl, D.R.; Balfour, H.H., Jr. Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann. Intern. Med. 1990, 112, 416–422. [Google Scholar] [CrossRef]
- Clementi, N.; Criscuolo, E.; Cappelletti, F.; Burioni, R.; Clementi, M.; Mancini, N. Novel therapeutic investigational strategies to treat severe and disseminated hsv infections suggested by a deeper understanding of in vitro virus entry processes. Drug Discov. Today 2016, 21, 682–691. [Google Scholar] [CrossRef]
- Xie, D.; Yao, C.; Wang, L.; Min, W.; Xu, J.; Xiao, J.; Huang, M.; Chen, B.; Liu, B.; Li, X. An albumin-conjugated peptide exhibits potent anti-hiv activity and long in vivo half-life. Antimicrob. Agents Chemother. 2010, 54, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, S.A.; Jackson, J.O.; Jardetzky, T.S.; Longnecker, R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 2011, 9, 369–381. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Tarallo, R.; Russo, L.; Galdiero, E.; Cantisani, M.; Morelli, G.; Galdiero, M. Peptide inhibitors against herpes simplex virus infections. J. Pept. Sci. 2013, 19, 148–158. [Google Scholar] [CrossRef]
- Falanga, A.; Tarallo, R.; Vitiello, G.; Vitiello, M.; Perillo, E.; Cantisani, M.; D’Errico, G.; Galdiero, M.; Galdiero, S. Biophysical characterization and membrane interaction of the two fusion loops of glycoprotein b from herpes simplex type i virus. PLoS ONE 2012, 7, e32186. [Google Scholar] [CrossRef] [Green Version]
- Cantisani, M.; Falanga, A.; Incoronato, N.; Russo, L.; De Simone, A.; Morelli, G.; Berisio, R.; Galdiero, M.; Galdiero, S. Conformational modifications of gb from herpes simplex virus type 1 analyzed by synthetic peptides. J. Med. Chem. 2013, 56, 8366–8376. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Zannella, C.; Folliero, V.; Martora, F.; Galdiero, M.; Galdiero, S.; Morelli, G. Infectivity inhibition by overlapping synthetic peptides derived from the gh/gl heterodimer of herpes simplex virus type 1. J. Pept. Sci. 2017, 23, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarallo, R.; Carberry, T.P.; Falanga, A.; Vitiello, M.; Galdiero, S.; Galdiero, M.; Weck, M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int. J. Nanomed. 2013, 8, 521–534. [Google Scholar]
- Galdiero, S.; Falanga, A.; Vitiello, M.; D’Isanto, M.; Cantisani, M.; Kampanaraki, A.; Benedetti, E.; Browne, H.; Galdiero, M. Peptides containing membrane-interacting motifs inhibit herpes simplex virus type 1 infectivity. Peptides 2008, 29, 1461–1471. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; D’Isanto, M.; Collins, C.; Orrei, V.; Browne, H.; Pedone, C.; Galdiero, M. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein h in membrane fusion and virus inhibition. Chembiochem 2007, 8, 885–895. [Google Scholar] [CrossRef]
- Galdiero, S.; Vitiello, M.; D’Isanto, M.; Falanga, A.; Collins, C.; Raieta, K.; Pedone, C.; Browne, H.; Galdiero, M. Analysis of synthetic peptides from heptad-repeat domains of herpes simplex virus type 1 glycoproteins h and b. J. Gen. Virol. 2006, 87, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Falanga, A.; Del Genio, V.; Palomba, L.; Galdiero, M.; Franci, G.; Galdiero, S. A boost to the antiviral activity: Cholesterol tagged peptides derived from glycoprotein b of herpes simplex virus type i. Int. J. Biol. Macromol. 2020, 162, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Jacob Silva, P.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2017, 17, 195. [Google Scholar] [CrossRef]
- Carberry, T.P.; Tarallo, R.; Falanga, A.; Finamore, E.; Galdiero, M.; Weck, M.; Galdiero, S. Dendrimer functionalization with a membrane-interacting domain of herpes simplex virus type 1: Towards intracellular delivery. Chem. A Eur. J. 2012, 18, 13678–13685. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.A.; Tarallo, R.; Elacqua, E.; Carberry, T.P.; Weck, M. Synthesis of well-defined bifunctional newkome-type dendrimers. Macromolecules 2017, 50, 4897–4905. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the mtt assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef]


| Compounds | Peptide | Sequence | MW | Charge |
|---|---|---|---|---|
| gH | PrA-gH493-511 | NH2-PrA-AAHLIDALYAEFLGGRVLT-CONH2 | 2124 | −1 |
| Cys-gH | Cys-gH493-511 | Ac-C-AAHLIDALYAEFLGGRVLT-CONH2 | 2174 | −1 |
| gB | gB503-523-PrA | NH2- FARLQFTYNHIQRHVRDMEGR-PrA-CONH2 | 2769 | +2 |
| DendrimerA | Monofunctional dendrimer | 3430 | ||
| DendrimerB | Bifunctional dendrimer | 3043 |
| ζ-Potential (mV) | Std. Dev (mV) | |
|---|---|---|
| DendrimerB | −11.0 | ±1.6 |
| DendrimerB + gB/gH | 5.17 | ±0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falanga, A.; Del Genio, V.; Kaufman, E.A.; Zannella, C.; Franci, G.; Weck, M.; Galdiero, S. Engineering of Janus-Like Dendrimers with Peptides Derived from Glycoproteins of Herpes Simplex Virus Type 1: Toward a Versatile and Novel Antiviral Platform. Int. J. Mol. Sci. 2021, 22, 6488. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126488
Falanga A, Del Genio V, Kaufman EA, Zannella C, Franci G, Weck M, Galdiero S. Engineering of Janus-Like Dendrimers with Peptides Derived from Glycoproteins of Herpes Simplex Virus Type 1: Toward a Versatile and Novel Antiviral Platform. International Journal of Molecular Sciences. 2021; 22(12):6488. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126488
Chicago/Turabian StyleFalanga, Annarita, Valentina Del Genio, Elizabeth A. Kaufman, Carla Zannella, Gianluigi Franci, Marcus Weck, and Stefania Galdiero. 2021. "Engineering of Janus-Like Dendrimers with Peptides Derived from Glycoproteins of Herpes Simplex Virus Type 1: Toward a Versatile and Novel Antiviral Platform" International Journal of Molecular Sciences 22, no. 12: 6488. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126488







