Sex Steroids and the Shaping of the Peripubertal Brain: The Sexual-Dimorphic Set-Up of Adult Neurogenesis
Abstract
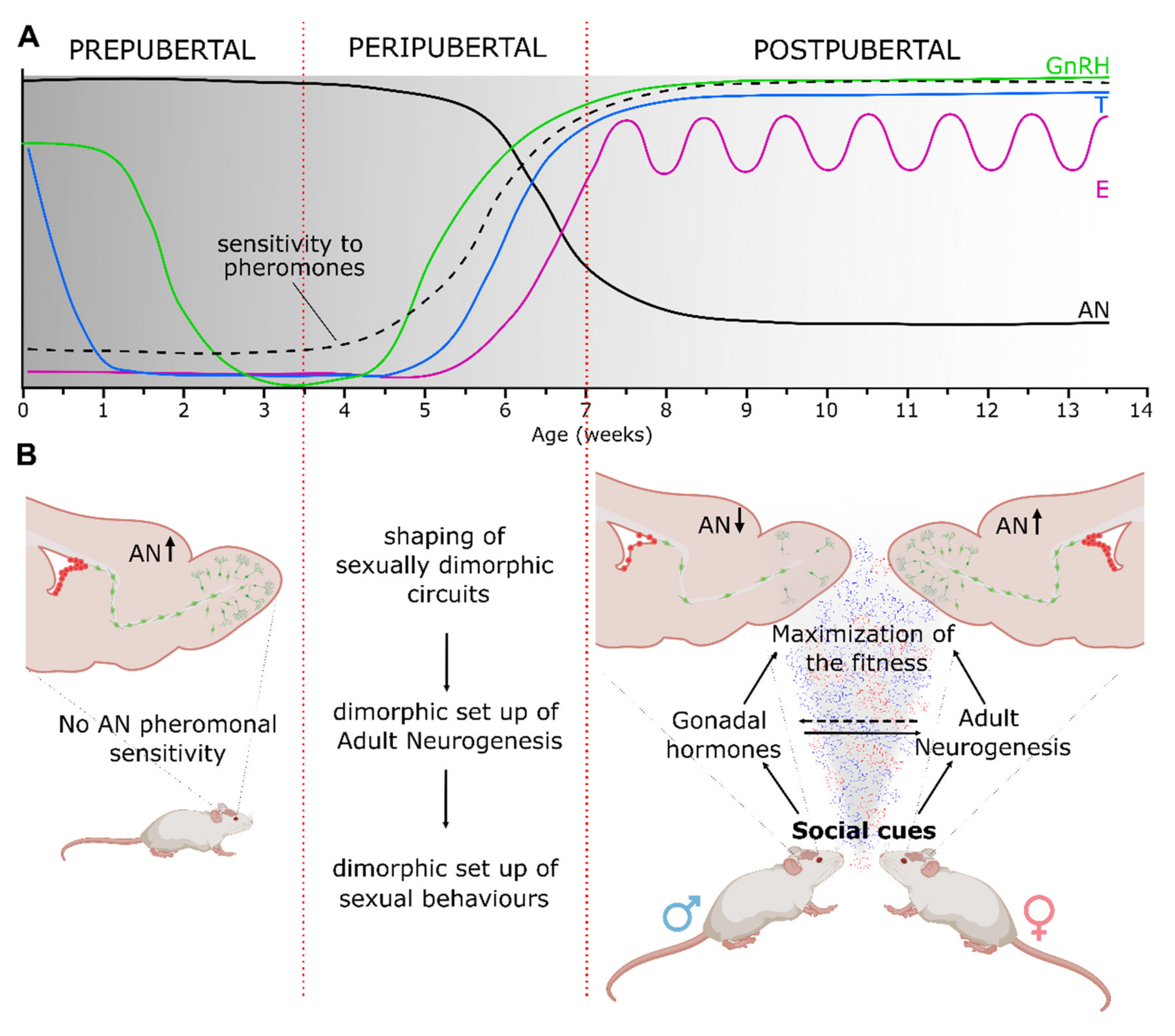
:1. Introduction
2. Sex Steroid-Dependent Refinement of Brain Circuits and Behaviors during Peripuberty
3. Mechanisms Underlying the Sex Steroid-Dependent Refinement of the Brain at Peripubertal Ages
4. The Interplay among Pheromones, Hormones and Adult Neurogenesis in the Regulation of Reproductive Activities
5. Role of Hormones on the Sexually Dimorphic Set-Up of Adult Neurogenesis at Puberty
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, S.E.; Levitt, P.; Nelson, C.A. How the timing and quality of early experiences influence the development of brain architecture. Child. Dev. 2011, 81, 28–40. [Google Scholar] [CrossRef]
- Stoka, A.M. Phylogeny and evolution of chemical communication: An endocrine approach. J. Mol. Endocrinol. 1999, 22, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef]
- Bakker, J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J. Neuroendocrinol. 2003, 15, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, M.J.; Bakker, J. Roles of sex and gonadal steroids in mammalian pheromonal communication. Front. Neuroendocrinol. 2013, 34, 4. [Google Scholar] [CrossRef] [Green Version]
- Mccarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, J.; Busby, E.R.; Kirilov, M.; Schütz, G.; Sherwood, N.M.; Herbison, A.E. Systems/circuits sexual differentiation of the brain requires perinatal kisspeptin-gnRH neuron signaling. J. Neurosci. 2014, 34, 15297–15305. [Google Scholar] [CrossRef] [Green Version]
- Romeo, R.D. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. J. Neuroendocrinol. 2003, 15, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.D.; Richardson, H.N.; Sisk, C.L. Puberty and the maturation of the male brain and sexual behavior: Recasting a behavioral potential. Neurosci. Biobehav. Rev. 2002, 26, 381–391. [Google Scholar] [CrossRef]
- Petrulis, A. Chemosignals and hormones in the neural control of mammalian sexual behavior. Front. Neuroendocrinol. 2013, 34, 255–267. [Google Scholar] [CrossRef]
- Petrulis, A. Chemosignals, hormones and mammalian reproduction. Horm. Behav. 2013, 63, 723–741. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Chamero, P.; Pru, J.K.; Chien, M.S.; Ibarra-Soria, X.; Spencer, K.R.; Logan, D.W.; Matsunami, H.; Peluso, J.J.; Stowers, L. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell 2015, 161, 1334–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelliher, K.R.; Chang, Y.-M.; Wersinger, S.R.; Baum, M.J. Sex difference and testosterone modulation of pheromone-induced neuronal fos in the ferret’s main olfactory bulb and hypothalamus. Biol. Reprod. 1998, 59, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Oboti, L.; Trova, S.; Schellino, R.; Marraudino, M.; Peretto, P. Activity dependent modulation of granule cell survival in the accessory olfactory bulb at puberty. Front. Neuroanat. 2017, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, C.; Wang, Z. Hormonal regulation of mammalian adult neurogenesis: A multifaceted mechanism. Biomolecules 2020, 10, 1151. [Google Scholar] [CrossRef]
- Mahmoud, R.; Wainwright, S.R.; Galea, L.A.M. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocrinol. 2016, 41, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Schellino, R.; Trova, S.; Cimino, I.; Farinetti, A.; Jongbloets Pasterkamp, R.J.; Panzica, G.; Giacobini, P.; De Marchis, S.; Peretto, P. Opposite-sex attraction in male mice requires testosterone-dependent regulation of adult olfactory bulb neurogenesis. Sci. Rep. 2016, 6, 36063. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Nottebohm, F. Migration of young neurons in adult avian brain. Nature 1988, 335, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feierstein, C.E. Linking adult olfactory neurogenesis to social behavior. Front. Neurosci. 2012, 6, 173. [Google Scholar] [CrossRef] [Green Version]
- Peretto, P.; Schellino, R.; De Marchis, S.; Fasolo, A. The Interplay between reproductive social stimuli and adult olfactory bulb neurogenesis. Neural Plast. 2014, 2014, 497657. [Google Scholar] [CrossRef]
- Feierstein, C.E.; Lazarini, F.; Wagner, S.; Gabellec, M.M.; de Chaumont, F.; Olivo-Marin, J.C.; Boussin, F.D.; Lledo, P.M.; Gheusi, G. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front. Behav. Neurosci. 2010, 4, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oboti, L.; Schellino, R.; Giachino, C.; Chamero, P.; Pyrski, M.; Leinders-Zufall, T.; Zufall, F.; Fasolo, A.; Peretto, P. Newborn interneurons in the accessory olfactory bulb promote mate recognition in female mice. Front. Neurosci. 2011, 5, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, G.K.; Enwere, E.K.; Gregg, C.; Pakarainen, T.; Poutanen, M.; Huhtaniemi, I.; Weiss, S. Male pheromone-stimulated neurogenesis in the adult female brain: Possible role in mating behavior. Nat. Neurosci. 2007, 10, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Oboti, L.; Savalli, G.; Giachino, C.; De Marchis, S.; Panzica, G.C.; Fasolo, A.; Peretto, P. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur. J. Neurosci. 2009, 29, 679–692. [Google Scholar] [CrossRef]
- Liberles, S.D. Mammalian pheromones. Annu. Rev. Physiol. 2014, 76, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Ponti, G.; Farinetti, A.; Marraudino, M.; Panzica, G.C.; Gotti, S. Sex steroids and adult neurogenesis in the ventricular-subventricular zone. Front. Endocrinol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Messina, A.; Ferraris, N.; Wray, S.; Cagnoni, G.; Donohue, D.E.; Casoni, F.; Kramer, P.R.; Derijck, A.A.; Adolfs, Y.; Fasolo, A.; et al. Dysregulation of Semaphorin7A/β1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility. Hum. Mol. Genet. 2011, 20, 4759–4774. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Langlet, F.; Chachlaki, K.; Roa, J.; Rasika, S.; Jouy, N.; Gallet, S.; Gaytan, F.; Parkash, J.; Tena-Sempere, M.; et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci. 2016, 19, 835–844. [Google Scholar] [CrossRef]
- Bronson, F.H.; Rissman, E.F. The biology of puberty. Biol. Rev. Camb. Philos. Soc. 1986, 61, 157–195. [Google Scholar] [CrossRef]
- Ebling, F.J.P.; Cronin, A.S. The neurobiology of reproductive development. Neuroreport 2000, 11, R23–R33. [Google Scholar] [CrossRef] [PubMed]
- Sisk, C.L.; Foster, D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004, 7, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.J.; Hayflick, J.S.; Zoeller, R.T.; Young, W.S.; Phillips, H.S.; Nikolics, K.; Seeburg, P.H. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science 1986, 234, 1366–1371. [Google Scholar] [CrossRef]
- Dellovade, T.; Schwanzel-Fukuda, M.; Gordan, J.; Pfaff, D. Aspects of GnRH neurobiology conserved across vertebrate forms. Gen. Comp. Endocrinol. 1998, 112, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokininn B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef]
- Uenoyama, Y.; Inoue, N.; Nakamura, S.; Tsukamura, H. Central mechanism controlling pubertal onset in mammals: A triggering role of kisspeptin. Front. Endocrinol. 2010, 10, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisk, C.L.; Richardson, H.N.; Chappell, P.E.; Levine, J.E. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 2001, 142, 2929–2936. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Lomniczi, A.; Loche, A.; Matagne, V.; Kaidar, G.; Sandau, U.S.; Dissen, G.A. The Transcriptional Control of Female Puberty. Brain Res. 2010, 1364, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Harris, G.C.; Levine, J.E. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology 2003, 144. [Google Scholar] [CrossRef] [PubMed]
- Prevot, V. Puberty in mice and rats. In Knobil and Neil’s Physiology of Reproduction; Plant, T.M., Zeleznik, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1395–1439. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol. Cell. Endocrinol. 2006, 254, 120–126. [Google Scholar] [CrossRef]
- Sisk, C.L.; Schulz, K.M.; Zehr, J.L. Puberty: A finishing school for male social behavior. Ann. N. Y. Acad. Sci. 2003, 1007, 189–198. [Google Scholar] [CrossRef]
- Schulz, K.M.; Molenda-Figueira, H.A.; Sisk, C.L. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009, 55, 597–604. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.M.; Herold, K.; Stockman, S.L. Fast, furious and enduring: Sensitive versus critical periods in sexual differentiation of the brain. Physiol. Behav. 2018, 187, 13–19. [Google Scholar] [CrossRef]
- Wallen, K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005, 26, 7–26. [Google Scholar] [CrossRef]
- Piekarski, D.J.; Boivin, J.R.; Wilbrecht, L. Ovarian hormones organize the maturation of inhibitory neurotransmission in the frontal cortex at puberty onset in female mice. Curr. Biol. 2017, 27, 1735–1745. [Google Scholar] [CrossRef] [Green Version]
- Juraska, J.M.; Willing, J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2017, 1654, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Koss, W.A.; Lloyd, M.M.; Sadowski, R.N.; Wise, L.M.; Juraska, J.M. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev. Psychobiol. 2015, 57, 305–312. [Google Scholar] [CrossRef]
- Laube, C.; van den Bos, W.; Fandakova, Y. The relationship between pubertal hormones and brain plasticity: Implications for cognitive training in adolescence. Dev. Cogn. Neurosci. 2020, 42, 100753. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 2016, 70, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.M.; Richardson, H.N.; Zehr, J.L.; Osetek, A.J.; Menard, T.A.; Sisk, C.L. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm. Behav. 2004, 45, 242–249. [Google Scholar] [CrossRef]
- Schulz, K.M.; Menard, T.A.; Smith, D.A.; Albers, H.E.; Sik, C.L. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm. Behav. 2006, 50, 477–483. [Google Scholar] [CrossRef]
- Wu, Y.C.; Du, X.; van den Buuse, M.; Hill, R.A. Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: A role for estradiol. Psychoneuroendocrinology 2014, 45, 167–178. [Google Scholar] [CrossRef]
- Piekarski, D.J.; Johnson, C.M.; Boivin, J.R.; Thomas, A.W.; Lin, W.C.; Delevich, K.; Galarce, K.; Wilbrecht, L. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res. 2017, 1654, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Hier, D.B.; Crowley, W.F., Jr. Spatial ability in androgen-deficient men. New Engl. J. Med. 1982, 306, 1202–1205. [Google Scholar] [CrossRef]
- Mueller, S.C.; Mandell, D.; Leschek, E.W.; Pine, D.S.; Merke, D.P.; Ernst, M. Early hyperandrogenism affects the development of hippocampal function: Preliminary evidence from a functional magnetic resonance imaging study of boys with familial male precocious puberty. J. Child Adolesc. Psychopharmacol. 2009, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.A.; Juraska, J.M. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp. Neurol. 2008, 209, 284–287. [Google Scholar] [CrossRef] [Green Version]
- Willing, J.; Juraska, J.M. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience 2015, 301, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Markham, J.A.; Morris, J.R.; Juraska, J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience 2007, 144, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.A.; Belden, C.E.; Hristov, A.D.; Juraska, J.M. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse 2014, 68, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Luna, B.; Garver, K.E.; Urban, T.A.; Lazar, N.A.; Sweeney, J.A. Maturation of cognitive processes from late childhood to adulthood. Child. Dev. 2004, 75, 1357–1372. [Google Scholar] [CrossRef]
- Peper, J.S.; Dahl, R.E. Surging hormones: Brain-behavior interactions during puberty. Curr. Dir. Psychol. Sci. 2013, 22, 134–139. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Wolf, D.H.; Erus, G.; Ruparel, K.; Elliott, M.A.; Gennatas, E.D.; Hopson, R.; Jackson, C.; Prabhakaran, K.; Bilker, W.B.; et al. Functional maturation of the executive system during adolescence. J. Neurosci. 2013, 33, 16249–16261. [Google Scholar] [CrossRef] [Green Version]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Dorrn, A.L.; Yuan, K.; Barker, A.J.; Schreiner, C.E.; Froemke, R.C. Developmental sensory experience balances cortical excitation and inhibition. Nature 2010, 465, 932–936. [Google Scholar] [CrossRef]
- Ahmed, E.I.; Zehr, J.L.; Schulz, K.M.; Lorenz, B.H.; DonCarlos, L.L.; Sisk, C.L. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008, 11, 995–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beale, K.E.; Kinsey-Jones, J.S.; Gardiner, J.V.; Harrison, E.K.; Thompson, E.L.; Hu, M.H.; Sleeth, M.L.; Sam, A.H.; Greenwood, H.C.; McGavigan, A.K.; et al. The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinology 2014, 155, 1091–1098. [Google Scholar] [CrossRef]
- He, Z.; Ferguson, S.A.; Cui, L.; Greenfield, L.J.; Paule, M.G. Development of the sexually dimorphic nucleus of the preoptic area and the influence of estrogen-like compounds. Neural Regen. Res. 2013, 8, 2763–2774. [Google Scholar] [CrossRef]
- Tsukahara, S.; Morishita, M. Sexually dimorphic formation of the preoptic area and the bed nucleus of the stria terminalis by neuroestrogens. Front. Neurosci. 2020, 14, 797. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.C.; Krauthamer, S.; Essenberg, J.M.; Holy, T.E. Inhibition shapes sex selectivity in the mouse accessory olfactory bulb. J. Neurosci. 2008, 28, 12523–12534. [Google Scholar] [CrossRef]
- Brennan, P.A. Outstanding issues surrounding vomeronasal mechanisms of pregnancy block and individual recognition in mice. Behav. Brain Res. 2009, 200, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mucignat-Caretta, C.; Caretta, A.; Cavaggioni, A. Acceleration of puberty onset in female mice by male urinary proteins. J. Physiol. 1995, 486 Pt 2, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Pierman, S.; Douhard, Q.; Baum, M.J.; Bakker, J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 2006, 23, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Bonfanti, L.; Peretto, P.; Merighi, A.; Fasolo, A. Newly-generated cells from the rostral migratory stream in the accessory olfactory bulb of the adult rat. Neuroscience 1997, 81, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Peretto, P.; Giachino, C.; Panzica, G.C.; Fasolo, A. Sexually dimorphic neurogenesis is topographically matched with the anterior accessory olfactory bulb of the adult rat. Cell Tissue Res. 2001, 306, 385–389. [Google Scholar] [CrossRef]
- Hoffman, E.; Pickavance, L.; Thippeswamy, T.; Beynon, R.J.; Hurst, J.L. The male sex pheromone darcin stimulates hippocampal neurogenesis and cell proliferation in the subventricular zone in female mice. Front. Behav. Neurosci. 2015, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galea, L.A.M. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Rev. 2008, 57, 332–341. [Google Scholar] [CrossRef]
- Larsen, C.M.; Grattan, D.R. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 2010, 151, 3805–3814. [Google Scholar] [CrossRef] [Green Version]
- Imayoshi, I.; Sakamoto, M.; Ohtsuka, T.; Takao, K.; Miyakawa, T.; Yamaguchi, M.; Mori Ikeda, T.; Itohara, S.; Kageyama, R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008, 11, 1153–1161. [Google Scholar] [CrossRef]
- Bruce, H.M. Smell as an exteroceptive factor. J. Anim. Sci. 1966, 25, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Brennan, P.; Keverne, E.B. Biological complexity and adaptability of simple mammalian olfactory memory systems. Neurosci. Biobehav. Rev. 2014, 50, 29–40. [Google Scholar] [CrossRef]
- Li, C.S.; Kaba, H.; Saito, H.; Seto, K. Excitatory influence of the accessory olfactory bulb on tuberoinfundibular arcuate neurons of female mice and its modulation by oestrogen. Neuroscience 1989, 29, 201–208. [Google Scholar] [CrossRef]
- Brennan, P.A.; Zufall, F. Pheromonal communication in vertebrates. Nature 2006, 444, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.A.M.; Wainwright, S.R.; Roes, M.M.; Chow, C.; Hamson, D.K. Sex, hormones and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. Neuroendocrinology 2013, 1039–1061. [Google Scholar] [CrossRef] [Green Version]
- Trova, S.; Bovetti, S.; Pellegrino, G.; Bonzano, S.; Giacobini, P.; Peretto, P. HPG-dependent peri-pubertal regulation of adult neurogenesis in mice. Front. Neuroanat. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J.; Baker PSohnius, U.; Haavisto, A.-M.; Charlton, H.M.; Huhtaniemi, I. Fetal development of leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology 1998, 139, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Glanowska, K.M.; Burger, L.L.; Moenter, S.M. Development of gonadotropin-releasing hormone secretion and pituitary response. J. Neurosci. 2014, 34, 15060–15069. [Google Scholar] [CrossRef] [Green Version]
- Nunez-Parra, A.; Pugh, V.; Araneda, R.C. Regulation of adult neurogenesis by behavior and age in the accessory olfactory bulb. Mol. Cell. Neurosci. 2011, 47, 274–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banasr, M.; Hery, M.; Brezun, J.M.; Daszuta, A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur. J. Neurosci. 2001, 14, 1417–1424. [Google Scholar] [CrossRef]
- Leuner, B.; Mirescu CNoiman, L.; Gould, E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus 2007, 17, 434–442. [Google Scholar] [CrossRef]
- Crews, L.; Adame, A.; Patrick, C.; DeLaney, A.; Pham, E.; Rockenstein, E.; Hansen, L.; Masliah, E. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J. Neurosci. 2010, 30, 12252–12262. [Google Scholar] [CrossRef]
- Epp, J.R.; Beasley, C.L.; Galea, L.A. Increased hippocampal neurogenesis and p21 expression in depression: Dependent on antidepressants, sex, age, and antipsychotic exposure. Neuropsychopharmacology 2013, 38, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Díaz, D.; Muñoz-Castañeda, R.; Ávila-Zarza, C.; Carretero, J.; Alonso, J.R.; Weruaga, E. Olfactory bulb plasticity ensures proper olfaction after severe impairment in postnatal neurogenesis. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Pasterkamp, R.J.; Peschon, J.J.; Spriggs, M.K.; Kolodkin, A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003, 424, 398–405. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trova, S.; Bovetti, S.; Bonzano, S.; De Marchis, S.; Peretto, P. Sex Steroids and the Shaping of the Peripubertal Brain: The Sexual-Dimorphic Set-Up of Adult Neurogenesis. Int. J. Mol. Sci. 2021, 22, 7984. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22157984
Trova S, Bovetti S, Bonzano S, De Marchis S, Peretto P. Sex Steroids and the Shaping of the Peripubertal Brain: The Sexual-Dimorphic Set-Up of Adult Neurogenesis. International Journal of Molecular Sciences. 2021; 22(15):7984. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22157984
Chicago/Turabian StyleTrova, Sara, Serena Bovetti, Sara Bonzano, Silvia De Marchis, and Paolo Peretto. 2021. "Sex Steroids and the Shaping of the Peripubertal Brain: The Sexual-Dimorphic Set-Up of Adult Neurogenesis" International Journal of Molecular Sciences 22, no. 15: 7984. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22157984




