Immunity in Atherosclerosis: Focusing on T and B Cells
Abstract
:1. Introduction
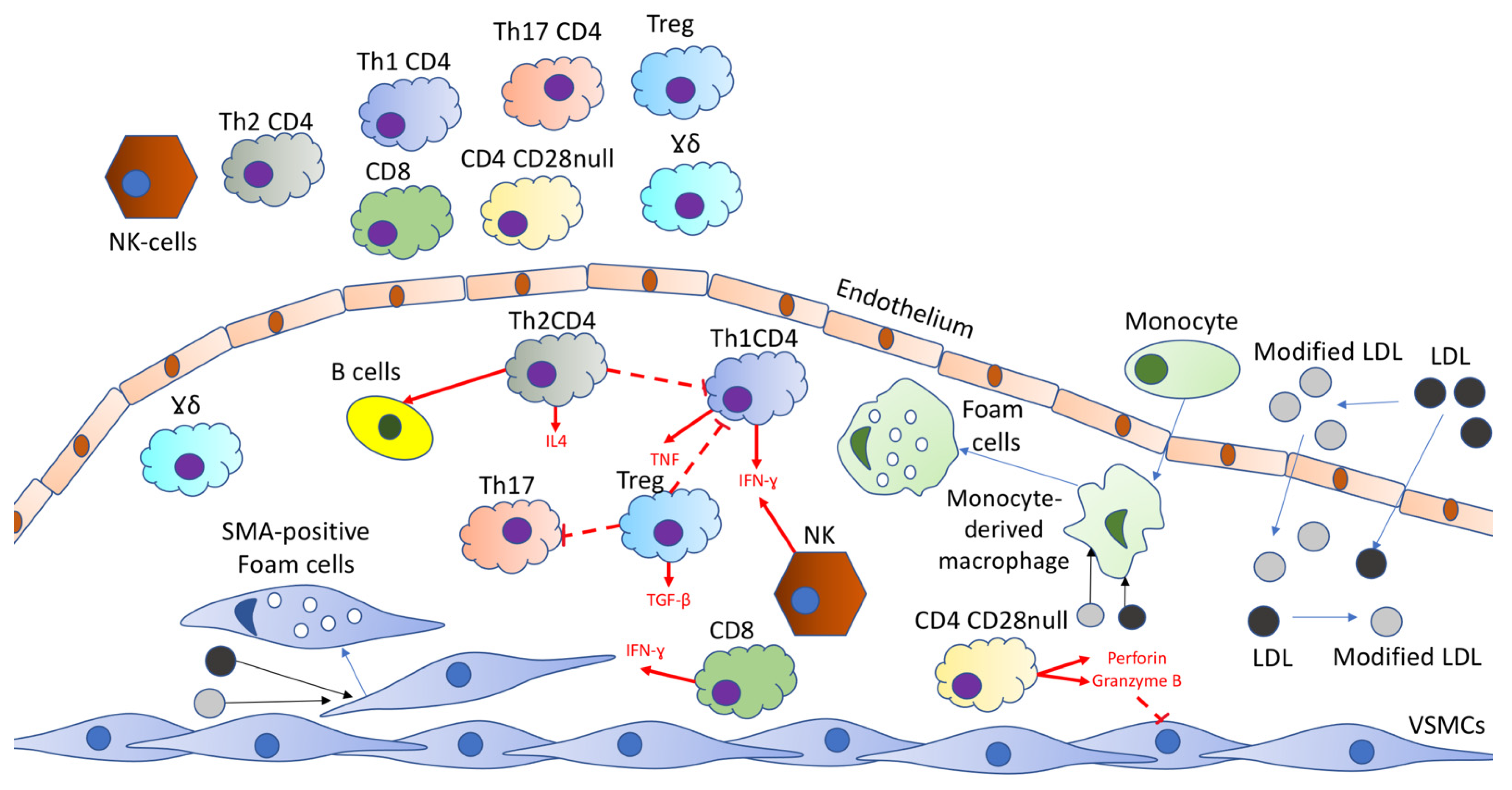
2. T-Cells
2.1. T Cells within Plaque
2.2. Circulating T Cell Subpopulations
3. B-Cells
4. T-Cell Based Therapy
5. B-Cell Based Therapy
5.1. Rituximab
5.2. Modulating B-Cell Receptor Signaling
5.3. Targeting B-Cell Costimulation and Immune Checkpoint Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Tokgozoglu, L.; Hekimsoy, V.; Costabile, G.; Calabrese, I.; Riccardi, G. Diet, Lifestyle, Smoking. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M.; et al. Food Consumption and Its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report From the Workshop Convened by the World Heart Federation. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef] [Green Version]
- Conti, P.; Shaik-Dasthagirisaeb, Y. Atherosclerosis: A chronic inflammatory disease mediated by mast cells. Cent. Eur. J. Immunol. 2015, 40, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yang, X.; Feng, Y.; Yu, J. Trained Immunity: An Underlying Driver of Inflammatory Atherosclerosis. Front. Immunol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero-Fernandez, B.; Gomez-Bris, R.; Somovilla-Crespo, B.; Gonzalez-Granado, J.M. Immunobiology of Atherosclerosis: A Complex Net of Interactions. Int. J. Mol. Sci. 2019, 20, 5293. [Google Scholar] [CrossRef] [Green Version]
- Crux, N.B.; Elahi, S. Human Leukocyte Antigen (HLA) and Immune Regulation: How Do Classical and Non-Classical HLA Alleles Modulate Immune Response to Human Immunodeficiency Virus and Hepatitis C Virus Infections? Front. Immunol. 2017, 8, 832. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [Green Version]
- van Duijn, J.; Kuiper, J.; Slütter, B. The many faces of CD8+ T cells in atherosclerosis. Curr. Opin. Lipidol. 2018, 29, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moroni, F.; Magnoni, M.; Camici, P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015, 179, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Piñon-Esteban, P.; Núñez, L.; Moure, R.; Marrón-Liñares, G.M.; Flores-Rios, X.; Aldama-Lopez, G.; Salgado-Fernandez, J.; Calviño-Santos, R.; Rebollal-Leal, F.; Pan-Lizcano, R.; et al. Presence of bacterial DNA in thrombotic material of patients with myocardial infarction. Sci. Rep. 2020, 10, 16299. [Google Scholar] [CrossRef]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B lymphocytes. In Autoimmunity: From Bench to Bedside [Internet]; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 2013; Chapter 5. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK459471/ (accessed on 25 May 2021).
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dézsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and During Myocardial Infarction. Front. Immunol. 2018, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Zernecke, A. CD8+ T Cells in Atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef]
- Lei, T.Y.; Ye, Y.Z.; Zhu, X.Q.; Smerin, D.; Gu, L.J.; Xiong, X.X.; Zhang, H.F.; Jian, Z.H. The immune response of T cells and therapeutic targets related to regulating the levels of T helper cells after ischaemic stroke. J. Neuroinflamm. 2021, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Amemiya, K.; Dankmeyer, J.L.; Bearss, J.J.; Zeng, X.; Stonier, S.W.; Soffler, C.; Cote, C.K.; Welkos, S.L.; Fetterer, D.P.; Chance, T.B.; et al. Dysregulation of TNF-α and IFN-γ expression is a common host immune response in a chronically infected mouse model of melioidosis when comparing multiple human strains of Burkholderia pseudomallei. BMC Immunol. 2020, 21, 5. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [Green Version]
- McLeod, O.; Silveira, A.; Valdes-Marquez, E.; Björkbacka, H.; Almgren, P.; Gertow, K.; Gådin, J.R.; Bäcklund, A.; Sennblad, B.; Baldassarre, D.; et al. Genetic loci on chromosome 5 are associated with circulating levels of interleukin-5 and eosinophil count in a European population with high risk for cardiovascular disease. Cytokine 2016, 81, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bax, L.; van der Graaf, Y.; Rabelink, A.J.; Algra, A.; Beutler, J.J.; Mali, W.P. SMART Study Group. Influence of atherosclerosis on age-related changes in renal size and function. Eur. J. Clin. Investig. 2003, 33, 34–40. [Google Scholar] [CrossRef]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arter. Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Bano, A.; Pera, A.; Almoukayed, A.; Clarke, T.H.S.; Kirmani, S.; Davies, K.A.; Kern, F. CD28 null CD4 T-cell expansions in autoimmune disease suggest a link with cytomegalovirus infection. F1000Research 2019, 8, 327. [Google Scholar] [CrossRef]
- Dumitriu, I.E. The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology 2015, 146, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.H.; de Jong, A.; Jukema, J.W.; de Vries, M.R.; Arens, R.; Quax, P.H.A. T cell co-stimulation and co-inhibition in cardiovascular disease: A double-edged sword. Nat. Rev. Cardiol. 2019, 16, 325–343. [Google Scholar] [CrossRef]
- Xu, A.; Liu, Y.; Chen, W.; Wang, J.; Xue, Y.; Huang, F.; Rong, L.; Lin, J.; Liu, D.; Yan, M.; et al. TGF-β-Induced Regulatory T Cells Directly Suppress B Cell Responses through a Noncytotoxic Mechanism. J. Immunol. 2016, 196, 3631–3641. [Google Scholar] [CrossRef] [Green Version]
- Rohm, I.; Atiskova, Y.; Drobnik, S.; Fritzenwanger, M.; Kretzschmar, D.; Pistulli, R.; Zanow, J.; Krönert, T.; Mall, G.; Figulla, H.R.; et al. Decreased regulatory T cells in vulnerable atherosclerotic lesions: Imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediat. Inflamm. 2015, 2015, 364710. [Google Scholar] [CrossRef] [Green Version]
- Ou, H.X.; Guo, B.B.; Liu, Q.; Li, Y.K.; Yang, Z.; Feng, W.J.; Mo, Z.C. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharm. Sin. 2018, 39, 1249–1258. [Google Scholar] [CrossRef]
- Li, F.; Guo, X.; Chen, S.Y. Function and Therapeutic Potential of Mesenchymal Stem Cells in Atherosclerosis. Front. Cardiovasc. Med. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Foks, A.C.; Lichtman, A.H.; Kuiper, J. Treating atherosclerosis with regulatory T cells. Arter. Thromb. Vasc. Biol. 2015, 35, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Bayati, F.; Mohammadi, M.; Valadi, M.; Jamshidi, S.; Foma, A.M.; Sharif-Paghaleh, E. The Therapeutic Potential of Regulatory T Cells: Challenges and Opportunities. Front. Immunol. 2021, 11, 585819. [Google Scholar] [CrossRef]
- Tse, K.; Tse, H.; Sidney, J.; Sette, A.; Ley, K. T cells in atherosclerosis. Int. Immunol. 2013, 25, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Van Kaer, L. Natural killer T cells in health and disease. Front. Biosci. 2011, 3, 236–251. [Google Scholar] [CrossRef] [Green Version]
- Kott, K.A.; Vernon, S.T.; Hansen, T.; de Dreu, M.; Das, S.K.; Powell, J.; Fazekas de St Groth, B.; Di Bartolo, B.A.; McGuire, H.M.; Figtree, G.A. Single-Cell Immune Profiling in Coronary Artery Disease: The Role of State-of-the-Art Immunophenotyping with Mass Cytometry in the Diagnosis of Atherosclerosis. J. Am. Heart Assoc. 2020, 9, e017759. [Google Scholar] [CrossRef]
- Riazi Rad, F.; Ajdary, S.; Omranipour, R.; Alimohammadian, M.H.; Hassan, Z.M. Comparative analysis of CD4+ and CD8+ T cells in tumor tissues, lymph nodes and the peripheral blood from patients with breast cancer. Iran. Biomed. J. 2015, 19, 35–44. [Google Scholar] [CrossRef]
- Rattik, S.; Engelbertsen, D.; Wigren, M.; Ljungcrantz, I.; Östling, G.; Persson, M.; Nordin Fredrikson, G.; Bengtsson, E.; Nilsson, J.; Björkbacka, H. Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diabetes Vasc. Dis. Res. 2019, 16, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Gencer, S.; Evans, B.R.; van der Vorst, E.P.C.; Döring, Y.; Weber, C. Inflammatory Chemokines in Atherosclerosis. Cells 2021, 10, 226. [Google Scholar] [CrossRef]
- Olson, N.C.; Doyle, M.F.; Jenny, N.S.; Huber, S.A.; Psaty, B.M.; Kronmal, R.A.; Tracy, R.P. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: The multi-ethnic study of atherosclerosis. PLoS ONE 2013, 8, e71498. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Rubba, P.; Gentile, M.; Mallardo, V.; Calcaterra, I.; Bresciani, A.; Covetti, G.; Cuomo, G.; Merone, P.; Di Lorenzo, A.; et al. Carotid Atherosclerosis, Ultrasound and Lipoproteins. Biomedicines 2021, 9, 521. [Google Scholar] [CrossRef]
- Gasper, D.J.; Tejera, M.M.; Suresh, M. CD4 T-cell memory generation and maintenance. Crit. Rev. Immunol. 2014, 34, 121–146. [Google Scholar] [CrossRef]
- Saraiva, D.P.; Jacinto, A.; Borralho, P.; Braga, S.; Cabral, M.G. HLA-DR in Cytotoxic T Lymphocytes Predicts Breast Cancer Patients’ Response to Neoadjuvant Chemotherapy. Front. Immunol. 2018, 9, 2605. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Moore, X.L.; Dart, A.M.; Wang, L.M. Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [CrossRef]
- Eikendal, A.L.; Groenewegen, K.A.; Anderson, T.J.; Britton, A.R.; Engström, G.; Evans, G.W.; de Graaf, J.; Grobbee, D.E.; Hedblad, B.; Holewijn, S.; et al. Common carotid intima-media thickness relates to cardiovascular events in adults aged < 45 years. Hypertension 2015, 65, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleby, L.J.; Nausch, N.; Heard, F.; Erskine, L.; Bourke, C.D.; Midzi, N.; Mduluza, T.; Allen, J.E.; Mutapi, F. Down Regulation of the TCR Complex CD3ζ-Chain on CD3+ T Cells: A Potential Mechanism for Helminth-Mediated Immune Modulation. Front. Immunol. 2015, 6, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolich-Žugich, J. Author Correction: The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 1146. [Google Scholar] [CrossRef]
- Seyda, M.; Elkhal, A.; Quante, M.; Falk, C.S.; Tullius, S.G. T Cells Going Innate. Trends Immunol. 2016, 37, 546–556. [Google Scholar] [CrossRef] [Green Version]
- Lichtman, A.H.; Binder, C.J.; Tsimikas, S.; Witztum, J.L. Adaptive immunity in atherogenesis: New insights and therapeutic approaches. J. Clin. Investig. 2013, 123, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Garza-Reyes, M.G.; Mora-Ruíz, M.D.; Chávez-Sánchez, L.; Madrid-Miller, A.; Cabrera-Quintero, A.J.; Maravillas-Montero, J.L.; Zentella-Dehesa, A.; Moreno-Ruíz, L.; Pastor-Salgado, S.; Ramírez-Arias, E.; et al. Effect of Interleukin-17 in the Activation of Monocyte Subsets in Patients with ST-Segment Elevation Myocardial Infarction. J. Immunol. Res. 2020, 2020, 5692829. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, D.M.; Camirand, G. New insights into the mechanisms of Treg function. Curr. Opin. Organ. Transplant. 2015, 20, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, C.C. Lymphocytes in atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, C.W.; Vasan, R.S. Cohort Profile: The Framingham Heart Study (FHS): Overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol. 2015, 44, 1800–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graver, J.C.; Boots, A.M.H.; Haacke, E.A.; Diepstra, A.; Brouwer, E.; Sandovici, M. Massive B-Cell Infiltration and Organization into Artery Tertiary Lymphoid Organs in the Aorta of Large Vessel Giant Cell Arteritis. Front. Immunol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stebegg, M.; Kumar, S.D.; Silva-Cayetano, A.; Fonseca, V.R.; Linterman, M.A.; Graca, L. Regulation of the Germinal Center Response. Front. Immunol. 2018, 9, 2469. [Google Scholar] [CrossRef] [Green Version]
- Fairfax, K.C.; Everts, B.; Amiel, E.; Smith, A.M.; Schramm, G.; Haas, H.; Randolph, G.J.; Taylor, J.J.; Pearce, E.J. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J. Immunol. 2015, 194, 2999–3010. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Kawamura, M.; Kochi, I.; Imai, M.; Murata, Y.; Suzuki, T.; Chen, Y.; Hashimoto, K.; Kihara, S. Serum Anti-Apo B Antibody Level as Residual CVD Marker in DM Patients under Statin Treatment. J. Atheroscler. Thromb. 2019, 26, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Willems, S.; van der Velden, D.; Quax, P.H.; de Borst, G.J.; de Vries, J.P.; Moll, F.L.; Kuiper, J.; Toes, R.E.; de Jager, S.C.; de Kleijn, D.P.; et al. Circulating immunoglobulins are not associated with intraplaque mast cell number and other vulnerable plaque characteristics in patients with carotid artery stenosis. PLoS ONE 2014, 9, e88984. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Meier, L.A.; Binstadt, B.A. The Contribution of Autoantibodies to Inflammatory Cardiovascular Pathology. Front. Immunol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7, 324. [Google Scholar] [CrossRef] [Green Version]
- Allman, D.; Wilmore, J.R.; Gaudette, B.T. The continuing story of T-cell independent antibodies. Immunol. Rev. 2019, 288, 128–135. [Google Scholar] [CrossRef]
- Lui, M.M.; Sau-Man, M. OSA and atherosclerosis. J. Thorac. Dis. 2012, 4, 164–172. [Google Scholar] [CrossRef]
- Bullenkamp, J.; Dinkla, S.; Kaski, J.C.; Dumitriu, I.E. Targeting T cells to treat atherosclerosis: Odyssey from bench to bedside. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 194–199. [Google Scholar] [CrossRef] [Green Version]
- van Leuven, S.I.; van Wijk, D.F.; Volger, O.L.; de Vries, J.P.; van der Loos, C.M.; de Kleijn, D.V.; Horrevoets, A.J.; Tak, P.P.; van der Wal, A.C.; de Boer, O.J.; et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis 2010, 211, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chapman, N.M.; Karmaus, P.W.; Zeng, H.; Chi, H. mTOR and metabolic regulation of conventional and regulatory T cells. J. Leukoc. Biol. 2015, 97, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, M.; Yamashita, T.; Sasaki, N.; Nakajima, K.; Kita, T.; Shinohara, M.; Ishida, T.; Hirata, K. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arter. Thromb. Vasc. Biol. 2010, 30, 2495–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Li, S.Q.; Wang, W.J.; Liu, L.S.; Jiang, Y.G.; Feng, P.N.; Wang, Y.Q.; Wang, S.M. Oral FTY720 administration induces immune tolerance and inhibits early development of atherosclerosis in apolipoprotein E-deficient mice. Int. J. Immunopathol. Pharmacol. 2012, 25, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Yuan, Z.; Liu, Y.; Liu, W.; Zhang, W.; Xue, J.; Shen, Y.; Liang, X.; Chen, T.; Kishimoto, C. Pioglitazone modulates the balance of effector and regulatory T cells in apolipoprotein E deficient mice. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 25–32. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Li, J.; Dong, M.; Yang, J.; An, G.; Qin, W.; Gao, F.; Zhang, C.; Zhang, Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol. Med. 2012, 18, 598–605. [Google Scholar] [CrossRef]
- Jiagang, D.; Li, C.; Wang, H.; Hao, E.; Du, Z.; Bao, C.; Lv, J.; Wang, Y. Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells. Biochem. Biophys. Res. Commun. 2011, 411, 523–529. [Google Scholar] [CrossRef]
- Dinh, T.N.; Kyaw, T.S.; Kanellakis, P.; To, K.; Tipping, P.; Toh, B.H.; Bobik, A.; Agrotis, A. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation 2012, 126, 1256–1266. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, K.; Sasaki, N.; Yamashita, T.; Kita, T.; Yodoi, K.; Sasaki, Y.; Takeda, M.; Hirata, K. CD3 antibody and IL-2 complex combination therapy inhibits atherosclerosis by augmenting a regulatory immune response. J. Am. Heart Assoc. 2014, 3, e000719. [Google Scholar] [CrossRef] [Green Version]
- Worthington, J.J.; Kelly, A.; Smedley, C.; Bauché, D.; Campbell, S.; Marie, J.C.; Travis, M.A. Integrin αvβ8-Mediated TGF-β Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity 2015, 42, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Perobelli, S.M.; Mercadante, A.C.; Galvani, R.G.; Gonçalves-Silva, T.; Alves, A.P.; Pereira-Neves, A.; Benchimol, M.; Nóbrega, A.; Bonomo, A. G-CSF-Induced Suppressor IL-10+ Neutrophils Promote Regulatory T Cells That Inhibit Graft-Versus-Host Disease in a Long-Lasting and Specific Way. J. Immunol. 2016, 197, 3725–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilhan, F.; Kalkanli, S.T. Atherosclerosis and the role of immune cells. World J. Clin. Cases 2015, 3, 345–352. [Google Scholar] [CrossRef]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; Grossman, A.; Hershman, J.M.; Hofland, J.; et al. (Eds.) The Role of Lipids and Lipoproteins in Atherosclerosis; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK343489/ (accessed on 21 June 2021).
- Li, M.; Wang, X.; Li, X.; Chen, H.; Hu, Y.; Zhang, X.; Tang, X.; Miao, Y.; Tian, G.; Shang, H. Statins for the Primary Prevention of Coronary Heart Disease. BioMed Res. Int. 2019, 2019, 4870350. [Google Scholar] [CrossRef] [PubMed]
- Soulaidopoulos, S.; Nikiphorou, E.; Dimitroulas, T.; Kitas, G.D. The Role of Statins in Disease Modification and Cardiovascular Risk in Rheumatoid Arthritis. Front. Med. 2018, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- de Punder, Y.M.; Fransen, J.; Kievit, W.; Houtman, P.M.; Visser, H.; van de Laar, M.A.; van Riel, P.L. The prevalence of clinical remission in RA patients treated with anti-TNF: Results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Rheumatology 2012, 51, 1610–1617. [Google Scholar] [CrossRef] [Green Version]
- Novikova, D.S.; Popkova, T.V.; Nasonov, E.L. The effect of anti-B-cell therapy on the development of atherosclerosis in patients with rheumatoid arthritis. Curr. Pharm. Des. 2012, 18, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Efremov, D.G.; Turkalj, S.; Laurenti, L. Mechanisms of B Cell Receptor Activation and Responses to B Cell Receptor Inhibitors in B Cell Malignancies. Cancers 2020, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.D.; Mussbacher, M.; Galkina, E.V. Functional Role of B Cells in Atherosclerosis. Cells 2021, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.A.; Tai, X.; Hodes, R.J. CD28-CD80/86 and CD40-CD40L Interactions Promote Thymic Tolerance by Regulating Medullary Epithelial Cell and Thymocyte Development. Crit. Rev. Immunol. 2015, 35, 59–76. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [Green Version]
- Porsch, F.; Binder, C.J. Impact of B-Cell-Targeted Therapies on Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 1705–1714. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef]
- Yap, H.Y.; Tee, S.Z.; Wong, M.M.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Drug Name | Effects | Mechanisms | Reference |
|---|---|---|---|
| Mycophenolate mofetil | Atherosclerosis suppression | Enhancing the number of Tregs Decreasing the activation of T cells, NKs, macrophages and DCs Decreasing the MMP and cathepsin generation Stimulating the expression of lipid metabolism-associated genes | [67,68] |
| Rapamycin | Immunotherapy in T cell-mediated CVD | Immunosuppressive activity Triggering Treg expansion and lowering T effector cells | [69] |
| Orally activated vitamin D3 | Atherosclerosis suppression | Enhancing Treg levels Triggering tolerogenic DCs Lowering IL-12 expression, and enhancing IL-10 expression in mice | [70,71] |
| Pioglitazone | Atherosclerosis suppression | Modulating the of Th1/Th2 cells balance Increasing Treg response Enhancing the SMC level and collagen content in ApoE-deficient mice | [72] |
| Simvastatin | Beneficial in ACS patients | Enhancing Treg levels Stimulating TGFβ, IL-10, and FOXP3 expression in the atherosclerotic plaques of ApoE−/− mice Inducing Treg expansion in ACS patients | [73] |
| Atorvastatin | Beneficial in ACS patients | TriggeringTreg expansion Inducing FOXP3 expression in humans but not in C57BL/6 mice | [73] |
| Amygdalin (vitamin B17) | Atherosclerosis suppression | Modulating the lipid metabolism by lowering plasma total cholesterol (TC), triglyceride (TG), and LDL levels Triggering Treg expansion and upregulating IL-10 and TGF-β expression in ApoE−/− mice | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poznyak, A.V.; Bezsonov, E.E.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Immunity in Atherosclerosis: Focusing on T and B Cells. Int. J. Mol. Sci. 2021, 22, 8379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168379
Poznyak AV, Bezsonov EE, Popkova TV, Starodubova AV, Orekhov AN. Immunity in Atherosclerosis: Focusing on T and B Cells. International Journal of Molecular Sciences. 2021; 22(16):8379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168379
Chicago/Turabian StylePoznyak, Anastasia V., Evgeny E. Bezsonov, Tatyana V. Popkova, Antonina V. Starodubova, and Alexander N. Orekhov. 2021. "Immunity in Atherosclerosis: Focusing on T and B Cells" International Journal of Molecular Sciences 22, no. 16: 8379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168379






