Preparation and Size Control of Efficient and Safe Nanopesticides by Anodic Aluminum Oxide Templates-Assisted Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the BNPs
2.2. Optimization of Preparation Parameters
2.3. Release Behavior
2.4. Stability
2.5. Promotion and Application
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Selection of Organic Solvent
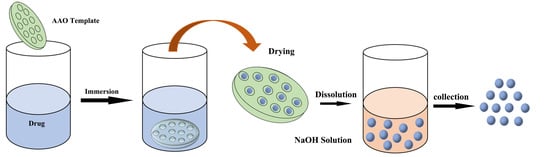
3.2.2. Preparation of Buprofezin Nanoparticles (BNPs) by AAO Method
3.2.3. Analysis of BNPs Collection Methods
3.2.4. Optimization of Preparation Process of BNPs
3.2.5. Characterization of BNPs
3.2.6. In Vitro Drug-Release
3.2.7. Stability
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop. Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Campos, E.V.R.; De Oliveira, J.L.; Fraceto, L.F. Applications of Controlled Release Systems for Fungicides, Herbicides, Acaricides, Nutrients, and Plant Growth Hormones: A Review. Adv. Sci. Eng. Med. 2014, 6, 373–387. [Google Scholar] [CrossRef]
- Rossall, S. Fungicide Resistance in Crop Protection: Risk and Management. Plant Pathol. 2012, 61, 820. [Google Scholar] [CrossRef]
- Murdande, S.B.; Shah, D.A.; Dave, R.H. Impact of Nanosizing on Solubility and Dissolution Rate of Poorly Soluble Pharma-ceuticals. J. Pharm. Sci. 2015, 104, 2094–2102. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Raj, S.N.; Anooj, E.; Rajendran, K.; Vallinayagam, S. A comprehensive review on regulatory invention of nano pesticides in Agricultural nano formulation and food system. J. Mol. Struct. 2021, 1239, 130517. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Maggi, F.; Petrelli, R.; Nicoletti, M. Commentary: Making Green Pesticides Greener? The Potential of Plant Products for Nanosynthesis and Pest Control. J. Clust. Sci. 2017, 28, 3–10. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, H.X.; Wang, Y.; Cui, B.; Zeng, Z.H. Development strategies and prospects of nano-based smart pesticide for-mulation. J. Agric. Food. Chem. 2017, 66, 6504–6512. [Google Scholar] [CrossRef]
- Mishra, P.; Tyagi, B.K.; Chandrasekaran, N.; Mukherjee, A. Biological nanopesticides: A greener approach towards the mos-quito vector control. Environ. Sci. Pollut. Res. Int. 2017, 25, 1–13. [Google Scholar]
- Shang, Y.; Hasan, K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.X.; Guo, L.; Yao, J.W.; Wang, A.Q.; Gao, F.; Zhao, X.; Zeng, Z.H.; Wang, Y.; Sun, C.J.; Cui, H.X. Preparation, char-acterization and antifungal activity of pyraclostrobin solid nanodispersion by self-mulsifying technique. Pest. Manag. Sci. 2019, 75, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Lv, Y.; Gao, F.; Wang, C.; Zeng, Z.; Wang, Y.; Sun, C.; Zhao, X.; Shen, Y.; Liu, G.; et al. Improving abamectin bioavailability via nanosuspension constructed by wet milling technique. Pest Manag. Sci. 2019, 75, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, X.; Chen, Y.; Yang, W.; Du, X. User-safe and efficient chitosan-gated porous carbon nanopesticides and nanoherbicides. J. Colloid Interface Sci. 2021, 594, 20–34. [Google Scholar] [CrossRef]
- Wang, C.; Cui, B.; Guo, L.; Wang, A.; Zhao, X.; Wang, Y.; Sun, C.; Zeng, Z.; Zhi, H.; Chen, H.; et al. Fabrication and Evaluation of Lambda-Cyhalothrin Nanosuspension by One-Step Melt Emulsification Technique. Nanomaterials 2019, 9, 145. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Sun, C.; Xue, Y.; Liu, C.; Qiu, D.; Cui, B.; Zhang, Y.; Cui, H.; Zeng, Z. Tannic acid-based nanopesticides coating with highly improved foliage adhesion to enhance foliar retention. RSC Adv. 2019, 9, 27096–27104. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Cui, B.; Zhao, X.; Zhi, H.; Zeng, Z.; Wang, Y.; Sun, C.; Liu, G.; Gao, J.; Cui, H. Antagonistic Effect of Azoxystrobin Poly (Lactic Acid) Microspheres with Controllable Particle Size on Colletotrichum higginsianum Sacc. Nanomaterials 2018, 8, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Zhou, Z.; Niu, S.; Cao, C.; Li, X.; Shan, Y.; Huang, Q. Positive-Charge Functionalized Mesoporous Silica Nanoparticles as Nanocarriers for Controlled 2,4-Dichlorophenoxy Acetic Acid Sodium Salt Release. J. Agric. Food Chem. 2018, 66, 6594–6603. [Google Scholar] [CrossRef]
- Baba, K.; Pudavar, H.E.; Roy, I.; Ohulchanskyy, T.Y.; Chen, Y.; Pandey, R.K.; Prasad, P.N. New method for delivering a hy-drophobic drug for photodynamic therapy using pure nanocrystal form of the drug. Mol. Pharm. 2006, 4, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, P.; Kumar, D.; Yadav, K.; Yadav, A. Nanopesticides: Synthesis, Formulation and Application in Agriculture. Nanobiotechnol. Appl. Plant. Prot. 2019, 7, 129–143. [Google Scholar]
- Liu, F.; Wen, L.-X.; Li, Z.-Z.; Yu, W.; Sun, H.-Y.; Chen, J.-F. Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mater. Res. Bull. 2006, 41, 2268–2275. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants. Molecules 2017, 22, 817. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Sun, C.; Zhao, X.; Cui, B. Construction and evaluation of controlled-release delivery system of Abamectin using porous silica nanoparticles as carriers. Nanoscale Res. Lett. 2014, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, J.; Wiley, J.B. Fabrication of thick porous anodized aluminum oxide templates. J. Solid State Electrochem. 2015, 19, 1447–1452. [Google Scholar] [CrossRef]
- Jo, H.; Haberkorn, N.; Pan, J.-A.; Vakili, M.; Nielsch, K.; Theato, P. Fabrication of Chemically Tunable, Hierarchically Branched Polymeric Nanostructures by Multi-branched Anodic Aluminum Oxide Templates. Langmuir 2016, 32, 6437–6444. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, Q.; Yang, Z.; Miao, Q.; Gao, X.; Zhou, G.; Zhang, Z. Size-Controlled Growth of High-Density Ordered Na-nomagnet Arrays by Template-Assisted Method. J. Nanosci. Nanotechn. 2016, 16, 12231–12236. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; An, F.-F.; Zhang, X.; Chen, X.; Lee, C.-S. Preparation and Size Control of Sub-100 nm Pure Nanodrugs. Nano Lett. 2015, 15, 313–318. [Google Scholar] [CrossRef]
- Martin, N.; Workman, P. Buprofezin: A selective pesticide for greenhouse whitefly control. In Proceedings of the New Zealand Weed and Pest Control Conference, Quality Inn, Palmerston North, New Zealand, 8 January 1986; Volume 39, pp. 234–236. [Google Scholar] [CrossRef]
- Ishaaya, I.; Degheele, D. Buprofezin: A Novel Chitin Synthesis Inhibitor Affecting Specifically Planthoppers. In Whiteflies and Scale Insects; Springer: Berlin/Heidelberg, Germany, 1998; Volume 5, pp. 74–91. [Google Scholar]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Skrbek, K.; Bartněk, V.; Lojka, M.; Sedmidubsk, D.; Jankovsk, O. Synthesis and Characterization of the Properties of Ceria Nanoparticles with Tunable Particle Size for the Decomposition of Chlorinated Pesticides. Appl. Sci. 2020, 10, 5224. [Google Scholar] [CrossRef]
- Kolaib, E.; Sharma, R.K. Nanodispersions Platform for Solubility Improvement. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 636–643. [Google Scholar]
- Dinh, H.T.; Tran, P.; Duan, W.; Lee, B.-J.; Tran, T.T. Nano-sized solid dispersions based on hydrophobic-hydrophilic conjugates for dissolution enhancement of poorly water-soluble drugs. Int. J. Pharm. 2017, 533, 93–98. [Google Scholar] [CrossRef]
- Knieke, C.; Rawtani, A.; Davé, R.N. Concentrated fenofibrate nanoparticle suspensions from melt emulsification for en-hanced drug dissolution. Chem. Eng. Technol. 2014, 37, 157–167. [Google Scholar] [CrossRef]
- Du, X.; Xu, S.H.; Sun, Z.W.; Aa, Y. Effect of the hydrodynamic radius of colloid microspheres on the Estimation of the coag-ulation rate constant. Acta. Phys. Chim. Sin. 2010, 26, 2807–2812. [Google Scholar]
- Zhou, H.; Sun, X.; Zhang, L.; Zhang, P.; Li, J.; Liu, Y.-N. Fabrication of Biopolymeric Complex Coacervation Core Micelles for Efficient Tea Polyphenol Delivery via a Green Process. Langmuir 2012, 28, 14553–14561. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.M.; Zhu, X.Y.; Chen, F.L. Release performance and sustained-release efficacy of emamectin benzoate-loaded polylactic acid microspheres. J. Integr. Agric. 2018, 17, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Zou, G.; Wang, J. The affect of suitable compatibility of auxiliary to increase the suspensibility of pesticide wettable powder. Jiangxi. Chem. Ind. 2002, 4, 134–136. [Google Scholar]
- Kwok, P.; Chan, H.-K. Nanotechnology Versus other Techniques in Improving Drug Dissolution. Curr. Pharm. Des. 2014, 20, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Jelvehgari, M.; Valizadeh, H.; Montazam, S.H.; Abbaszadeh, S. Experimental Design to Predict Process Variables in the Mi-crocrystals of Celecoxib for Dissolution Rate Enhancement Using Response Surface Methodology. Adv. Pharm. Bull. 2015, 5, 237. [Google Scholar] [CrossRef]
- Mohamed, F.; Roberts, M.; Seton, L.; Ford, J.L.; Levina, M.; Siahboomi, A. The effect of HPMC particle size on the drug re-lease rate and the percolation threshold in extended-release mini-tablets. Drug. Dev. Ind. Pharm. 2015, 41, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Luckham, P.F. Physical stability of suspension concentrates with particular reference to pharmaceutical and pesticide formu-lations. Pest Manag Sci. 2010, 25, 25–34. [Google Scholar] [CrossRef]
- Yang, M.; Ma, H. Effect of polydispersity on the relative stability of hard-sphere crystals. J. Chem. Phys. 2008, 128, 134510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Liu, X.; Jiang, J.; Qian, Y.; Zhang, N.; Wu, Q. Stability of triazophos in self-nanoemulsifying pesticide delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2009, 350, 57–62. [Google Scholar] [CrossRef]
- Müller, E.; Vogelsberger, W.; Fritsche, H.-G. The dependence of the surface energy of regular clusters and small crystallites on the particle size. Cryst. Res. Technol. 1988, 23, 1153–1159. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Cui, B.; Wang, Y.; Wang, M.; Zeng, Z.; Gao, F.; Sun, C.; Guo, L.; Zhao, X.; Cui, H. Preparation and Size Control of Efficient and Safe Nanopesticides by Anodic Aluminum Oxide Templates-Assisted Method. Int. J. Mol. Sci. 2021, 22, 8348. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22158348
Wang C, Cui B, Wang Y, Wang M, Zeng Z, Gao F, Sun C, Guo L, Zhao X, Cui H. Preparation and Size Control of Efficient and Safe Nanopesticides by Anodic Aluminum Oxide Templates-Assisted Method. International Journal of Molecular Sciences. 2021; 22(15):8348. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22158348
Chicago/Turabian StyleWang, Chunxin, Bo Cui, Yan Wang, Mengjie Wang, Zhanghua Zeng, Fei Gao, Changjiao Sun, Liang Guo, Xiang Zhao, and Haixin Cui. 2021. "Preparation and Size Control of Efficient and Safe Nanopesticides by Anodic Aluminum Oxide Templates-Assisted Method" International Journal of Molecular Sciences 22, no. 15: 8348. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22158348