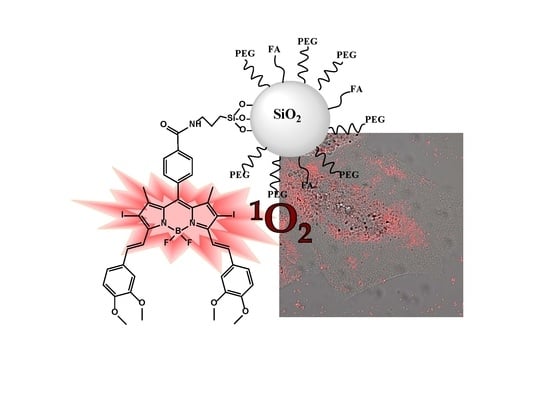
Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Silica Nanoparticles Characterization
2.2. Optimization of the Functionalization of Silica Nanoparticles with Rose Bengal as PS
2.3. Photosensitized Silica Nanoparticles with Other Photosensitizers
2.4. In Vitro Experiments in HeLa Cells
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of New BODIPY-Based PSs
3.2.1. General
3.2.2. General Procedure for the Formation of Amides
3.2.3. Synthesis of BDP3
3.2.4. Synthesis of BDP4
3.2.5. Synthesis of BDP5
3.2.6. Synthesis of BDP6
3.2.7. Synthesis of BDP7
3.3. Synthesis of the MSNs
3.4. Grafting of Molecules (PS, PEG and FA) on the MSN Surface
- -
- RB-PEG-NP-a: RB (0.03 mmol), previously silylated with an equimolar ratio of APTMS (0.03 mmol) in 20 mL of CH3CN under stirring for 1 h under inert atmosphere [109], was directly coupled to the structural hydroxyl groups of MSN (40 mg) added afterward. The reaction was kept for 3 h and the nanoparticles were collected by filtration. Then, RB-MSNs were re-suspended in 20 mL of CH3CN and then, NHS ester-activated PEG (0.03 mmol), NHS-PEG (Figure 1), was added to react for 3 h with the external primary amine group of NH-MSN to yield stable amide bonds and releasing N-hydroxysuccinimide group (NHS). In this type of MSN, the pelygation procedure of the external surface was carried out with three different PEG chains of 750, 2000, and 5000 Da. The corresponding samples were named RB-PEG750-NP-a, RB-PEG2000-NP-a and RB-PEG5000-NP-a, respectively.
- -
- RB-PEG-NP-b: RB was linked through its carboxylic group to the amine groups of NH-MSN (40 mg) by the carbodiimide method. This method has been previously described [105,109]. Briefly, NHS/EDC is added to RB in solution (0.03 mmol in 20 mL of CH3CN) in equimolar concentration and stirring for 1 h in an inert atmosphere. Then NH-MSN (40 mg) was directly added to the reaction mixture and kept stirring for 3 h, and nanoparticles were collected. In a second step, PEG (0.03 mmol) with a silylated group at one edge (Si-PEG, Figure 1) was externally anchored to the inherent hydroxyl groups of RB-MSN (40 mg) by direct condensation reaction during 3 h.
- -
- RB-PEG-NP-c: both RB (0.03 mmol), previously silylated according to the process previously described in sample RB-PEG-NP-a, and Si-PEG (0.03 mmol) were simultaneously bound in CH3CN to the external hydroxyl groups of MSN (40 mg) following the procedure previously mentioned.
- -
- RB-NP-d: FA (0.03 mg) was added to RB-PEG-NP-c (40 mg), previously re-suspended in CH3CN (20 mL), and was linked to the amine groups of RB-PEG-NP-c sample through the carbodiimide method cited above.
3.5. Physicochemical and Photophysical Characterization
3.6. In Vitro Assays
3.6.1. Cell Culture
3.6.2. Sample Preparation and In Vitro Exposures
3.6.3. Confocal Microscopy
3.6.4. Cell Viability (MTT) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K.; Gibbins, S. A Surgical View of Photodynamic Therapy in Oncology: A Review. Surg. J. 2015, 1, e1–e15. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wan, M.T. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- DeRosa, M. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Nonell, S.; Flors, C. (Eds.) Singlet Oxygen, Applications in Biosciences and Nanosciences; Royal Society of Chemistry: Cambridge, UK, 2016; ISBN 978-1-78262-038-9. [Google Scholar]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Stallivieri, A.; Colombeau, L.; Jetpisbayeva, G.; Moussaron, A.; Myrzakhmetov, B.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. Folic acid conjugates with photosensitizers for cancer targeting in photodynamic therapy: Synthesis and photophysical properties. Bioorg. Med. Chem. 2017, 25, 1–10. [Google Scholar] [CrossRef]
- Lacombe, S.; Pigot, T. Materials for selective photo-oxygenation vs. photocatalysis: Preparation, properties and applications in environmental and health fields. Catal. Sci. Technol. 2016, 6, 1571–1592. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Yin, R.; Hamblin, M. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1604053. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Álvarez-Fernández, D.; Prieto Montero, R.; García-Moreno, I.; Martínez-Martínez, V.; Bañuelos, J.; Sáenz-de-Santa-María, I.; Chiara, M.D.; Chiara, J.L. A general modular approach for the solubility tagging of BODIPY dyes. Dye. Pigment. 2019, 170, 107545. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yang, X. Photosensitizer that selectively generates singlet oxygen in nonpolar environments: Photophysical mechanism and efficiency for a covalent BODIPY dimer. J. Phys. Chem. B 2013, 117, 9050–9055. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zhou, L.; Li, Y.; Dong, Y. Diiodo-Bodipy-Encapsulated Nanoscale Metal–Organic Framework for pH-Driven Selective and Mitochondria Targeted Photodynamic Therapy. Inorg. Chem. 2018, 57, 10137–10145. [Google Scholar] [CrossRef]
- Sánchez-Arroyo, A.J.; Palao, E.; Agarrabeitia, A.R.; Ortiz, M.J.; García-Fresnadillo, D. Towards improved halogenated BODIPY photosensitizers: Clues on structural designs and heavy atom substitution patterns. Phys. Chem. Chem. Phys. 2017, 19, 69–72. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; Moya, S.; Martínez-Martínez, V. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef]
- López Arbeloa, F.; Bañuelos, J.; Martínez, V.; Arbeloa, T.; López Arbeloa, I. Structural, photophysical and lasing properties of pyrromethene dyes. Int. Rev. Phys. Chem. 2005, 24, 339–374. [Google Scholar] [CrossRef]
- Prieto, J.B.; Arbeloa, F.L.; Martínez, V.M.; López, T.A.; Arbeloa, I.L. Photophysical properties of the pyrromethene 597 dye: Solvent effect. J. Phys. Chem. A 2004, 108, 5503–5508. [Google Scholar] [CrossRef]
- Wang, D.G.; Zhang, L.N.; Li, Q.; Yang, Y.; Wu, Y.; Fan, X.; Song, M.; Kuang, G.C. Dimeric BODIPYs with different linkages: A systematic investigation on structure-properties relationship. Tetrahedron 2017, 73, 6894–6900. [Google Scholar] [CrossRef]
- Esnal, I.; Valois-Escamilla, I.; Gõmez-Durán, C.F.; Urías-Benavides, A.; Betancourt-Mendiola, M.L.; Lõpez-Arbeloa, I.; Bañuelos, J.; García-Moreno, I.; Costela, A.; Peña-Cabrera, E. Blue-to-orange color-tunable laser emission from tailored boron-dipyrromethene dyes. ChemPhysChem 2013, 14, 4134–4142. [Google Scholar] [CrossRef]
- Epelde-Elezcano, N.; Martínez-Martínez, V.; Peña-Cabrera, E.; Gómez-Durán, C.F.A.; López-Arbeloa, I.; Lacombe, S. Modulation of singlet oxygen generation in halogenated BODIPY dyes by substitution at their meso position: Towards a solvent-independent standard in the vis region. RSC Adv. 2016, 6, 41991–41998. [Google Scholar] [CrossRef] [Green Version]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Palao-Utiel, E.; Montalvillo-Jiménez, L.; Esnal, I.; Prieto-Montero, R.; Agarrabeitia, A.R.; García-Moreno, I.; Bañuelos, J.; López-Arbeloa, I.; de la Moya, S.; Ortiz, M.J. Controlling Vilsmeier-Haack processes in meso-methylBODIPYs: A new way to modulate finely photophysical properties in boron dipyrromethenes. Dye. Pigment. 2017, 141, 286–298. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with BODIPY dyes. Coord. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Zhang, X.F.; Feng, N. Photoinduced Electron Transfer-based Halogen-free Photosensitizers: Covalent meso-Aryl (Phenyl, Naphthyl, Anthryl, and Pyrenyl) as Electron Donors to Effectively Induce the Formation of the Excited Triplet State and Singlet Oxygen for BODIPY Compounds. Chem. An Asian J. 2017, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Prieto-Montero, R.; Maroto, B.L.; Moreno, F.; Ortiz, M.J.; Oliden-Sánchez, A.; López-Arbeloa, I.; Martínez-Martínez, V.; de la Moya, S. Manipulating Charge-Transfer States in BODIPYs: A Model Strategy to Rapidly Develop Photodynamic Theragnostic Agents. Chem. A Eur. J. 2020, 26, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Sui, B.; Yue, X.; Tang, S.; Tichy, M.G.; Belfield, K.D. In Vitro Photodynamic Studies of a BODIPY-Based Photosensitizer. European J. Org. Chem. 2017, 2017, 25–28. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Haefele, A.; Harriman, A. An artificial light-harvesting array constructed from multiple bodipy dyes. J. Am. Chem. Soc. 2013, 135, 11330–11344. [Google Scholar] [CrossRef]
- Gorbe, M.; Costero, A.M.; Sancenón, F.; Martínez-Máñez, R.; Ballesteros-Cillero, R.; Ochando, L.E.; Chulvi, K.; Gotor, R.; Gil, S. Halogen-containing BODIPY derivatives for photodynamic therapy. Dye. Pigment. 2019, 160, 198–207. [Google Scholar] [CrossRef]
- Atilgan, S.; Ekmekci, Z.; Dogan, A.L.; Guc, D.; Akkaya, E.U. Water soluble distyryl-boradiazaindacenes as efficient photosensitizers for photodynamic therapy. Chem. Commun. 2006, 4398–4400. [Google Scholar] [CrossRef]
- Ke, M.R.; Yeung, S.L.; Ng, D.K.P.; Fong, W.P.; Lo, P.C. Preparation and in vitro photodynamic activities of folate-conjugated distyryl boron dipyrromethene based photosensitizers. J. Med. Chem. 2013, 56, 8475–8483. [Google Scholar] [CrossRef]
- Xiong, H.; Zhou, K.; Yan, Y.; Miller, J.B.; Siegwart, D.J. Tumor-Activated Water-Soluble Photosensitizers for Near-Infrared Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 16335–16343. [Google Scholar] [CrossRef]
- He, H.; Lo, P.C.; Yeung, S.L.; Fong, W.P.; Ng, D.K.P. Synthesis and in vitro photodynamic activities of pegylated distyryl boron dipyrromethene derivatives. J. Med. Chem. 2011, 54, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.C.S.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated Photosensitizers for Imaging and PDT in Cancer Research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef] [PubMed]
- Khuong Mai, D.; Kang, B.; Pegarro Vales, T.; Badon, I.W.; Cho, S.; Lee, J.; Kim, E.; Kim, H.-J. Synthesis and Photophysical Properties of Tumor-Targeted Water-Soluble BODIPY Photosensitizers for Photodynamic Therapy. Molecules 2020, 25, 3340. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2018, 20, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, V.; Calatayud, D.G.; Arrowsmith, R.L.; Ge, H.; Pascu, S.I. Metallic nanoparticles as synthetic building blocks for cancer diagnostics: From materials design to molecular imaging applications. J. Mater. Chem. B 2015, 3, 5657–5672. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhao, L.; Zheng, L.; Hu, Y.; Ding, L.; Li, X.; Liu, C.; Zhao, J.; Shi, X.; Guo, R. LAPONITE-Polyethylenimine Based Theranostic Nanoplatform for Tumor-Targeting CT Imaging and Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 431–442. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. JBIC J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adumeau, L.; Genevois, C.; Roudier, L.; Schatz, C.; Couillaud, F.; Mornet, S. Impact of surface grafting density of PEG macromolecules on dually fluorescent silica nanoparticles used for the in vivo imaging of subcutaneous tumors. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1587–1596. [Google Scholar] [CrossRef] [Green Version]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.; Yang, S.M.; Yi, G.; Roh, Y.J.; Park, H.; Park, J.M.; Choi, M.; Koo, H. Folate-modified PLGA nanoparticles for tumor-targeted delivery of pheophorbide a in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 523–528. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F.; Nguyen, K.T.; Ma, X.; Wang, X.; Xing, B.; Zhao, Y. Multifunctional Mesoporous Silica Nanoparticles for Cancer-Targeted and Controlled Drug Delivery. Adv. Funct. Mater. 2012, 22, 5144–5156. [Google Scholar] [CrossRef]
- Villaverde, G.; Baeza, A.; Melen, G.J.; Alfranca, A.; Ramirez, M.; Vallet-Regí, M. A new targeting agent for the selective drug delivery of nanocarriers for treating neuroblastoma. J. Mater. Chem. B 2015, 3, 4831–4842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, T.; Prazeres, T.J.V.; Moffitt, M.; Farinha, J.P.S. Enhanced Photoluminescence from Micellar Assemblies of Cadmium Sulfide Quantum Dots and Gold Nanoparticles. J. Phys. Chem. C 2013, 117, 3122–3133. [Google Scholar] [CrossRef]
- Pérez, N.; Ruiz-Rubio, L.; Vilas, J.L.; Rodríguez, M.; Martinez-Martinez, V.; León, L.M. Synthesis and characterization of near-infrared fluorescent and magnetic iron zero-valent nanoparticles. J. Photochem. Photobiol. A Chem. 2016, 315, 1–7. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvindi, M.A.; Brunetti, V.; Vecchio, G.; Galeone, A.; Cingolani, R.; Pompa, P.P. SiO2 nanoparticles biocompatibility and their potential for gene delivery and silencing. Nanoscale 2012, 4, 486–495. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Wang, J.; Baker, J.; Mathias, L.J. Synthesis and characterization of covalently functionalized laponite clay. Chem. Mater. 2005, 17, 3012–3018. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Q.; He, S.; Liu, Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef]
- Chen, K.; Chang, C.; Liu, Z.; Zhou, Y.; Xu, Q.; Li, C.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2020, 194. [Google Scholar] [CrossRef]
- Lyles, Z.K.; Tarannum, M.; Mena, C.; Inada, N.M.; Bagnato, V.S.; Vivero-Escoto, J.L. Biodegradable Silica-Based Nanoparticles with Improved and Safe Delivery of Protoporphyrin IX for the In Vivo Photodynamic Therapy of Breast Cancer. Adv. Ther. 2020, 3, 2000022. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalised with a photoactive ruthenium(ii) complex: Exploring the formulation of a metal-based photodynamic therapy photosensitiser. Dalt. Trans. 2019, 48, 5940–5951. [Google Scholar] [CrossRef]
- Figueira, F.; Cavaleiro, J.A.S.; Tomé, J.P.C. Silica nanoparticles functionalized with porphyrins and analogs for biomedical studies. J. Porphyr. Phthalocyanines 2011, 15, 517–533. [Google Scholar] [CrossRef]
- Martins Estevão, B.; Miletto, I.; Marchese, L.; Gianotti, E. Optimized Rhodamine B labeled mesoporous silica nanoparticles as fluorescent scaffolds for the immobilization of photosensitizers: A theranostic platform for optical imaging and photodynamic therapy. Phys. Chem. Chem. Phys. 2016, 18, 9042–9052. [Google Scholar] [CrossRef] [PubMed]
- Ronzani, F.; Costarramone, N.; Blanc, S.; Benabbou, A.K.; Le Bechec, M.; Pigot, T.; Oelgemöller, M.; Lacombe, S. Visible-light photosensitized oxidation of α-terpinene using novel silica-supported sensitizers: Photooxygenation vs. photodehydrogenation. J. Catal. 2013, 303, 164–174. [Google Scholar] [CrossRef]
- Gianotti, E.; Martins Estevão, B.; Cucinotta, F.; Hioka, N.; Rizzi, M.; Renò, F.; Marchese, L. An Efficient Rose Bengal Based Nanoplatform for Photodynamic Therapy. Chem. A Eur. J. 2014, 20, 10921–10925. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lee, C.-H.; Yang, C.-S.; Tseng, F.-G.; Mou, C.-Y.; Lo, L. Mesoporous silica nanoparticles functionalized with an oxygen-sensing probe for cell photodynamic therapy: Potential cancer theranostics. J. Mater. Chem. 2009, 19, 1252. [Google Scholar] [CrossRef]
- Martins Estevão, B.; Cucinotta, F.; Hioka, N.; Cossi, M.; Argeri, M.; Paul, G.; Marchese, L.; Gianotti, E. Rose Bengal incorporated in mesostructured silica nanoparticles: Structural characterization, theoretical modeling and singlet oxygen delivery. Phys. Chem. Chem. Phys. 2015, 17, 26804–26812. [Google Scholar] [CrossRef]
- Kan, J.L.; Jiang, Y.; Xue, A.; Yu, Y.H.; Wang, Q.; Zhou, Y.; Dong, Y. Bin Surface Decorated Porphyrinic Nanoscale Metal-Organic Framework for Photodynamic Therapy. Inorg. Chem. 2018, 57, 5420–5428. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Kim, G.; Hah, H.J.; Lee, Y.E.K.; Kopelman, R.; Ethirajan, M.; Gupta, A.; Goswami, L.N.; Pera, P.; et al. Novel methods to incorporate photosensitizers into nanocarriers for cancer treatment by photodynamic therapy. Lasers Surg. Med. 2011, 43, 686–695. [Google Scholar] [CrossRef] [Green Version]
- Tada, D.B.; Baptista, M.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Ma, H.; Hou, B.; Zhao, H.; Ji, Y.; Jiang, R.; Hu, X.; Lu, X.; Zhang, L.; Tang, Y.; et al. Engineering Lysosome-Targeting BODIPY Nanoparticles for Photoacoustic Imaging and Photodynamic Therapy under Near-Infrared Light. ACS Appl. Mater. Interfaces 2016, 8, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhu, M.; Niu, N.; Ren, J.; Yang, N.; Yu, C. Aza-BODIPY Probe-Decorated Mesoporous Black TiO2Nanoplatform for the Highly Efficient Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 41071–41078. [Google Scholar] [CrossRef] [PubMed]
- Mangalath, S.; Saneesh Babu, P.S.; Nair, R.R.; Manu, P.M.; Krishna, S.; Nair, S.A.; Joseph, J. Graphene Quantum Dots Decorated with Boron Dipyrromethene Dye Derivatives for Photodynamic Therapy. ACS Appl. Nano Mater. 2021, 4, 4162–4171. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Song, K.; Liu, T.; Zhou, L.; Bao, B.; Wang, R.; Wang, L. The self-assembly of a hybrid photosensitizer for the synergistically enhanced photodynamic/photothermal therapy. Biomater. Sci. 2021, 9, 2115–2123. [Google Scholar] [CrossRef]
- Li, R.; Du, Y.; Guo, W.; Su, Y.; Meng, Y.; Shan, Z.; Feng, Y.; Meng, S. Methotrexate coated AZA-BODIPY nanoparticles for chemotherapy, photothermal and photodynamic synergistic therapy. Dye. Pigment. 2020, 179, 108351. [Google Scholar] [CrossRef]
- Bartelmess, J.; Milcovich, G.; Maffeis, V.; d’Amora, M.; Bertozzi, S.M.; Giordani, S. Modulation of Efficient Diiodo-BODIPY in vitro Phototoxicity to Cancer Cells by Carbon Nano-Onions. Front. Chem. 2020, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- González-Béjar, M.; Liras, M.; Francés-Soriano, L.; Voliani, V.; Herranz-Pérez, V.; Duran-Moreno, M.; Garcia-Verdugo, J.M.; Alarcon, E.I.; Scaiano, J.C.; Pérez-Prieto, J. NIR excitation of upconversion nanohybrids containing a surface grafted Bodipy induces oxygen-mediated cancer cell death. J. Mater. Chem. B 2014, 2, 4554. [Google Scholar] [CrossRef]
- Chauhan, P.; Yan, N. Novel bodipy—Cellulose nanohybrids for the production of singlet oxygen. RSC Adv. 2016, 6, 32070–32073. [Google Scholar] [CrossRef]
- Ruan, Z.; Liu, L.; Jiang, W.; Li, S.; Wang, Y.; Yan, L. NIR imaging-guided combined photodynamic therapy and chemotherapy by a pH-responsive amphiphilic polypeptide prodrug. Biomater. Sci. 2017, 5, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, X.; Fan, J.; Du, J.; Peng, X. Boron Dipyrromethene Nano-Photosensitizers for Anticancer Phototherapies. Small 2019, 1804927, 1–25. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Hoop, M.; Shamsudhin, N.; Huang, T.; Özkale, B.; Li, Q.; Siringil, E.; Mushtaq, F.; Di Tizio, L.; Nelson, B.J.; et al. Hybrid Magnetoelectric Nanowires for Nanorobotic Applications: Fabrication, Magnetoelectric Coupling, and Magnetically Assisted In Vitro Targeted Drug Delivery. Adv. Mater. 2017, 29, 1605458–1605465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, Y.; Wang, X.J.; Xu, J.L.; Ye, Z.; Wang, S.; Seeberger, P.H.; Yin, J. Targeted Photodynamic Killing of Breast Cancer Cells Employing Heptamannosylated β-Cyclodextrin-Mediated Nanoparticle Formation of an Adamantane-Functionalized BODIPY Photosensitizer. ACS Appl. Mater. Interfaces 2016, 8, 33405–33411. [Google Scholar] [CrossRef]
- Zong, J.; Peng, H.; Qing, X.; Fan, Z.; Xu, W.; Du, X.; Shi, R.; Zhang, Y. pH-Responsive Pluronic F127–Lenvatinib-Encapsulated Halogenated Boron-Dipyrromethene Nanoparticles for Combined Photodynamic Therapy and Chemotherapy of Liver Cancer. ACS Omega 2021, 6, 12331–12342. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, M.; Li, C.; Liu, G.; Sun, Q.; Luo, X.; Wu, F. Single molecular-based nanoparticles with aggregation-induced emission characteristics for fluorescence imaging and efficient cancer phototherapy. Dye. Pigment. 2021, 187, 109130. [Google Scholar] [CrossRef]
- Das, A.; Shome, A.; Manna, U. Porous and reactive polymeric interfaces: An emerging avenue for achieving durable and functional bio-inspired wettability. J. Mater. Chem. A 2021, 9, 824–856. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Cao, Z.; Wu, Y.; Chen, J.A.; Gao, J.; Wang, Z.; Guo, W.; Gu, X. Mitochondria-targeting BODIPY-loaded micelles as novel class of photosensitizer for photodynamic therapy. Eur. J. Med. Chem. 2018, 157, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Ye, J.H.; Qi, F.; Fang, H.; Lin, F.; Wang, S.; Mu, C.; Zhang, W.; He, W. A PEGylated photosensitizer-core pH-responsive polymeric nanocarrier for imaging-guided combination chemotherapy and photodynamic therapy. New J. Chem. 2021, 45, 6180–6185. [Google Scholar] [CrossRef]
- Perumal, D.; Golla, M.; Pillai, K.S.; Raj, G.; Anusree Krishna, P.K.; Varghese, R. Biotin-decorated NIR-absorbing nanosheets for targeted photodynamic cancer therapy. Org. Biomol. Chem. 2021, 19, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. BODIPY-doped silica nanoparticles with reduced dye leakage and enhanced singlet oxygen generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Song, N.; Chen, L.; Xie, Z. Reduction responsive BODIPY decorated mesoporous silica nanoscale platforms for photodynamic therapy. Microporous Mesoporous Mater. 2021, 311, 110689. [Google Scholar] [CrossRef]
- Alexander, W. American society of clinical oncology, 2010 annual meeting and Rose Bengal: From a wool dye to a cancer therapy. Pharm. Ther. 2010, 35, 469–478. [Google Scholar]
- Gualdesi, M.S.; Vara, J.; Aiassa, V.; Alvarez Igarzabal, C.I.; Ortiz, C.S. Thionine in the design of new photosensitizers: Bromination and vehiculization in polymeric nanoparticles. J. Mol. Liq. 2020, 310, 113247. [Google Scholar] [CrossRef]
- Tuite, E.M.; Kelly, J.M. New trends in photobiology. Photochemical interactions of methylene blue and analogues with DNA and other biological substrates. J. Photochem. Photobiol. B Biol. 1993, 21, 103–124. [Google Scholar] [CrossRef]
- Zahraei, M.; Marciello, M.; Lazaro-Carrillo, A.; Villanueva, A.; Herranz, F.; Talelli, M.; Costo, R.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; et al. Versatile theranostics agents designed by coating ferrite nanoparticles with biocompatible polymers. Nanotechnology 2016, 27, 255702. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Shi, J.; Zhu, Z.; Zhang, L.; Bu, W.; Guo, L.; Chen, Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials 2010, 31, 1085–1092. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Vijay Fernández, M.; Javaida, F.; Chudasama, V. Advances in targeting the folate receptor in thetreatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, A.M.; Ribeiro, T.; Rodrigues, A.S.; Ribeiro, B.; Frade, R.F.M.; Baleizão, C.; Farinha, J.P.S. Multifunctional Hybrid Silica Nanoparticles with a Fluorescent Core and Active Targeting Shell for Fluorescence Imaging Biodiagnostic Applications. Eur. J. Inorg. Chem. 2015, 2015, 4579–4587. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.G.; Van Der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor- α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Katsumiti, A.; Cajaraville, M.P.; Lopez Arbeloa, I.; Martinez-Martinez, V. Functionalized Fluorescent Silica Nanoparticles for Bioimaging of Cancer Cells. Sensors 2020, 20, 5590. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.X.; Meng, H. Mesoporous silica nanoparticles: A multifunctional nano therapeutic system. Integr. Biol. 2013, 5, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Arulprakasajothi, M.; Elangovan, K.; Chandrasekhar, U.; Suresh, S. Performance Study of Conical Strip Inserts in Tube Heat. Therm. Sci. 2018, 22, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Epelde-Elezcano, N.; Prieto-Montero, R.; Martínez-Martínez, V.; Ortiz, M.J.; Prieto-Castañeda, A.; Peña-Cabrera, E.; Belmonte-Vázquez, J.L.; López-Arbeloa, I.; Brown, R.; Lacombe, S. Adapting BODIPYs to singlet oxygen production on silica nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 13746–13755. [Google Scholar] [CrossRef]
- Maurel, M.; Montheil, T.; Martin, J.; Chaar, L.; Guzman-Gonzalez, V.; Couvet, M.; Jacquet, T.; Jia, T.; Eymin, B.; Parra, K.; et al. Design of pegylated three ligands silica nanoparticles for multi-receptor targeting. Nanomaterials 2021, 11, 177. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Figdor, C.G. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials 2011, 32, 6791–6803. [Google Scholar] [CrossRef]
- Chithrani, D.B. Polyethylene Glycol Density and Length Affects Nanoparticle Uptake by Cancer Cells. J. Nanomed. Res. 2014, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Off, M.K.; Steindal, A.E.; Porojnicu, A.C.; Juzeniene, A.; Vorobey, A.; Johnsson, A.; Moan, J. Ultraviolet photodegradation of folic acid. J. Photochem. Photobiol. B Biol. 2005, 80, 47–55. [Google Scholar] [CrossRef]
- Duman, S.; Cakmak, Y.; Kolemen, S.; Akkaya, E.U.; Dede, Y. Heavy atom free singlet oxygen generation: Doubly substituted configurations dominate S 1 states of bis-BODIPYs. J. Org. Chem. 2012, 77, 4516–4527. [Google Scholar] [CrossRef]
- Tao, J.; Sun, D.; Sun, L.; Li, Z.; Fu, B.; Liu, J.; Zhang, L.; Wang, S.; Fang, Y.; Xu, H. Tuning the photo-physical properties of BODIPY dyes: Effects of 1, 3, 5, 7- substitution on their optical and electrochemical behaviours. Dye. Pigment. 2019, 168, 166–174. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Zhou, Z.; Kessel, D.; Fronczek, F.R.; Pakhomova, S.; Vicente, M.G.H. Synthesis, spectroscopic, and in vitro investigations of 2,6-diiodo-BODIPYs with PDT and bioimaging applications. J. Photochem. Photobiol. B Biol. 2015, 145, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Awuah, S.G.; You, Y. Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv. 2012, 2, 11169. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Ballatore, M.B.; Durantini, A.M.; Durantini, E.N.; Tomé, A.C. BODIPYs in antitumoral and antimicrobial photodynamic therapy: An integrating review. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 21–48. [Google Scholar] [CrossRef]
- Lim, S.H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J.; Van Den Bergh, H.; Wagnières, G.; Burgess, K.; Lee, H.B. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Ventura, B.; Marconi, G.; Bröring, M.; Krüger, R.; Flamigni, L. Bis(BF2)-2,2′-bidipyrrins, a class of BODIPY dyes with new spectroscopic and photophysical properties. New J. Chem. 2009, 33, 428. [Google Scholar] [CrossRef]
- Epelde-Elezcano, N.; Palao, E.; Manzano, H.; Prieto-Castañeda, A.; Agarrabeitia, A.R.; Tabero, A.; Villanueva, A.; de la Moya, S.; López-Arbeloa, Í.; Martínez-Martínez, V.; et al. Rational Design of Advanced Photosensitizers Based on Orthogonal BODIPY Dimers to Finely Modulate Singlet Oxygen Generation. Chem. A Eur. J. 2017, 23, 4837–4848. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Zhang, X.-F.; Zhou, J.; Yu, C.; Hao, E.; Jiao, L. Modulating the singlet oxygen generation property of meso–β directly linked BODIPY dimers. Chem. Commun. 2012, 48, 5437. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cui, X.; Zhao, J. Hetero Bodipy-dimers as heavy atom-free triplet photosensitizers showing a long-lived triplet excited state for triplet–triplet annihilation upconversion. Chem. Commun. 2013, 49, 9009–9011. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Moorthy, M.S.; Manivasagan, P.; Seo, H.; Lee, K.D.; Oh, J. Chlorin e6 conjugated silica nanoparticles for targeted and effective photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 212–220. [Google Scholar] [CrossRef]
- Fraix, A.; Blangetti, M.; Guglielmo, S.; Lazzarato, L.; Marino, N.; Cardile, V.; Graziano, A.C.E.; Manet, I.; Fruttero, R.; Gasco, A.; et al. Light-tunable generation of singlet oxygen and nitric oxide with a bichromophoric molecular hybrid: A bimodal approach to killing cancer cells. ChemMedChem 2016, 11, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zeng, F.; Wu, S. A water-soluble and specific BODIPY-based fluorescent probe for hypochlorite detection and cell imaging. Anal. Methods 2013, 5, 5589–5596. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, H.; Huang, L.; Guo, Z.; Xiong, G.; Zhao, J. Porous material-immobilized iodo-Bodipy as an efficient photocatalyst for photoredox catalytic organic reaction to prepare pyrrolo[2,1-a]isoquinoline. Chem. Commun. 2013, 49, 8689–8702. [Google Scholar] [CrossRef]
- Nad, S.; Kumbhakar, M.; Pal, H. Photophysical Properties of Coumarin-152 and Coumarin-481 Dyes:Unusual Behavior in Nonpolar and in Higher Polarity Solvents. J. Phys. Chem. A 2003, 4808–4816. [Google Scholar] [CrossRef]
- Magde, D.; Brannon, J.H.; Cremers, T.L.; Olmsted, J. Absolute luminescence yield of cresyl violet. A standard for the red. J. Phys. Chem. 1979, 83, 696–699. [Google Scholar] [CrossRef]
- Vincett, P.S.; Voigt, E.M.; Rieckhoff, K.E. Phosphorescence and Fluorescence of Phthalocyanines. J. Chem. Phys. 1971, 55, 4131–4140. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Sola-Llano, R.; Montero, R.; Longarte, A.; Arbeloa, T.; López-Arbeloa, I.; Martínez-Martínez, V.; Lacombe, S. Methylthio BODIPY as a standard triplet photosensitizer for singlet oxygen production: A photophysical study. Phys. Chem. Chem. Phys. 2019, 21, 20403–20414. [Google Scholar] [CrossRef]








| Name | Shell | DLS (nm) | Z Pot (mV) |
|---|---|---|---|
| NH-MSN | NH2/OH | 71 | −3.96 |
| CN-MSN | CN/OH | 280 | −7.06 |
| COOH-MSN | COOH/OH | 66 | −39.7 |
| System | Characteristic | PEG Length (Da) | DLS Size (nm) | ZPOT (mV) | [RB] (μmol/g) |
|---|---|---|---|---|---|
| RB-PEG750-NP-a | RB-OH-MSN PEG-NH2-MSN | 750 | 130 | −4.3 | 20 |
| RB-PEG2000-NP-a | RB-OH-MSN PEG-NH2-MSN | 2000 | 99 | −25.0 | 20 |
| RB-PEG5000-NP-a | RB-OH-MSN PEG-NH2-MSN | 5000 | 114 | −25.0 | 20 |
| RB-PEG-NP-b | RB-NH2-MSN PEG-OH-MSN | 2000 | 95 | −29.0 | 10 |
| RB-PEG-NP-c | RB-OH-MSN PEG-OH-MSN | 2000 | 88 | −31.0 | 20 |
| System | Characteristic | [PS] (μmol/g) | λab (nm) | ΦΔ |
|---|---|---|---|---|
| BDP1-NP | BDP1-OH-MSN PEG-OH-MSN FA-NH2-MNS | 30 | 435.0 | 0.62 |
| BDP2-NP | BDP2-NH2-MSN PEG-OH-MSN FA-NH2-MNS | 3 | 527.0 | - |
| BDP3-NP | BDP3-OH-MSN PEG-OH-MSN FA-NH2-MNS | 40 | 528.0 | 0.69 |
| BDP4-NP | BDP4-NH2-MSN PEG-OH-MSN FA-NH2-MNS | 5 | 513.0 | 0.81 |
| BDP5-NP | BDP5-OH-MSN PEG-OH-MSN FA-NH2-MNS | 11 | 511.0 | 0.73 |
| BDP6-NP | BDP6-NH2-MSN PEG-OH-MSN FA-NH2-MNS | 7 | 670.0 | 0.50 |
| BDP7-NP | BDP7-OH-MSN PEG-OH-MSN FA-NH2-MNS | 3 | 669.5 | - |
| C6-NP | C6-NH2-MSN PEG-OH-MSN FA-NH2-MNS | 6 | 662.0 | 0.82 |
| Th-NP | Th-COOH-MSN PEG-OH-MSN FA-HDA-COOH-MNS | 15 | 599.0 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126618
Prieto-Montero R, Prieto-Castañeda A, Katsumiti A, Cajaraville MP, Agarrabeitia AR, Ortiz MJ, Martínez-Martínez V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. International Journal of Molecular Sciences. 2021; 22(12):6618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126618
Chicago/Turabian StylePrieto-Montero, Ruth, Alejandro Prieto-Castañeda, Alberto Katsumiti, Miren P. Cajaraville, Antonia R. Agarrabeitia, María J. Ortiz, and Virginia Martínez-Martínez. 2021. "Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer" International Journal of Molecular Sciences 22, no. 12: 6618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126618