DMA Study of the Molecular Structure of Porcine Fat in-Water Emulsions Stabilised by Potato Starch
Abstract
:1. Introduction
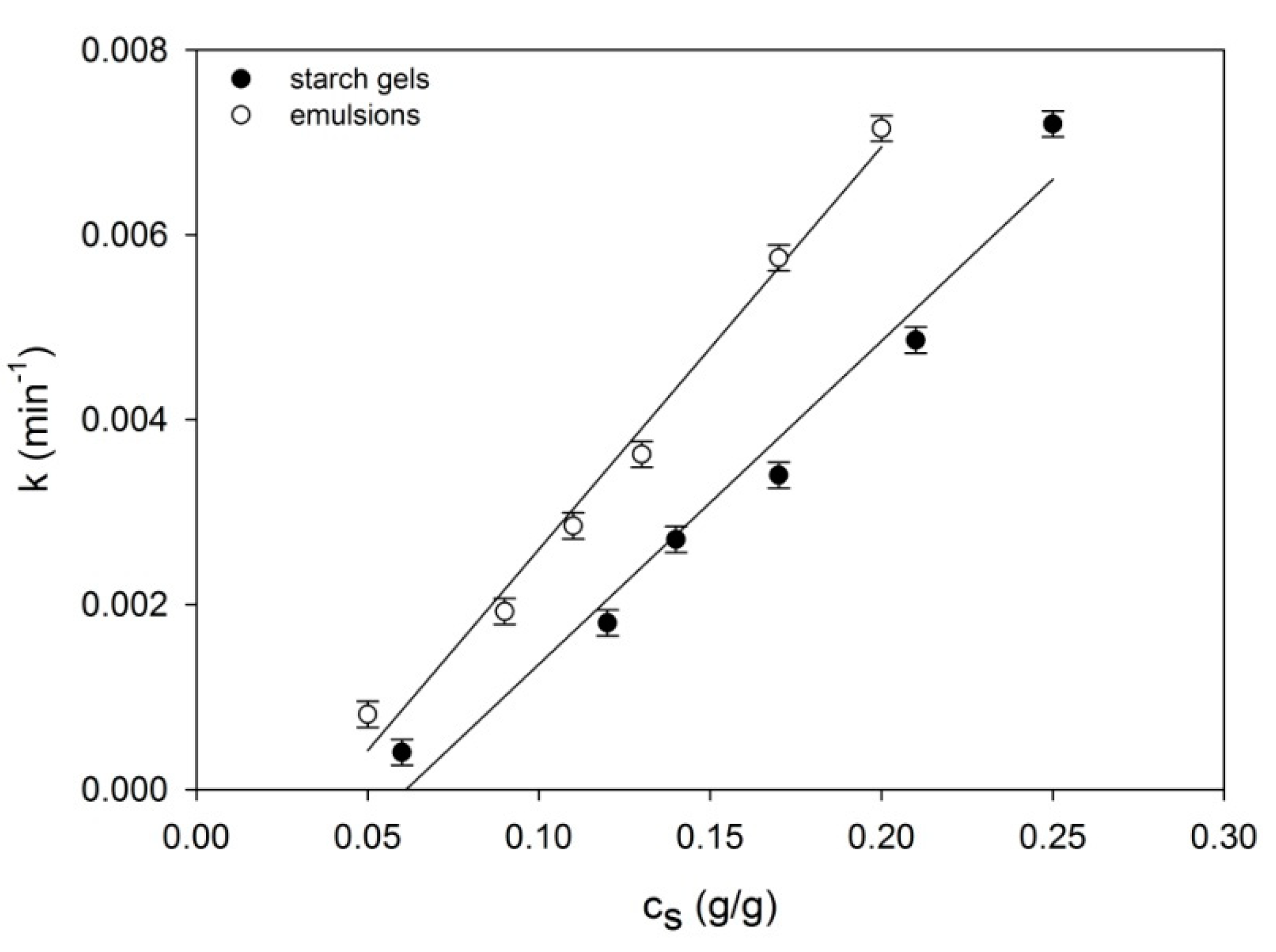
2. Results and Discussion
2.1. Rheomechanical Properties
2.2. Texture
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Rheomechanical Properties
3.3. Composition of Fatty Acids
3.4. Amylose Content and Complex Formation
3.5. Texture Analysis Method
- -
- head speed before compression: 1 mm/s,
- -
- head speed during compression: 1 mm/s,
- -
- head speed after compression: 1 mm/s,
- -
- disc penetration depth: 30 mm,
- -
- trigger force: 5 mm,
- -
- PPS (points per second): 250.
3.6. Sample Preparation for Texture Analysis
3.7. Statistical Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobin, B.D.; O’Sullivan, M.G.; Hamill, R.M.; Kerry, J.P. The impact of salt and fat level variation on the physiochemical properties and sensory quality of pork breakfast sausages. Meat Sci. 2013, 93, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, K.; Rezler, R.; Baranowska, H.M.; Stangierski, J. Influence of fish oil and microencapsulated fish oil additives on water binding and the rheological properties of poultry sausage batters. J. Sci. Food Agric. 2021, 101, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Bi, J.; Dai, R.; Li, X. Effects of fat/oil type and ethylcellulose on the gel characteristic of pork batter. Food Res. Int. 2020, 138, 109788. [Google Scholar] [CrossRef] [PubMed]
- Roullet, M.; Clegg, P.S.; Frith, W.J. Rheology of protein-stabilised emulsion gels envisioned as composite networks 1—Comparison of pure droplet gels and protein gels. J. Colloid Interface Sci. 2020, 579, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Atashkar, M.; Hojjatoleslamy, M.; Sedaghat Boroujeni, L. The influence of fat substitution with κ-carrageenan, konjac, and tragacanth on the textural properties of low-fat sausage. Food Sci. Nutr. 2018, 6, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Melro, E.; Antunes, F.; Cruz, I.; Ramos, P.E.; Carvalho, F.; Alves, L. Morphological, textural and physico-chemical characterization of processed meat products during their shelf life. Food Struct. 2020, 26, 100164. [Google Scholar] [CrossRef]
- Totosaus, A.; Pérez-Chabela, M.; Lourdes, S. Textural properties and microstructure of low-fat and sodium-reduced meat batters formulated with gellan gum and dicationic salts. LW Food Sci. Technol. 2009, 42, 563–569. [Google Scholar] [CrossRef]
- Varga-Visi, É.; Toxanbayeva, B.; Andrássyné Baka, G.; Romvári, R. Textural properties of turkey sausage using pea fiber or potato starch as fat replacers. Acta Aliment. 2018, 47, 36–43. [Google Scholar] [CrossRef]
- Resurreccion, A.V. Sensory aspects of consumer choices for meat and meat products. Meat Sci. 2004, 66, 11–20. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M. Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- Tomasik, P.; Schilling, C.H. Complexes of starch with organic guests. Adv. Carbohyd. Chem. 1998, 53, 345–426. [Google Scholar]
- Pareyt, B.; Finnie, S.; Putseys, J.; Delcour, J. Lipids in bread making: Sources, interactions, and the impact on bread quality. J. Cereal Sci. 2011, 54, 266–279. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.S.; Chen, H.H.; Li, Q.Q.; Wang, Q. The gelatinization and retrogradation properties of wheat starch with the addition of stearic acid and sodium alginate. Food Hydrocoll. 2018, 81, 77–86. [Google Scholar] [CrossRef]
- Dun, H.; Liang, H.; Zhan, F.; Wei, X.; Chen, Y.; Wan, J.; Ren, Y.; Hu, Li.; Li, B. Influence of O/W emulsion on gelatinization and retrogradation properties of rice starch. Food Hydrocoll. 2020, 103, 105652. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of food gum and starch dispersions. In Rheology of Fluid, Semisolid, and Solid Foods; Springer: Berlin/Heidelberg, Germany, 2014; pp. 161–229. [Google Scholar]
- Vilgis, T.A. Gels: Model systems for soft matter food physics. Curr. Opin. Food Sci. 2015, 3, 71–84. [Google Scholar] [CrossRef]
- Joyner, H.S. Explaining food texture through rheology. Curr. Opin. Food Sci. 2018, 21, 7–14. [Google Scholar] [CrossRef]
- Sadeghi-Mehr, A.; Raudsepp, P.; Brüggemann, D.A.; Lautenschlaeger, R.; Drusch, S. Dynamic rheology, microstructure and texture properties of model porcine meat batter as affected by different cold-set binding systems. Food Hydrocoll. 2018, 77, 937–944. [Google Scholar] [CrossRef]
- Barbut, S. Effects of regular and modified potato and corn starches on frankfurter type products prepared with vegetable oil. Ital. J. Food Sci. 2018, 30, 801–808. [Google Scholar]
- Campo, L.; Tovar, C. Influence of the starch content in the viscoelastic properties of surimi gels. J. Food Eng. 2008, 84, 140–147. [Google Scholar] [CrossRef]
- Shon, S.R.; Chin, K.B. Evaluation of rheological properties of pork myofibrillar protein with tapioca starch and its utilization to the pork model sausages. Korean J. Food Sci. Anim. 2012, 32, 323–329. [Google Scholar] [CrossRef] [Green Version]
- De Souza Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Stangierski, J.; Rezler, R.; Kawecki, K.; Pepli´nska, B. Effect of microencapsulated fish oil powder on selected quality characteristics of chicken sausages. J. Sci. Food Agric. 2020, 100, 2043–2051. [Google Scholar] [CrossRef]
- Rezler, R.; Krzywdzińska-Bartkowiak, M.; Piątek, M. The influence of the substitution of fat with modified starch on the quality of pork liver pâtés. LWT Food Sci. Technol. 2021, 135, 110264. [Google Scholar] [CrossRef]
- Kumar, Y. Development of low-fat/reduced-fat processed meat products using fat replacers and analogues. Food Rev. Int. 2019, 37, 1–17. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Ge, Q.; Yu, H.; Xiong, Y.L. Rheology and microstructure of myofibrillar protein-starch composite gels: Comparison of native and modified starches. Int. J. Biol. Macromol. 2018, 118, 988–996. [Google Scholar] [CrossRef]
- Toledo, O.; Totosaus, A. Raw and cooked meat emulsion stability as affected by starches determined by principal component analysis. Emir. J. Food Agric. 2020, 32, 786–795. [Google Scholar] [CrossRef]
- Lee, C.H.; Chin, K.B. Changes in physicochemical properties of pork myofibrillar protein combined with corn starch and application to low-fat pork patties. Int. J. Food Sci. Technol. 2020, 55, 157–164. [Google Scholar] [CrossRef]
- Aktaş, N.; Genccelep, H. Effect of starch type and its modifications on physicochemical properties of bologna-type sausage produced with sheep tail fat. Meat Sci. 2006, 74, 404–408. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley and Sons: New York, NY, USA, 1970. [Google Scholar]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Matignon, A.; Tecante, A. Starch retrogradation: From starch components to cereal products. Food Hydrocoll. 2017, 68, 43–52. [Google Scholar] [CrossRef]
- Biais, B.; Le Bail, P.; Robert, P.; Pontoire, B.; Buléon, A. Structural and stoichiometric studies of complexes between aroma compounds and amylose. Polymorphic transitions and quantification in amorphous and crystalline areas. Carbohydr. Polym. 2006, 66, 306–315. [Google Scholar] [CrossRef]
- Mariscal-Moreno, R.M.; Figueroa-Cárdenas, J.D.D.; Santiago-Ramos, D.; Rayas-Duarte, P. Amylose lipid complexes formation as an alternative to reduce amylopectin retrogradation and staling of stored tortillas. Int. J. Food Sci. Technol. 2019, 54, 1651–1657. [Google Scholar] [CrossRef]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Tang, M.C.; Copeland, L. Analysis of complexes between lipids and wheat starch. Carbohydr. Polym. 2007, 67, 80–85. [Google Scholar] [CrossRef]
- Lu, X.; Shi, C.; Zhu, J.; Li, Y.; Huang, Q. Structure of starch-fatty acid complexes produced via hydrothermal treatment. Food Hydrocoll. 2019, 88, 58–67. [Google Scholar] [CrossRef]
- Kong, L.; Yucel, U.; Yoksan, R.; Elias, R.J.; Ziegler, G.R. Characterization of amylose inclusion complexes using electron paramagnetic resonance spectroscopy. Food Hydrocoll. 2018, 82, 82–88. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Physical properties, microstructure and digestion behavior of amylose-lipid powder complexes prepared using conventional and spray-drying based methods. Food Biosci. 2020, 37, 100724. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Van Duynhoven, J.P.; Acevedo, N.C.; Nicholson, R.A.; Patel, A.R. Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matter 2020, 16, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The role of hydrogen bonds in TAG derivative-based oleogel structure and properties. Food Chem. 2021, 334, 127585. [Google Scholar] [CrossRef] [PubMed]
- Zabar, S.; Lesmes, U.; Katz, I.; Shimoni, E.; Bianco-Peled, H. Studying different dimensions of amylose-long chain fatty acid complexes: Molecular, nano and micro level characteristics. Food Hydrocoll. 2009, 23, 1918–1925. [Google Scholar] [CrossRef]
- Lesmes, U.; McClements, D.J. Structure-function relationships to guide rational design and fabrication of particulate food delivery systems. Trends Food Sci. Technol. 2009, 20, 448–457. [Google Scholar] [CrossRef]
- Bourne, M.C. Basic principles of food texture measurement. In Dough Rheology and Baked Product Texture; Springer: Boston, MA, USA, 1990; pp. 331–341. [Google Scholar]
- Wilkinson, C.; Dijksterhuis, G.B.; Minekus, M. From food structure to texture. Trends Food Sci. Technol. 2000, 11, 442–450. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Kohyama, K. Chapter 1. Food Texture—Sensory Evaluation and Instrumental Measurement. In Textural Characteristics of World Foods; Nishinari, K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–13. [Google Scholar]
- Morris, V.J. Starch gelation and retrogradation. Trends Food Sci. Technol. 1990, 1, 2–6. [Google Scholar] [CrossRef]
- Civille, G.V.; Szczesniak, A.S. Guidelines to training a texture profile panel. J. Texture Stud. 1973, 4, 204–223. [Google Scholar] [CrossRef]
- Karam, L.B.; Ferrero, C.; Martino, M.N.; Zaritzky, N.E.; Grossmann, M.V.E. Thermal, microstructural and textural characterisation of gelatinised corn, cassava and yam starch blends. Int. J. Food Sci. Technol. 2006, 41, 805–812. [Google Scholar] [CrossRef]
- Roman, L.; Yee, J.; Hayes, A.M.; Hamaker, B.R.; Bertoft, E.; Martinez, M.M. On the role of the internal chain length distribution of amylopectins during retrogradation: Double helix lateral aggregation and slow digestibility. Carbohydr. Polym. 2020, 246, 116633. [Google Scholar] [CrossRef]
- Seo, T.R.; Kim, J.Y.; Lim, S.T. Preparation and characterization of crystalline complexes between amylose and C18 fatty acids. LWT Food Sci. Technol. 2015, 64, 889–897. [Google Scholar] [CrossRef]
- Garcia-Santos, M.D.S.L.; Conceicao, F.S.; Villas Boas, F.; Salotti De Souza, B.M.; Barretto, A.C.D.S. Effect of the addition of resistant starch in sausage with fat reduction on the physicochemical and sensory properties. Food Sci. Technol. 2019, 39, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Hu, H.; Xing, L.; Zhang, W.; Zhou, G. Influence of rice flour, glutinous rice flour, and tapioca starch on the functional properties and quality of an emulsion-type cooked sausage. Foods 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, W.R.; Laignele, B. An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Rezler, R.; Poliszko, S. Temperature dependence of starch gel rheological properties. Food Hydrocoll. 2010, 24, 570–577. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Przybylski, R.; Klensporf-Pawlik, D.; Anwar, F.; Rudzinska, M. Lipid Components of the North American Wild Rice (Zizania palustris). J. Am. Oil Chem. Soc. 2009, 86, 553–559. [Google Scholar] [CrossRef]



| cs, g/g | CI, % |
|---|---|
| 0.20 | 20.48 ± 0.89 |
| 0.17 | 27.64 ± 0.98 |
| 0.13 | 32.48 ± 0.74 |
| 0.11 | 40.20 ± 1.35 |
| 0.09 | 43.50 ± 1.12 |
| 0.05 | 45.24 ± 1.24 |
| Fatty Acids | Content, % |
|---|---|
| C10:0 | 0.06 |
| C12:0 | 0.07 |
| C13:0 | – |
| C14:0 | 1.37 |
| C14:1 c-9 | 0.07 |
| C15:0 | 0.12 |
| C16:0 | 23.76 |
| C16:1 c-9 | 2.34 |
| C17:0 | 0.52 |
| C18:0 | 17.86 |
| C18:1 c-9 | 41.57 |
| C18:2 c-9 c-12 | 7.38 |
| C18:3 c-9 c-12 c-15 | 0.70 |
| C20:0 | 0.23 |
| C20:1 c-9 | 1.03 |
| cs g/g | Break Point N | Firmness N | Consistency N | Cohesiveness N | Index of Viscosity Ns | G′ Pas | G′′ Pas | ||
|---|---|---|---|---|---|---|---|---|---|
| after one day | starch gels | 0.25 | 37.60 ± 0.90 | 69.49 ± 0.65 | 1659.09 ± 42.12 | −24.04 ± 0.36 | 1254.93 ± 42.19 | 7200.8 ± 354.2 | 6001.2 ± 439.5 |
| 0.21 | 32.32 ± 0.30 | 44.55 ± 0.43 | 1008.10 ± 32.17 | −15.28 ± 0.22 | 725.80 ± 36.98 | 4860.0 ± 265.4 | 3075.4 ± 298.7 | ||
| 0.17 | 9.01 ± 0.46 | 16.37 ± 0.70 | 334.57 ± 11.58 | −5.10 ± 0.31 | 232.66 ± 8.49 | 3400.3 ± 189.1 | 2250.3 ± 192.1 | ||
| 0.14 | 4.21 ± 0.21 | 12.10 ± 0.63 | 140.21 ± 4.34 | −3.20 ± 0.15 | 113.24 ± 4.17 | 2050.1 ± 57.3 | 1325.6 ± 131.3 | ||
| 0.12 | 1.18 ± 0.07 | 1.01 ± 0.05 | 30.07 ± 1.29 | −0.31 ± 0.04 | 18.53 ± 0.54 | 600.8 ± 48.5 | 551.0 ± 32.8 | ||
| 0.06 | 0.32 ± 0.01 | 0.52 ± 0.02 | 7.35 ± 0.43 | −0.18 ± 0.02 | 4.11 ± 0.25 | 400.5 ± 11.7 | 350.6 ± 24.1 | ||
| starch-fat emulsions | 0.20 | 20.51 ± 0.82 | 21.31 ± 0.85 | 583.67 ± 17.23 | −9.54 ± 0.51 | 377.35 ± 10.93 | 7450.6 ± 345.1 | 6958.3 ± 248.7 | |
| 0.17 | 9.51 ± 0.35 | 11.92 ± 0.39 | 295.73 ± 6.96 | −5.75 ± 0.17 | 171.25 ± 3.51 | 5750.5 ± 147.5 | 4763.1 ± 327.6 | ||
| 0.13 | 1.41 ± 0.05 | 1.08 ± 0.02 | 110.47 ± 3.74 | −1.35 ± 0.06 | 25.80 ± 1.39 | 3625.4 ± 125.7 | 3084.2 ± 211.4 | ||
| 0.11 | 1.15 ± 0.04 | 0.76 ± 0.02 | 54.73 ± 2.01 | −0.52 ± 0.05 | 13.42 ±0.73 | 2850.9 ± 123.4 | 2658.7 ± 190.1 | ||
| 0.09 | 0.80 ± 0.03 | 0.75 ± 0.02 | 29.71 ± 1.61 | −0.49 ± 0.04 | 8.34 ± 0.50 | 1925.3 ± 83.2 | 1899.1 ± 128.5 | ||
| 0.05 | 1.10 ± 0.04 | 0.54 ± 0.01 | 11.19 ± 0.37 | −0.10 ± 0.02 | 5.20 ± 0.04 | 812.5 ± 35.4 | 7396.8 ± 43.8 | ||
| after seven day | starch gels | 0.25 | 84.53 ± 4.07 | 159.28 ± 4.90 | 4126.50 ± 103.69 | −22.62 ± 0.93 | 3830.44 ± 95.21 | 7800.7 ± 84.2 | 6240.5 ± 274.2 |
| 0.21 | 49.72 ± 3.21 | 75.31 ± 3.84 | 2022.23 ± 77.15 | −20.01 ± 0.84 | 1407.30 ± 51.37 | 5458.4 ± 59.3 | 4366.7 ± 271.3 | ||
| 0.17 | 18.24 ± 1.11 | 16.48 ± 1.43 | 313.51 ± 16.39 | −10.53 ± 0.41 | 213.45 ± 7.84 | 4190.6 ± 47.5 | 3352.4 ± 175.7 | ||
| 0.14 | 4.11 ± 0.63 | 3.34 ± 0.39 | 87.26 ± 10.02 | −4.51 ± 0.22 | 65.32 ± 2.79 | 3520.3 ± 31.1 | 2816.2 ± 145.3 | ||
| 0.12 | 1.85 ± 0.27 | 1.11 ± 0.12 | 46.78 ± 7.78 | −3.03 ± 0.19 | 26.38 ± 1.26 | 870.1 ± 11.5 | 696.0 ± 32.8 | ||
| 0.06 | 1.12 ± 0.17 | 0.80 ± 0.09 | 23.02 ± 3.25 | −0.6 ± 0.04 | 9.24 ± 0.68 | 600.9 ± 8.7 | 480.7 ± 21.4 | ||
| starch-fat emulsions | 0.20 | 62.74 ± 4.57 | 57.91 ± 0.75 | 1750.58 ± 29.39 | −14.78 ± 0.63 | 1610.40 ± 45.88 | 8469.6 ± 142.4 | 7775.6 ± 327.5 | |
| 0.17 | 26.62 ± 1.52 | 25.63 ± 0.46 | 773.85 ± 4.25 | −12.62 ± 0.51 | 504.69 ± 10.24 | 6289.8 ± 131.7 | 5331.8 ± 269.7 | ||
| 0.13 | 3.71 ± 0.21 | 2.94 ± 0.09 | 84.50 ± 2.65 | −3.73 ± 0.18 | 44.38 ± 1.95 | 4293.7 ± 93.6 | 3434.9 ± 164.3 | ||
| 0.11 | 2.93 ± 0.17 | 1.25 ± 0.05 | 68.73 ± 1.92 | −0.80 ± 0.05 | 21.25 ± 0.91 | 3075.0 ± 74.4 | 2760.0 ± 136.1 | ||
| 0.09 | 1.24 ± 0.09 | 0.99 ± 0.04 | 40.72 ± 1.46 | −0.66 ± 0.04 | 18.80 ± 0.78 | 2147.2 ± 65.2 | 1917.7 ± 87.3 | ||
| 0.05 | 1.30 ± 0.09 | 1.13 ± 0.05 | 25.09 ± 0.63 | −0.11 ± 0.02 | 13.60 ± 0.66 | 1078.1 ± 30.5 | 862.4 ± 45.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezler, R. DMA Study of the Molecular Structure of Porcine Fat in-Water Emulsions Stabilised by Potato Starch. Int. J. Mol. Sci. 2021, 22, 7276. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147276
Rezler R. DMA Study of the Molecular Structure of Porcine Fat in-Water Emulsions Stabilised by Potato Starch. International Journal of Molecular Sciences. 2021; 22(14):7276. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147276
Chicago/Turabian StyleRezler, Ryszard. 2021. "DMA Study of the Molecular Structure of Porcine Fat in-Water Emulsions Stabilised by Potato Starch" International Journal of Molecular Sciences 22, no. 14: 7276. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147276






