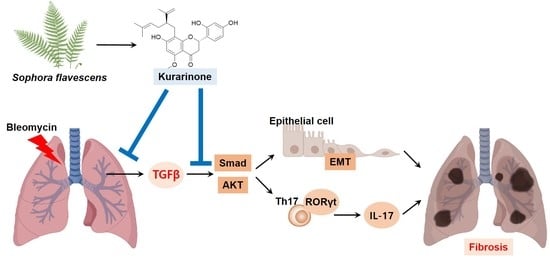
Kurarinone Attenuates BLM-Induced Pulmonary Fibrosis via Inhibiting TGF-β Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Murine Bleomycin-Induced Pulmonary Fibrosis Model
2.6. Pulmonary Mechanical Function Test
2.7. Staining for Histopathological Analysis
2.8. Hydroxyproline Assay
2.9. Quantitative Real-Time Reverse Transcription-PCR (qPCR)
2.10. Western Blot Analysis
2.11. Splenocyte Culture and Kurarinone Treatment
2.12. Measurement of Cytokine Secretion by ELISA
2.13. Measurement of Total TGF-β1 by ELISA
2.14. Statistical Analysis
3. Results
3.1. Kurarinone Inhibits TGF-β-Induced Epithelial–Mesenchymal Transition in BEAS-2B Cells
3.2. Kurarinone Improves the Mechanical Lung Function in Bleomycin-Induced Pulmonary Fibrosis
3.3. Kurarinone Attenuates Fibrotic Changes of Lung Tissues in BLM-Treated Mice
3.4. Kurarinone Suppressed Phophorylation of Smad2/3 and AKT Mediating the TGF-β Signaling Pathway In Vitro and In Vivo
3.5. Kurarinone Inhibited Th17 Differentiation in the Lungs of a BLM-Induced Pulmonary Fibrosis Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | alpha-smooth muscle actin |
| BLM | bleomycin |
| Col1α1 | collagen type 1 alpha 1 |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| HPLC | high-performance liquid chromatography |
| IL- | interleukin- |
| IPF | idiopathic pulmonary fibrosis |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein 1 |
| PAI-1 | plasminogen activator inhibitor 1 |
| PDGF | platelet-derived growth factor |
| PFD | pirfenidone |
| RORγt | PAR-related orphan receptor gamma |
| TGF-β | transforming growth factor β |
| TNF-α | tumor necrosis factor α |
References
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [Green Version]
- Galli, J.A.; Pandya, A.; Vega-Olivo, M.; Dass, C.; Zhao, H.; Criner, G.J. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions. Respirology 2017, 22, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Kasai, H.; Allen, J.T.; Mason, R.M.; Kamimura, T.; Zhang, Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir. Res. 2005, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Eitzman, D.T.; McCoy, R.D.; Zheng, X.; Fay, W.P.; Shen, T.; Ginsburg, D.; Simon, R.H. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J. Clin. Investig. 1996, 97, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Cutroneo, K.R.; White, S.L.; Phan, S.H.; Ehrlich, H.P. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J. Cell. Physiol. 2007, 211, 585–589. [Google Scholar] [CrossRef]
- Siegel, P.M.; Massagué, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef]
- Fintha, A.; Gasparics, Á.; Rosivall, L.; Sebe, A. Therapeutic Targeting of Fibrotic Epithelial-Mesenchymal Transition-An Outstanding Challenge. Front. Pharmacol. 2019, 10, 388. [Google Scholar] [CrossRef]
- Willis, B.C.; Borok, Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef] [Green Version]
- Gurczynski, S.J.; Moore, B.B. IL-17 in the lung: The good, the bad, and the ugly. Am. J. Physiol-Lung Cell. Mol. Physiol. 2018, 314, L6–L16. [Google Scholar] [CrossRef]
- Yao, Z.; Fanslow, W.C.; Seldin, M.F.; Rousseau, A.M.; Painter, S.L.; Comeau, M.R.; Cohen, J.I.; Spriggs, M.K. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. J. Immunol. 2011, 187, 4392–4402. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wu, Y.; Wei, H.; Xing, X.; Zhan, N.; Xiong, H.; Peng, B. IL-17R activation of human periodontal ligament fibroblasts induces IL-23 p19 production: Differential involvement of NF-κB versus JNK/AP-1 pathways. Mol. Immunol. 2011, 48, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Nuovo, G.J.; Hagood, J.S.; Magro, C.M.; Chin, N.; Kapil, R.; Davis, L.; Marsh, C.B.; Folcik, V.A. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod. Pathol. 2012, 25, 416–433. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.S.; Madala, S.K.; Ramalingam, T.R.; Gochuico, B.R.; Rosas, I.O.; Cheever, A.W.; Wynn, T.A. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010, 207, 535–552. [Google Scholar] [CrossRef] [Green Version]
- Cheresh, P.; Kim, S.J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta 2013, 1832, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Han, J.M.; Jin, Y.Y.; Kim, H.Y.; Park, K.H.; Lee, W.S.; Jeong, T.S. Lavandulyl flavonoids from Sophora flavescens suppress lipopolysaccharide-induced activation of nuclear factor-kappaB and mitogen-activated protein kinases in RAW264.7 cells. Biol. Pharm. Bull. 2010, 33, 1019–1023. [Google Scholar] [CrossRef] [Green Version]
- Bringardner, B.D.; Baran, C.P.; Eubank, T.D.; Marsh, C.B. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid. Redox Signal 2008, 10, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Furuie, H.; Yamasaki, H.; Suga, M.; Ando, M. Altered accessory cell function of alveolar macrophages: A possible mechanism for induction of Th2 secretory profile in idiopathic pulmonary fibrosis. Eur. Respir. J. 1997, 10, 787–794. [Google Scholar]
- Kim, B.H.; Na, K.M.; Oh, I.; Song, I.H.; Lee, Y.S.; Shin, J.; Kim, T.Y. Kurarinone regulates immune responses through regulation of the JAK/STAT and TCR-mediated signaling pathways. Biochem. Pharmacol. 2013, 85, 1134–1144. [Google Scholar] [CrossRef]
- Gao, H.Y.; He, X.F.; Shao, J.F. Effect of kurarinone on renal tubular epithelial cell-mesenchyma trans-differentiation in rats with renal interstitial fibrosis. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007, 27, 535–539. [Google Scholar]
- Kwon, M.; Ko, S.-K.; Jang, M.; Kim, G.-H.; Ryoo, I.-J.; Son, S.; Ryu, H.W.; Oh, S.-R.; Lee, W.-K.; Kim, B.Y.; et al. Inhibitory effects of flavonoids isolated from Sophora flavescens on indoleamine 2,3-dioxygenase 1 activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 1481–1488. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef]
- Xie, L.; Gong, W.; Chen, J.; Xie, H.W.; Wang, M.; Yin, X.P.; Wu, W. The flavonoid kurarinone inhibits clinical progression of EAE through inhibiting Th1 and Th17 cell differentiation and proliferation. Int. Immunopharmacol. 2018, 62, 227–236. [Google Scholar] [CrossRef]
- Mi, S.; Li, Z.; Yang, H.Z.; Liu, H.; Wang, J.P.; Ma, Y.G.; Wang, X.X.; Liu, H.Z.; Sun, W.; Hu, Z.W. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 2011, 187, 3003–3014. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Gregory, B.; Catravas, J.D. HSP90 Inhibition and Modulation of the Proteome: Therapeutical Implications for Idiopathic Pulmonary Fibrosis (IPF). Int. J. Mol. Sci. 2020, 21, 5286. [Google Scholar] [CrossRef]
- Sontake, V.; Wang, Y.; Kasam, R.K.; Sinner, D.; Reddy, G.B.; Naren, A.P.; McCormack, F.X.; White, E.S.; Jegga, A.G.; Madala, S.K. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight 2017, 2, e91454. [Google Scholar] [CrossRef] [Green Version]
- Bellaye, P.S.; Burgy, O.; Causse, S.; Garrido, C.; Bonniaud, P. Heat shock proteins in fibrosis and wound healing: Good or evil? Pharmacol. Ther. 2014, 143, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Liang, L.; Bi, Z.; Wang, Y.; Gao, S.; Wang, M.; Li, H.; Miao, Y.; Deng, R.; et al. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β signaling via targeting HSP90β. Biochem. Pharmacol. 2020, 178, 114097. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Nagai, S.; Ikejiri, A.; Ohtani, M.; Ichiyama, K.; Baba, Y.; Yamada, T.; Egami, S.; Hoshii, T.; Hirao, A.; et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep. 2012, 1, 360–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oku, H.; Shimizu, T.; Kawabata, T.; Nagira, M.; Hikita, I.; Ueyama, A.; Matsushima, S.; Torii, M.; Arimura, A. Antifibrotic action of pirfenidone and prednisolone: Different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur. J. Pharmacol. 2008, 590, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Reddy, S.P.; Yamamoto, M.; Kleeberger, S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004, 18, 1258–1260. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, A.; Scotton, C.J.; Chambers, R.C. Novel therapeutic approaches for pulmonary fibrosis. Br. J. Pharmacol. 2011, 163, 141–172. [Google Scholar] [CrossRef] [Green Version]
- Li, L.C.; Kan, L.D. Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J. Ethnopharmacol. 2017, 198, 45–63. [Google Scholar] [CrossRef]
- Nishikawa, S.; Inoue, Y.; Hori, Y.; Miyajima, C.; Morishita, D.; Ohoka, N.; Hida, S.; Makino, T.; Hayashi, H. Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway. Antioxidants 2020, 9, 842. [Google Scholar] [CrossRef]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.Q.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural Products as a Source for Antifibrosis Therapy. Trends Pharmacol. Sci. 2018, 39, 937–952. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Kim, T.-h.; Lee, K.; Kang, M.-A.; Jang, H.-J.; Ryu, H.-W.; Oh, S.-R.; Lee, H.-J. Kurarinone Attenuates BLM-Induced Pulmonary Fibrosis via Inhibiting TGF-β Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 8388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168388
Park S-J, Kim T-h, Lee K, Kang M-A, Jang H-J, Ryu H-W, Oh S-R, Lee H-J. Kurarinone Attenuates BLM-Induced Pulmonary Fibrosis via Inhibiting TGF-β Signaling Pathways. International Journal of Molecular Sciences. 2021; 22(16):8388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168388
Chicago/Turabian StylePark, Soo-Jin, Tae-hyoun Kim, Kiram Lee, Min-Ah Kang, Hyun-Jae Jang, Hyung-Won Ryu, Sei-Ryang Oh, and Hyun-Jun Lee. 2021. "Kurarinone Attenuates BLM-Induced Pulmonary Fibrosis via Inhibiting TGF-β Signaling Pathways" International Journal of Molecular Sciences 22, no. 16: 8388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168388