Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Location, Conservation and Pathogenicity Predictions of Four CYP1A1 Variants
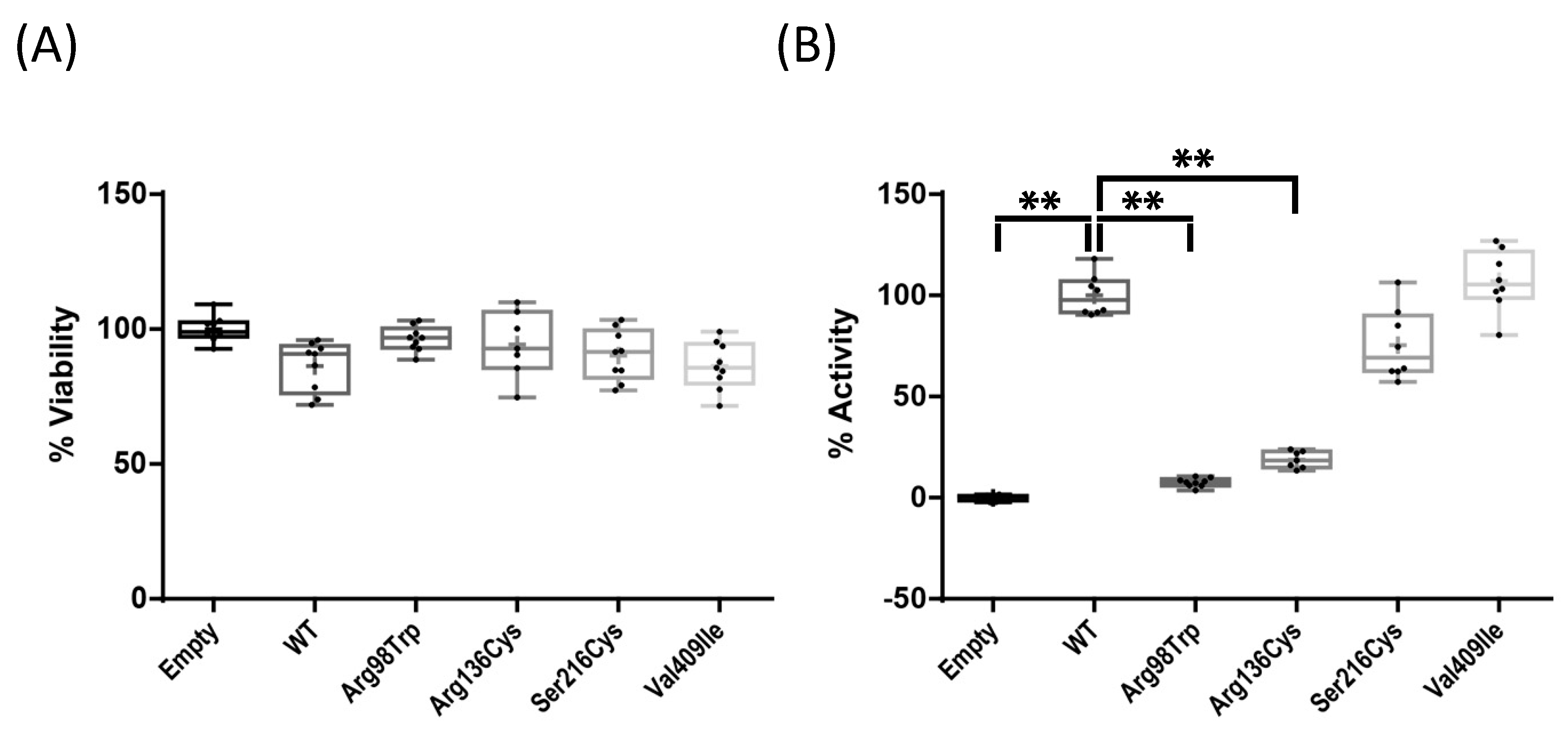
2.2. Enzyme Activity Assays of Four CYP1A1 Variants
2.3. Effect of BP Treatment on Wild-Type CYP1A1 Enzyme Activity
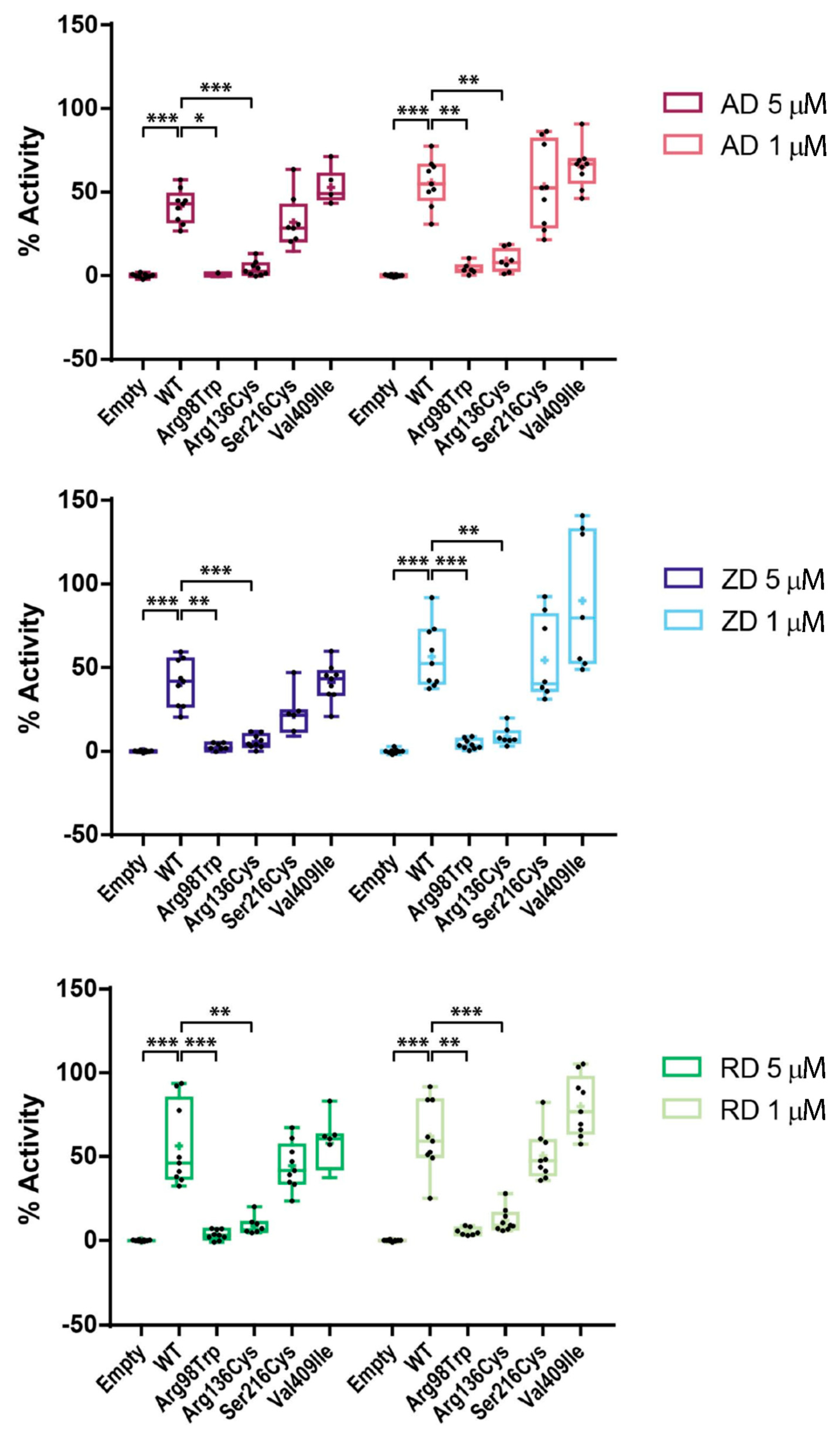
2.4. Effect of BP Treatment on Enzyme Activity of Four CYP1A1 Variants
2.5. AFF as an Oligogenic Phenotype; a Role for CYP1A1 Variants in Combination with BPs
3. Materials and Methods
3.1. In Silico Prediction of Protein Structure and Pathogenicity
3.2. Site-Directed Mutagenesis
3.3. Cell Culture and Transfection
3.4. RT-qPCR
3.5. Bisphosphonate Treatment and Enzymatic and Viability Assays
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, R.G.G.; Xia, Z.; Dunford, J.E.; Oppermann, U.; Kwaasi, A.; Hulley, P.A.; Kavanagh, K.L.; Triffitt, J.T.; Lundy, M.W.; Phipps, R.J.; et al. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann. N. Y. Acad. Sci. 2007, 1117, 209–257. [Google Scholar] [CrossRef] [PubMed]
- Chapurlat, R.D.; Delmas, P.D. Drug insight: Bisphosphonates for postmenopausal osteoporosis. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 211–219. [Google Scholar] [CrossRef]
- Freemantle, N.; Cooper, C.; Diez-Perez, A.; Gitlin, M.; Radcliffe, H.; Shepherd, S.; Roux, C. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: A meta-analysis. Osteoporos. Int. 2013, 24, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ma, X.; Wang, T.; Zhai, S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: A systematic review with network meta-analyses. Osteoporos. Int. 2016, 27, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, Y.; Chen, L.; Peng, K.; Xu, Z.; Zhang, D.; Xiang, Z. Bisphosphonates can prevent recurrent hip fracture and reduce the mortality in osteoporotic patient with hip fracture: A meta-analysis. Pak. J. Med. Sci. 2016, 32, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; van Rooij, J.G.J.; Ebeling, P.R.; Verkerk, A.J.M.H.; Zillikens, M.C. The genetics of atypical femur fractures—A systematic review. Curr. Osteoporos. Rep. 2021, 19, 123–130. [Google Scholar] [CrossRef]
- Shin, W.C.; Moon, N.H.; Jang, J.H.; Park, K.Y.; Suh, K.T. Anterolateral femoral bowing and loss of thigh muscle are associated with occurrence of atypical femoral fracture: Effect of failed tension band mechanism in mid-thigh. J. Orthop. Sci. 2017, 22, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Ishijima, M.; Mogami, A.; Kubota, M.; Baba, T.; Kaketa, T.; Nagao, M.; Sakamoto, Y.; Sakai, K.; Kato, R.; et al. The fracture sites of atypical femoral fractures are associated with the weight-bearing lower limb alignment. Bone 2014, 66, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Ishijima, M.; Mogami, A.; Kubota, M.; Baba, T.; Kaketa, T.; Nagao, M.; Sakamoto, Y.; Sakai, K.; Homma, Y.; et al. The incidence of and risk factors for developing atypical femoral fractures in Japan. J. Bone Miner. Metab. 2015, 33, 311–318. [Google Scholar] [CrossRef]
- Kim, D.; Sung, Y.K.; Cho, S.K.; Han, M.; Kim, Y.-S. Factors associated with atypical femoral fracture. Rheumatol. Int. 2016, 36, 65–71. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lakhani, A.; Shore-Lorenti, C.; Zebaze, R.; Vincent, A.J.; Milat, F.; Ebeling, P.R. Asian ethnicity is associated with atypical femur fractures in an Australian population study. Bone 2020, 135, 115319. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; van de Laarschot, D.M.; Verkerk, A.J.M.H.; Milat, F.; Zillikens, M.C.; Ebeling, P.R. Genetic risk factors for atypical femoral fractures (AFFs): A Systematic Review. JBMR Plus 2018, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca-Ayats, N.; Ying, P.; Garcia-Giralt, N.; Falcó-Mascaró, M.; Cozar, M.; Abril, J.F.; Quesada-Gómez, J.M.; Prieto-Alhambra, D.; Nogués, X.; Dunford, J.E.; et al. Functional characterization of a GGPPS variant identified in atypical femoral fracture patients and delineation of the role of GGPPS in bone-relevant cell types. J. Bone Miner. Res. 2018, 33, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Peris, P.; González-Roca, E.; Rodríguez-García, S.C.; López-Cobo, M.M.; Monegal, A.; Guañabens, N. Incidence of mutations in the ALPL, GGPS1, and CYP1A1 genes in patients with atypical femoral fractures. JBMR Plus 2019, 3, 29–36. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Human family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: An update. Arch. Toxicol. 2021, 95, 395–472. [Google Scholar] [CrossRef]
- Kisselev, P.; Schunck, W.H.; Roots, I.; Schwarz, D. Association of CYP1A1 polymorphisms with differential metabolic activation of 17β-estradiol and estrone. Cancer Res. 2005, 65, 2972–2978. [Google Scholar] [CrossRef] [Green Version]
- Leelawattana, R.; Ziambaras, K.; Roodman-Weiss, J.; Lyss, C.; Wagner, D.; Klug, T.; Armamento-Villareal, R.; Civitelli, R. The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J. Bone Miner. Res. 2000, 15, 2513–2520. [Google Scholar] [CrossRef]
- Napoli, N.; Villareal, D.T.; Mumm, S.; Halstead, L.; Sheikh, S.; Cagaanan, M.; Rini, G.B.; Armamento-Villareal, R. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J. Bone Miner. Res. 2005, 20, 232–239. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkonen, J.; Muñoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.T.; Cao, R.; Liang, P.H.; Ko, T.-P.; Chang, T.-H.; Hudock, M.P.; Jeng, W.-Y.; Chen, C.K.-M.; Zhang, Y.; Song, Y.; et al. Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. Proc. Natl. Acad. Sci. USA 2007, 104, 10022–10027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, D.L.; Watts, N.B.; Miller, P.D. Bisphosphonates pharmacology and use in the treatment of osteoporosis. In Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D.W., Cauley, J.A., Bouxsein, M.L., Cosman, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1721–1736. [Google Scholar]
- Nancollas, G.H.; Tang, R.; Phipps, R.J.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.G.G.; Ebetino, F.H. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Wang, L.; Jin, X.; Shen, Y.; Nan, C.; Liu, H. Minimally effective concentration of zoledronic acid to suppress osteoclasts. Exp. Ther. Med. 2018, 15, 5330–5336. [Google Scholar] [PubMed]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Such, E.; Cervera, J.; Terpos, E.; Bagán, J.V.; Avaria, A.; Gómez, I.; Margaix, M.; Ibañez, M.; Luna, I.; Cordón, L.; et al. CYP2C8 gene polymorphism and bisphosphonate-related osteonecrosis of the jaw in patients with multiple myeloma. Haematologica 2011, 96, 1557–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasquete, M.E.; García-Sanz, R.; Marin, L.; Alcoceba, M.; Chillón, M.C.; Balanzategui, A.; Santamaria, C.; Rosiñol, L.; de la Rubia, J.; Hernandez, M.T.; et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: A genome-wide single nucleotide polymorphism analysis. Blood 2008, 112, 2709–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef] [Green Version]
- Dutkiewicz, Z.; Mikstacka, R. Structure-based drug design for cytochrome P450 family 1 inhibitors. Bioinorg. Chem. Appl. 2018, 2018. [Google Scholar] [CrossRef]
- Bart, A.G.; Takahashi, R.H.; Wang, X.; Scott, E.E. Human cytochrome P450 1A1 adapts active site for atypical nonplanar substrate. Drug Metab. Dispos. 2020, 48, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.A.; Badros, A.; Salama, A.R. A novel bioassay model to determine clinically significant bisphosphonate levels. Support. Care Cancer 2009, 17, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugartondo, N.; Martínez-Gil, N.; Esteve, M.; Garcia-Giralt, N.; Roca-Ayats, N.; Ovejero, D.; Nogués, X.; Díez-Pérez, A.; Rabionet, R.; Grinberg, D.; et al. Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures. Int. J. Mol. Sci. 2021, 22, 7395. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147395
Ugartondo N, Martínez-Gil N, Esteve M, Garcia-Giralt N, Roca-Ayats N, Ovejero D, Nogués X, Díez-Pérez A, Rabionet R, Grinberg D, et al. Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures. International Journal of Molecular Sciences. 2021; 22(14):7395. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147395
Chicago/Turabian StyleUgartondo, Nerea, Núria Martínez-Gil, Mònica Esteve, Natàlia Garcia-Giralt, Neus Roca-Ayats, Diana Ovejero, Xavier Nogués, Adolfo Díez-Pérez, Raquel Rabionet, Daniel Grinberg, and et al. 2021. "Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures" International Journal of Molecular Sciences 22, no. 14: 7395. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147395





