The Role of Glycemic Variability in Cardiovascular Disorders
Abstract
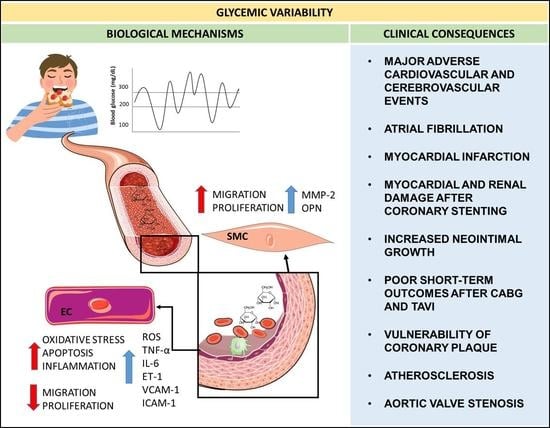
:1. Introduction
2. Glycemic Variability and Clinical Implications
2.1. Role of Glycemic Variability in Subclinical Atherosclerosis and CVD Risk
2.2. Glycemic Variability and Stable Coronary Artery Disease
2.3. Glycemic Variability and Coronary Plaque Vulnerability
2.4. Glycemic Variability and Acute Coronary Syndromes
2.5. Glycemic Variability in Patients with Type 1 Diabetes (T1DM) and Cardiovascular Complications
2.6. Possible Pharmacological Treatment to Control High Glycemic Variability Detrimental Effects
3. Animal Models of Glycemic Variability
4. In Vitro Studies of Glycemic Variability Effects on Human Cells
5. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Acronyms
| 3-NO-Tyr | nitrotyrosine |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| ACS | acute coronary syndrome |
| ADRR | average daily risk range |
| AF | atrial fibrillation |
| AMI | acute myocardial infarction |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CGM | continuous glucose monitoring |
| CONGA | continuous overlapping net glycemic action |
| CV | coefficient of variation |
| CVD | cardiovascular diseases |
| DM | diabetes mellitus |
| ET-1 | endothelin-1 |
| GV | glycemic variability |
| GPx-1 | glutathione peroxidase-1 |
| HbA1c | hemoglobin A1c |
| HBGI | high blood glucose index |
| ICAM-1 | intercellular adhesion molecule 1 |
| IMT | intimal medial thickness |
| IQR | interquartile range |
| LBGI | low blood glucose index |
| LI | glycemic lability index |
| MACCE | major adverse cardiovascular and cerebrovascular events |
| MAGE | mean amplitude glycemic excursion |
| MMP-2 | matrix-metalloprotease-2 |
| MODD | the mean of daily difference |
| NO | nitric oxide |
| NSTEMI | non-ST elevation myocardial infarction |
| OLEFT | Otsuka Long-Evans Tokushima Fatty |
| OPN | osteopontin |
| PCI | percutaneous coronary intervention |
| PI3K | phosphoinositide-3-kinase |
| PKC | phosphokinase C |
| ROS | reactive oxygen species |
| SMBG | self-measured blood glucose |
| SD | standard deviation of the mean glucose |
| SOD-1 | superoxide dismutase-1 |
| STEMI | ST-elevation myocardial infarction |
| STZ | streptozotocin |
| T2DM | type 2 diabetes mellitus |
| TAVI | transcatheter aortic valve implantation |
| TNF-α | tumor necrosis factor-alpha |
| VCAM-1 | vascular adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
References
- Monnier, L.; Colette, C.; Owens, D. The glycemic triumvirate and diabetic complications: Is the whole greater than the sum of its component parts? Diabetes Res. Clin. Pract. 2012, 95, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F. Do data in the literature indicate that glycaemic variability is a clinical problem? Glycaemic variability and vascular complications of diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. S2), 3–8. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease: The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Ceriello, A.; Monnier, L.; Owens, D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019, 7, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Roussel, R.; Steg, P.G.; Mohammedi, K.; Marre, M.; Potier, L. Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: A perspective on glucose-lowering interventions. Diabetes Obes. Metab. 2018, 20, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okada, Y.; Mori, H.; Tanaka, Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Mi, S.; Tao, H.; Li, Z.; Yang, H.; Zheng, H.; Zhou, Y.; Ma, C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci 2020, 21, 1835. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Xu, J.; Li, B.; Liu, Z.; Hao, H.; Yin, C.; Xu, D. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 466, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; He, L.J.; Cao, S.J.; Yang, Q.; Yang, S.W.; Zhou, Y.J. Effect of glycemic variability on short term prognosis in acute myocardial infarction subjects undergoing primary percutaneous coronary interventions. Diabetol. Metab. Syndr. 2014, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, B.; Lerner, A.; Novack, V.; Khabbaz, K.; Paryente-Wiesmann, M.; Hess, P.; Talmor, D. Increased glycemic variability in patients with elevated preoperative HbA1C predicts adverse outcomes following coronary artery bypass grafting surgery. Anesth. Analg. 2014, 118, 277–287. [Google Scholar] [CrossRef]
- Besch, G.; Pili-Floury, S.; Morel, C.; Gilard, M.; Flicoteaux, G.; du Mont, L.S.; Perrotti, A.; Meneveau, N.; Chocron, S.; Schiele, F.; et al. Impact of post-procedural glycemic variability on cardiovascular morbidity and mortality after transcatheter aortic valve implantation: A post hoc cohort analysis. Cardiovasc. Diabetol. 2019, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Shen, H.; Liu, H.; Wang, Y.; Bai, Y.; Han, P. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc. Diabetol. 2016, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Mazze, R.S.; Strock, E.; Wesley, D.; Borgman, S.; Morgan, B.; Bergenstal, R.; Cuddihy, R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol. Ther. 2008, 10, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Colette, C.; Monnier, L. Acute glucose fluctuations and chronic sustained hyperglycemia as risk factors for cardiovascular diseases in patients with type 2 diabetes. Horm. Metab. Res. 2007, 39, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Hu, S.; Xu, J.; Hao, H.; Yin, C.; Xu, D. The correlation between glucose fluctuation from self-monitored blood glucose and the major adverse cardiac events in diabetic patients with acute coronary syndrome during a 6-month follow-up by WeChat application. Clin. Chem. Lab. Med. 2018, 56, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.M.; Dong, Y.; Zhang, H.M.; Su, Q. Effects of intermittent high glucose on oxidative stress in endothelial cells. Acta Diabetol. 2010, 47 (Suppl. S1), 97–103. [Google Scholar] [CrossRef]
- Suh, S.; Kim, J.H. Glycemic Variability: How Do We Measure It and Why Is It Important? Diabetes Metab. J. 2015, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Miao, L.F.; Qian, L.L.; Wang, N.; Qi, M.M.; Zhang, Y.M.; Dang, S.P.; Wu, Y.; Wang, R.X. Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Front. Endocrinol. 2019, 10, 640. [Google Scholar] [CrossRef]
- Ceriello, A. Postprandial hyperglycemia and cardiovascular disease: Is the HEART2D study the answer? Diabetes Care 2009, 32, 521–522. [Google Scholar] [CrossRef] [Green Version]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Helleputte, S.; De Backer, T.; Lapauw, B.; Shadid, S.; Celie, B.; Van Eetvelde, B.; Vanden Wyngaert, K.; Calders, P. The relationship between glycaemic variability and cardiovascular autonomic dysfunction in patients with type 1 diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36, e3301. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, W.; Huang, R.; Zhang, X. Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis 2010, 210, 302–306. [Google Scholar] [CrossRef]
- Mo, Y.; Zhou, J.; Li, M.; Wang, Y.; Bao, Y.; Ma, X.; Li, D.; Lu, W.; Hu, C.; Jia, W. Glycemic variability is associated with subclinical atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc. Diabetol. 2013, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Di Flaviani, A.; Picconi, F.; Di Stefano, P.; Giordani, I.; Malandrucco, I.; Maggio, P.; Palazzo, P.; Sgreccia, F.; Peraldo, C.; Farina, F.; et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 2011, 34, 1605–1609. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Yin, H.; Wei, C.; Xie, L.; He, H.; Liu, X. Glucose variability for cardiovascular risk factors in type 2 diabetes: A meta-analysis. J. Diabetes Metab. Disord. 2017, 16, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mita, T.; Katakami, N.; Okada, Y.; Yoshii, H.; Osonoi, T.; Nishida, K.; Shiraiwa, T.; Torimoto, K.; Kurozumi, A.; Wakasugi, S.; et al. Protocol of a Prospective Observational Study on the Relationship Between Glucose Fluctuation and Cardiovascular Events in Patients with Type 2 Diabetes. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2019, 10, 1565–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, S.R.; Clements, M.A. Average daily risk range as a measure for clinical research and routine care. J. Diabetes Sci. Technol. 2013, 7, 1370–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergenstal, R.M. Glycemic Variability and Diabetes Complications: Does It Matter? Simply Put, There Are Better Glycemic Markers! Diabetes Care 2015, 38, 1615–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Su, G.; Mi, S.H.; Yang, H.X.; Xin, W.; Dai, W.L.; Liu, J.H. Association Between Blood Glucose Variability and the Characteristics of Vulnerable Plaque in Elderly Non-ST Segment Elevation Acute Coronary Syndrome Patients. Int. Heart J. 2019, 60, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.; Liu, Y.; Liu, H.; Yang, G.; Guo, Q.; Du, J.; Jin, N.; Zang, L.; Lv, Z.; Ba, J.; et al. Characteristics of glucose metabolism indexes and continuous glucose monitoring system (CGMS) in patients with insulinoma. Diabetol. Metab. Syndr. 2017, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Gomez, A.M.; Munoz, O.M.; Marin, A.; Fonseca, M.C.; Rondon, M.; Robledo Gomez, M.A.; Sanko, A.; Lujan, D.; Garcia-Jaramillo, M.; Leon Vargas, F.M. Different Indexes of Glycemic Variability as Identifiers of Patients with Risk of Hypoglycemia in Type 2 Diabetes Mellitus. J. Diabetes Sci. Technol. 2018, 12, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Kohnert, K.D.; Heinke, P.; Fritzsche, G.; Vogt, L.; Augstein, P.; Salzsieder, E. Evaluation of the mean absolute glucose change as a measure of glycemic variability using continuous glucose monitoring data. Diabetes Technol. Ther. 2013, 15, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Saboo, B.; Kesavadev, J.; Shankar, A.; Krishna, M.B.; Sheth, S.; Patel, V.; Krishnan, G. Time-in-range as a target in type 2 diabetes: An urgent need. Heliyon 2021, 7, e05967. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, E.; Darier, R.; Montaudon, M.; Beauvieux, M.C.; Coffin-Boutreux, C.; Coste, P.; Douard, H.; Ouattara, A.; Catargi, B. Glycemic Variability Is a Powerful Independent Predictive Factor of Midterm Major Adverse Cardiac Events in Patients With Diabetes With Acute Coronary Syndrome. Diabetes Care 2019, 42, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clement, K.C.; Alejo, D.; DiNatale, J.; Whitman, G.J.R.; Matthew, T.L.; Clement, S.C.; Lawton, J.S. Increased glucose variability is associated with atrial fibrillation after coronary artery bypass. J. Card. Surg. 2019, 34, 549–554. [Google Scholar] [CrossRef]
- Nusca, A.; Lauria Pantano, A.; Melfi, R.; Proscia, C.; Maddaloni, E.; Contuzzi, R.; Mangiacapra, F.; Palermo, A.; Manfrini, S.; Pozzilli, P.; et al. Glycemic Variability Assessed by Continuous Glucose Monitoring and Short-Term Outcome in Diabetic Patients Undergoing Percutaneous Coronary Intervention: An Observational Pilot Study. J. Diabetes Res. 2015, 2015, 250201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, M.; Shinke, T.; Otake, H.; Sugiyama, D.; Takaya, T.; Takahashi, H.; Terashita, D.; Uzu, K.; Tahara, N.; Kashiwagi, D.; et al. Effects of daily glucose fluctuations on the healing response to everolimus-eluting stent implantation as assessed using continuous glucose monitoring and optical coherence tomography. Cardiovasc. Diabetol. 2016, 15, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, K.; Jeon, Y.; Kim, W.H.; Jung, D.E.; Kwon, S.M.; Kang, P.; Cho, Y.J.; Kim, T.K. Intraoperative glucose variability, but not average glucose concentration, may be a risk factor for acute kidney injury after cardiac surgery: A retrospective study. Can. J. Anaesth. 2019, 66, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Hibi, K.; Gohbara, M.; Kataoka, S.; Takano, K.; Akiyama, E.; Matsuzawa, Y.; Saka, K.; Maejima, N.; Endo, M.; et al. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc. Diabetol. 2015, 14, 111. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Yamamoto, H.; Shinke, T.; Otake, H.; Kuroda, M.; Terashita, D.; Takahashi, H.; Sakaguchi, K.; Hirota, Y.; Emoto, T.; et al. Impact of CD14(++)CD16(+) monocytes on plaque vulnerability in diabetic and non-diabetic patients with asymptomatic coronary artery disease: A cross-sectional study. Cardiovasc. Diabetol. 2017, 16, 96. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, Y.; Hosoda, K.; Makino, H.; Matsubara, M.; Matsuo, M.; Ohata, Y.; Koezuka, R.; Tamanaha, T.; Tomita, T.; Honda-Kohmo, K.; et al. The efficacy of glycemic control with continuous glucose monitoring on atheroma progression: Rationale and design of the Observation of Coronary Atheroma Progression under Continuous Glucose Monitoring Guidance in Patients with Type 2 Diabetes Mellitus (OPTIMAL). Cardiovasc. Diagn. Ther. 2019, 9, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Shinke, T.; Sakaguchi, K.; Otake, H.; Takaya, T.; Hirota, Y.; Osue, T.; Kinutani, H.; Konishi, A.; Takahashi, H.; et al. Association between daily glucose fluctuation and coronary plaque properties in patients receiving adequate lipid-lowering therapy assessed by continuous glucose monitoring and optical coherence tomography. Cardiovasc. Diabetol. 2015, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otowa-Suematsu, N.; Sakaguchi, K.; Komada, H.; Nakamura, T.; Sou, A.; Hirota, Y.; Kuroda, M.; Shinke, T.; Hirata, K.I.; Ogawa, W. Comparison of the relationship between multiple parameters of glycemic variability and coronary plaque vulnerability assessed by virtual histology-intravascular ultrasound. J. Diabetes Investig. 2017, 9, 610–615. [Google Scholar] [CrossRef]
- Famulla, S.; Pieber, T.R.; Eilbracht, J.; Neubacher, D.; Soleymanlou, N.; Woerle, H.J.; Broedl, U.C.; Kaspers, S. Glucose Exposure and Variability with Empagliflozin as Adjunct to Insulin in Patients with Type 1 Diabetes: Continuous Glucose Monitoring Data from a 4-Week, Randomized, Placebo-Controlled Trial (EASE-1). Diabetes Technol. Ther. 2017, 19, 49–60. [Google Scholar] [CrossRef]
- Raz, I.; Ceriello, A.; Wilson, P.W.; Battioui, C.; Su, E.W.; Kerr, L.; Jones, C.A.; Milicevic, Z.; Jacober, S.J. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care 2011, 34, 1511–1513. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Sasso, F.C.; Cacciapuoti, F.; Portoghese, M.; Rizzo, M.R.; Siniscalchi, M.; Carbonara, O.; Ferraraccio, F.; Torella, M.; Petrella, A.; et al. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J. Clin. Endocrinol. Metab. 2012, 97, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, C.C.; Reynolds, C.; Fernandez, C.; Stocken, D.D.; Sharples, L.D.; Sathyapalan, T.; Heller, S.; Storey, R.F.; Ajjan, R.A. Rationale and design of the LIBERATES trial: Protocol for a randomised controlled trial of flash glucose monitoring for optimisation of glycaemia in individuals with type 2 diabetes and recent myocardial infarction. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120957934. [Google Scholar] [CrossRef]
- Greven, W.L.; Beulens, J.W.; Biesma, D.H.; Faiz, S.; de Valk, H.W. Glycemic variability in inadequately controlled type 1 diabetes and type 2 diabetes on intensive insulin therapy: A cross-sectional, observational study. Diabetes Technol. Ther. 2010, 12, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef]
- Motataianu, A.; Maier, S.; Bajko, Z.; Voidazan, S.; Balasa, R.; Stoian, A. Cardiac autonomic neuropathy in type 1 and type 2 diabetes patients. BMC Neurol. 2018, 18, 126. [Google Scholar] [CrossRef]
- Mathieu, C.; Dandona, P.; Phillip, M.; Oron, T.; Lind, M.; Hansen, L.; Thoren, F.; Xu, J.; Langkilde, A.M.; On behalf of the DEPICT-1 and DEPICT-2 Investigators. Glucose Variables in Type 1 Diabetes Studies with Dapagliflozin: Pooled Analysis of Continuous Glucose Monitoring Data From DEPICT-1 and -2. Diabetes Care 2019, 42, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szucs, G.; Attieh, Z.; Murlasits, Z.; Torok, S.; Posa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, J.; Mazumder, P.K.; Hu, P.; Chakrabarti, G.; Roberts, M.W.; Yun, U.J.; Cooksey, R.C.; Litwin, S.E.; Abel, E.D. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005, 146, 5341–5349. [Google Scholar] [CrossRef] [Green Version]
- Cindro, P.V.; Krnic, M.; Modun, D.; Smajic, B.; Vukovic, J. The differences between insulin glargine U300 and insulin degludec U100 in impact on the glycaemic variability, arterial stiffness and the lipid profiles in insulin naive patients suffering from type two diabetes mellitus—outcomes from cross-over open-label randomized trial. BMC Endocr. Disord. 2021, 21, 86. [Google Scholar] [CrossRef]
- Marso, S.P.; McGuire, D.K.; Zinman, B.; Poulter, N.R.; Emerson, S.S.; Pieber, T.R.; Pratley, R.E.; Haahr, P.M.; Lange, M.; Brown-Frandsen, K.; et al. Efficacy and Safety of Degludec versus Glargine in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 723–732. [Google Scholar] [CrossRef]
- Bolli, G.B.; Songini, M.; Trovati, M.; Del Prato, S.; Ghirlanda, G.; Cordera, R.; Trevisan, R.; Riccardi, G.; Noacco, C. Lower fasting blood glucose, glucose variability and nocturnal hypoglycaemia with glargine vs NPH basal insulin in subjects with Type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 571–579. [Google Scholar] [CrossRef]
- Rodbard, D. Continuous Glucose Monitoring: A Review of Recent Studies Demonstrating Improved Glycemic Outcomes. Diabetes Technol. Ther 2017, 19, S25–S37. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [Green Version]
- Carley, A.N.; Severson, D.L. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim. Biophys. Acta 2005, 1734, 112–126. [Google Scholar] [CrossRef]
- Hsueh, W.; Abel, E.D.; Breslow, J.L.; Maeda, N.; Davis, R.C.; Fisher, E.A.; Dansky, H.; McClain, D.A.; McIndoe, R.; Wassef, M.K.; et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ. Res. 2007, 100, 1415–1427. [Google Scholar] [CrossRef] [Green Version]
- McQueen, A.P.; Zhang, D.; Hu, P.; Swenson, L.; Yang, Y.; Zaha, V.G.; Hoffman, J.L.; Yun, U.J.; Chakrabarti, G.; Wang, Z.; et al. Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: Possible role of reduced capillary density. J. Mol. Cell. Cardiol. 2005, 39, 882–892. [Google Scholar] [CrossRef]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011, 2011, 497841. [Google Scholar] [CrossRef]
- Hayek, T.; Hussein, K.; Aviram, M.; Coleman, R.; Keidar, S.; Pavoltzky, E.; Kaplan, M. Macrophage foam-cell formation in streptozotocin-induced diabetic mice: Stimulatory effect of glucose. Atherosclerosis 2005, 183, 25–33. [Google Scholar] [CrossRef]
- Scatena, M.; Jackson, M.F.; Speer, M.Y.; Leaf, E.M.; Wallingford, M.C.; Giachelli, C.M. Increased Calcific Aortic Valve Disease in response to a diabetogenic, procalcific diet in the LDLr(-/-)ApoB(100/100) mouse model. Cardiovasc. Pathol. 2018, 34, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.M.; Benko, R.; Kiss, L.; Muranyi, M.; Pek, T.; Fekete, K.; Barany, T.; Somlai, A.; Csordas, A.; Szabo, C. Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia 2009, 52, 952–961. [Google Scholar] [CrossRef] [Green Version]
- Mita, T.; Otsuka, A.; Azuma, K.; Uchida, T.; Ogihara, T.; Fujitani, Y.; Hirose, T.; Mitsumata, M.; Kawamori, R.; Watada, H. Swings in blood glucose levels accelerate atherogenesis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2007, 358, 679–685. [Google Scholar] [CrossRef]
- Wang, J.S.; Yin, H.J.; Guo, C.Y.; Huang, Y.; Xia, C.D.; Liu, Q. Influence of high blood glucose fluctuation on endothelial function of type 2 diabetes mellitus rats and effects of Panax Quinquefolius Saponin of stem and leaf. Chin. J. Integr. Med. 2013, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Teshima, Y.; Fukui, A.; Kondo, H.; Nishio, S.; Nakagawa, M.; Saikawa, T.; Takahashi, N. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 2014, 104, 5–14. [Google Scholar] [CrossRef]
- Azuma, K.; Kawamori, R.; Toyofuku, Y.; Kitahara, Y.; Sato, F.; Shimizu, T.; Miura, K.; Mine, T.; Tanaka, Y.; Mitsumata, M.; et al. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2275–2280. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.H.; Yu, J.M.; Yoo, H.J.; Lee, S.J.; Kang, D.H.; Cho, Y.J.; Kim, D.M. Anti-Proliferative Effects of Rutin on OLETF Rat Vascular Smooth Muscle Cells Stimulated by Glucose Variability. Yonsei Med. J. 2016, 57, 373–381. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Dai, Z.; Sun, Y. Intermittent high glucose enhances proliferation of vascular smooth muscle cells by upregulating osteopontin. Mol. Cell. Endocrinol. 2009, 313, 64–69. [Google Scholar] [CrossRef]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef] [Green Version]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar]
- Garoffolo, G.; Madonna, R.; de Caterina, R.; Pesce, M. Cell based mechanosensing in vascular patho-biology: More than a simple go-with the flow. Vasc. Pharmacol. 2018, 111, 7–14. [Google Scholar] [CrossRef]
- Piconi, L.; Quagliaro, L.; Da Ros, R.; Assaloni, R.; Giugliano, D.; Esposito, K.; Szabo, C.; Ceriello, A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: The role of poly(ADP-ribose) polymerase. J. Thromb. Haemost. 2004, 2, 1453–1459. [Google Scholar] [CrossRef]
- Risso, A.; Mercuri, F.; Quagliaro, L.; Damante, G.; Ceriello, A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E924–E930. [Google Scholar] [CrossRef] [PubMed]
- Schisano, B.; Tripathi, G.; McGee, K.; McTernan, P.G.; Ceriello, A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia 2011, 54, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Dong, Y.; Zhong, J.; Cao, R.; Zhao, X.; Wen, G.; Liu, J. Adiponectin protects endothelial cells from the damages induced by the intermittent high level of glucose. Endocrine 2011, 40, 386–393. [Google Scholar] [CrossRef]
- Liu, T.; Gong, J.; Chen, Y.; Jiang, S. Periodic vs constant high glucose in inducing pro-inflammatory cytokine expression in human coronary artery endothelial cells. Inflamm. Res. 2013, 62, 697–701. [Google Scholar] [CrossRef]
- Liu, T.S.; Pei, Y.H.; Peng, Y.P.; Chen, J.; Jiang, S.S.; Gong, J.B. Oscillating high glucose enhances oxidative stress and apoptosis in human coronary artery endothelial cells. J. Endocrinol. Investig. 2014, 37, 645–651. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Sun, S.; Sun, Y.; Wang, X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: The role of mitochondrial reactive oxygen species. Mol. Cell. Biochem. 2010, 343, 27–35. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Micheloni, S.; Prattichizzo, F.; Ceriello, A. Glucose-sensing microRNA-21 disrupts ROS homeostasis and impairs antioxidant responses in cellular glucose variability. Cardiovasc. Diabetol. 2018, 17, 105. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Sang, Y.; Yin, T.; Wang, B.; Yang, W.; Li, X.; Li, H.; Kang, Y. miR-1273g-3p participates in acute glucose fluctuation-induced autophagy, dysfunction, and proliferation attenuation in human umbilical vein endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E734–E743. [Google Scholar] [CrossRef] [Green Version]


| GV Index | Definition | Reported Features | |
|---|---|---|---|
| ADRR | Average Daily Risk Range | The sum of the daily peak risks for hyperglycemia and hypoglycemia | It is equally sensitive in predicting future episodes of extreme hypoglycemia and hyperglycemia, and it is less sensitive to variability within the target blood glucose range [32] |
| CONGA | Continuous Overlapping Net Glycemic Action | Intraday (within-day) glycemic variation. The standard deviation of the differences of glucose readings for a defined period of hours | It is a parameter that reflects the variability of blood glucose over a certain time interval [20] |
| CV | Coefficient of Variation | The extent of variability in relation to the mean of the population. 100 * SD/mean of the observations | Less influenced when comparing data sets with widely different mean glucose values (or HbA1c) [6] |
| IQR | Interquartile Range | Distribution of glucose data at a given time-point calculated from non-parametric statistics. The difference between the 25–75 percentile. | Plotting the IQR (around the median glucose curve) on a modal day glucose profile makes it is easy to spot what time of day has the most GV and needs attention [33] |
| LI | Lability Index | It processes three glucose values to calculate a lability value and then moves to the next three glucose values | It can serve as an indicator of patients’ prognosis [34,35] |
| LBGI/HBGI | Low/High Blood Glucose Index | Implemented by converting glucose values into risk scores. If the risk score is below 0, then the risk is labeled LBGI; if it is above 0, HGBI. | They can assess the risk of severe hypoglycemia or hyperglycemia in diabetic patients [36] |
| MAG | Mean Absolute Glucose | Absolute differences between sequential readings divided by the time between the first and last blood glucose measurement | This measure includes minor as well as major glucose swings and a time axis as the coordinate; it does not permit assessment of the real magnitude of glycemic excursions but rather their kinetics [37] |
| MAGE | Mean Amplitude Glycemic Excursion | Average of all blood glucose excursions or swings (peak to trough) that are greater than 1 SD of all measures for a given glucose profile | The most common measure of glucose spikes, swings, or excursions as opposed to glucose dispersion [20] |
| Mean and SD | Mean and Standard Deviation | The amount of variation or dispersion of a data set. The SD of the data set is the square root of its variance | A variation measure that is the most familiar to clinicians and easy to calculate. Most accurate if values are “normally distributed around the mean,” which is often not the case [33] |
| MODD | Mean of Daily Difference | Interday (between-day) glycemic variation. The absolute value of the difference between glucose values taken on two consecutive days at the same time | It can be used to assess the continuous changes of blood glucose between different days [20] |
| TIR | Time in Range | The amount of time that glucose is in the target ranges between 3.9 and 10.0 mmol/L within 24 h | Early studies suggest that time-in-range is just as good a predictor of long-term diabetes complications [23,38] |
| CV Diagnosis | Patients Number | Intervention Type | Glucose Fluctuation Monitoring | Observed Effect(s) |
|---|---|---|---|---|
| ACS STEMI | 237 | p-PCI | MAGE, SMBG within 72 h after p-PCI | Increased GV associated with increased composite MACE and non-IRA revascularization during in-hospital and 30-day follow-up [12] |
| ACS STEMI, NSTEMI, UA | 864 | PCI/CABG | Mean and SD of blood glucose during hospitalization | Increased GV associated with 30-day increased incidence of MACCE and AF during hospitalization, and length of hospital stay [11] |
| ACS + DM | 262 | PCI/CABG | Mean and SD of blood glucose 6-months follow-up using the WeChat application | Increased GV associated with 2-fold increased MACE after 6 months of follow-up [18] |
| ACS + DM STEMI, NSTEMI | 327 | PCI/CABG/medical treatment | SD with the cut-off > 2.7 mmol/L, in hospital | A GV cut-off value of >2.70 mmol/L predicts mid-term MACE in patients after 16.9 months of follow-up [39] |
| CAD | 1461 | CAGB | Post-operative CV within 24 h | Increased post-operative GV associated with increased risk for in-hospital major adverse events [13] |
| CAD | 2073 | CAGB | Post-operative SD, CV, MAGE within 24 h | Increased 24 h post-operative GV was independently associated with AF incidence [40] |
| CAD + DM | 28 | PCI | SD, CV, MAGE, CONGA 12 h before and after PCI | Altered GV indexes associated with post-procedural impairment of renal function and myocardial damage [41] |
| CAD + DM | 50 | PCI | MAGE, 3 consecutive days before PCI | Larger glucose fluctuation is an independent risk factor for impaired uniform vessel healing after second-generation drug-eluting stent implantation after 9 months of follow-up and associated with MACE [42] |
| CV Diagnosis | Patients Number | Intervention Type | GV Monitoring | Observed Effect(s) |
|---|---|---|---|---|
| ACS STEMI, NSTEM | 57 | PCI | MAGE during hospital admission (at 10 ± 6 days) to minimize the influence of ACS | Higher GV is associated with increased lipid and decreased fibrous contents with larger plaque burden and higher remodeling index [44] |
| ACS NSTEMI, UA | 82 | PCI | MAGE, MODD, PPGE, LAGE post-procedural 48–72 h | MAGE and PPGE negatively correlated with the percent fibrous volume and positively with the percent necrotic volume [34] |
| CAD | 72 | PCI | MAGE, 3 consecutive days before PCI | Increased GV correlated with lipid-rich plaque formation [47] |
| CAD | 53 | PCI | SD, MAGE, CONGA, MODD before the procedure | All GV indexes associated with plaque vulnerability, MAGE, and ST had a higher correlation with coronary plaque vulnerability in comparison to others [48] |
| CAD + DM | 51 | PCI | MAGE, 3 consecutive days before PCI | Increased GV correlated with CD14++ CD16+ monocytes in non-DM patientsCD14++ CD16+ monocytes associated with plaque vulnerability [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, V.; Myasoedova, V.A.; Vinci, M.C.; Rondinelli, M.; Songia, P.; Massaiu, I.; Cosentino, N.; Moschetta, D.; Valerio, V.; Ciccarelli, M.; et al. The Role of Glycemic Variability in Cardiovascular Disorders. Int. J. Mol. Sci. 2021, 22, 8393. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168393
Alfieri V, Myasoedova VA, Vinci MC, Rondinelli M, Songia P, Massaiu I, Cosentino N, Moschetta D, Valerio V, Ciccarelli M, et al. The Role of Glycemic Variability in Cardiovascular Disorders. International Journal of Molecular Sciences. 2021; 22(16):8393. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168393
Chicago/Turabian StyleAlfieri, Valentina, Veronika A. Myasoedova, Maria Cristina Vinci, Maurizio Rondinelli, Paola Songia, Ilaria Massaiu, Nicola Cosentino, Donato Moschetta, Vincenza Valerio, Michele Ciccarelli, and et al. 2021. "The Role of Glycemic Variability in Cardiovascular Disorders" International Journal of Molecular Sciences 22, no. 16: 8393. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168393