Global Lysine Acetylome Analysis of LPS-Stimulated HepG2 Cells Identified Hyperacetylation of PKM2 as a Metabolic Regulator in Sepsis
Abstract
:1. Introduction
2. Results
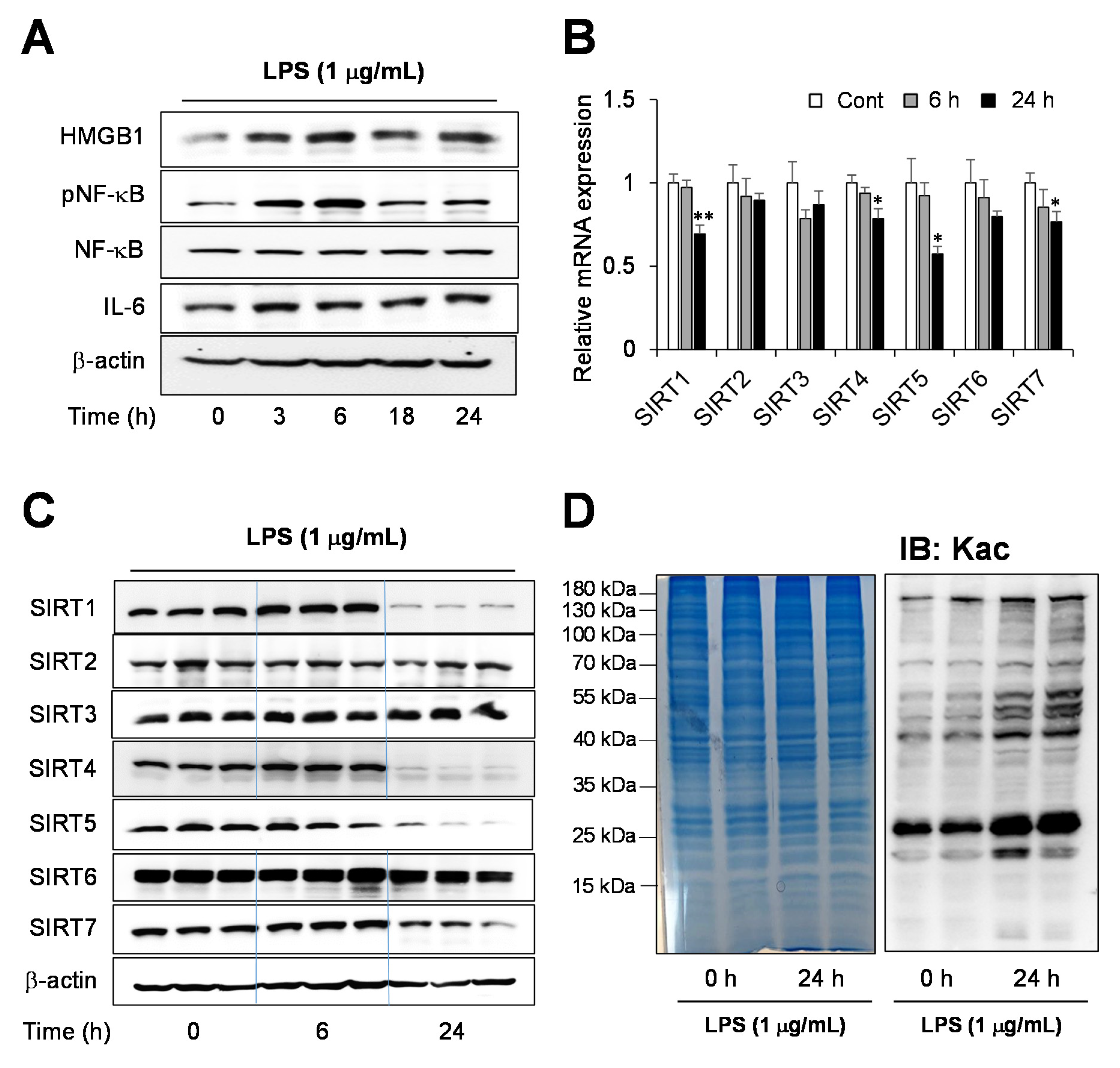
2.1. Endotoxin-Induced Inflammation Response in Human Hepatocytes
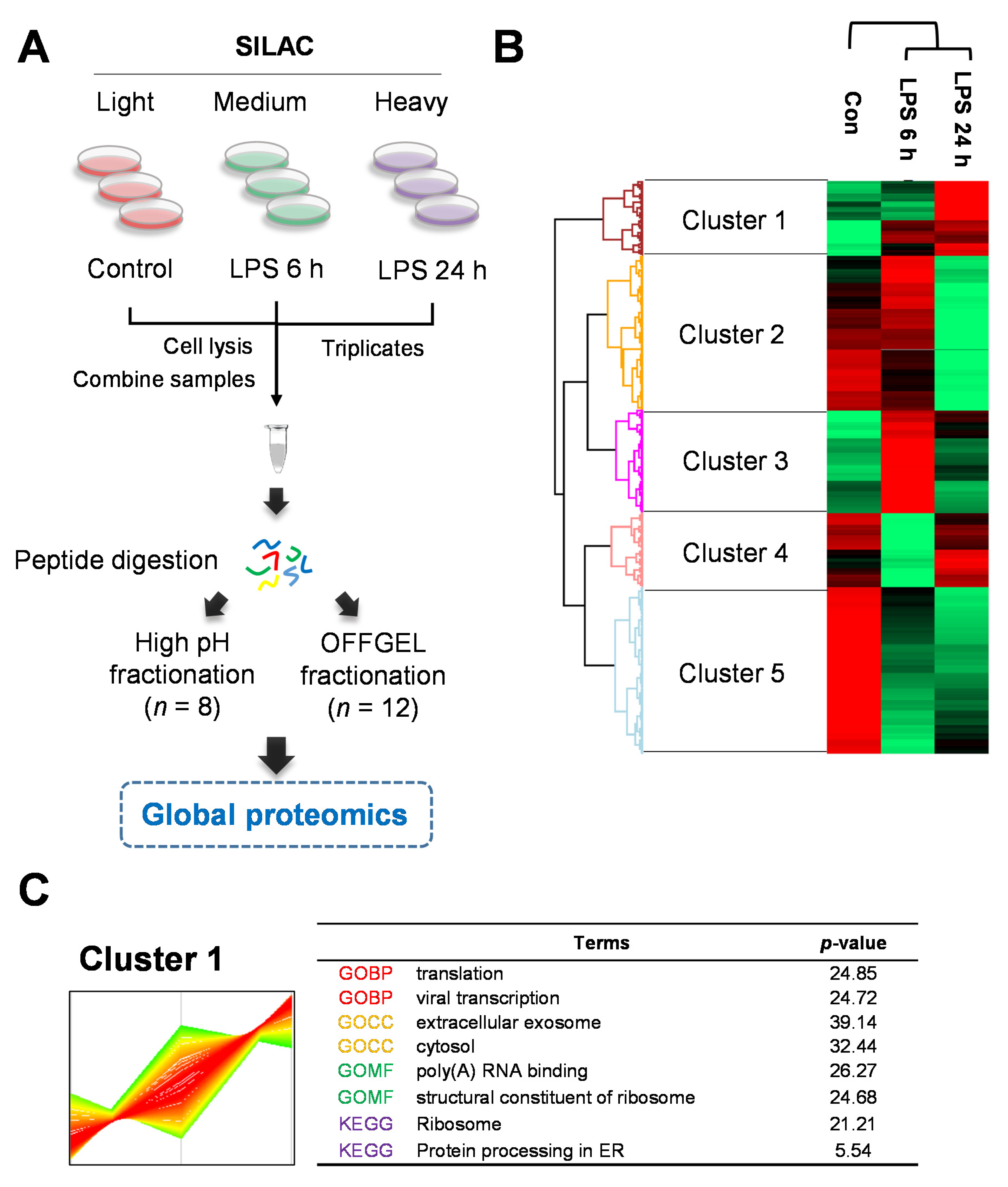
2.2. Quantitative Proteomics for LPS-Induced Sepsis in HepG2 Cells
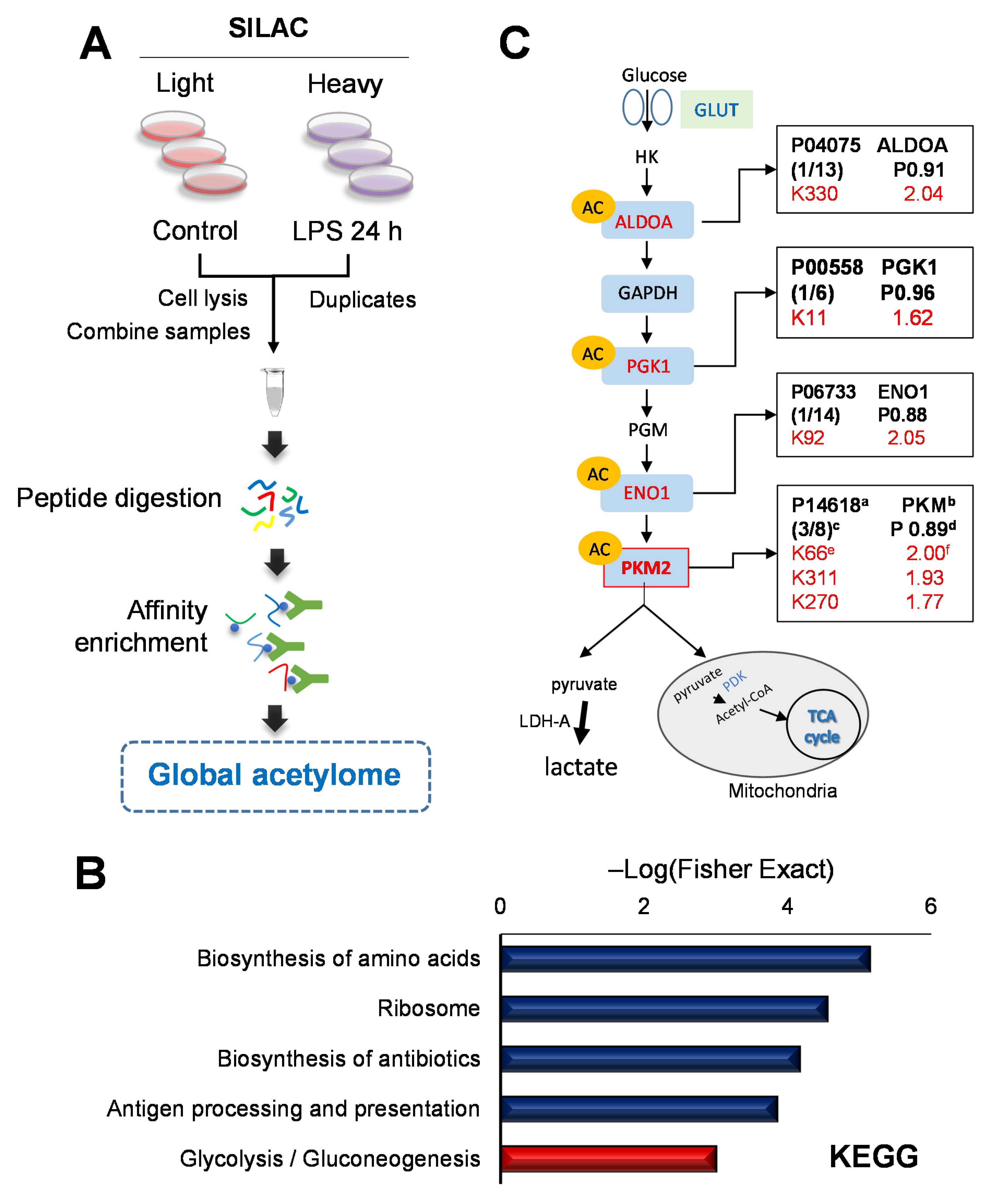
2.3. Quantitative Global Lysine Acetylome Profiling in LPS-Induced SILD
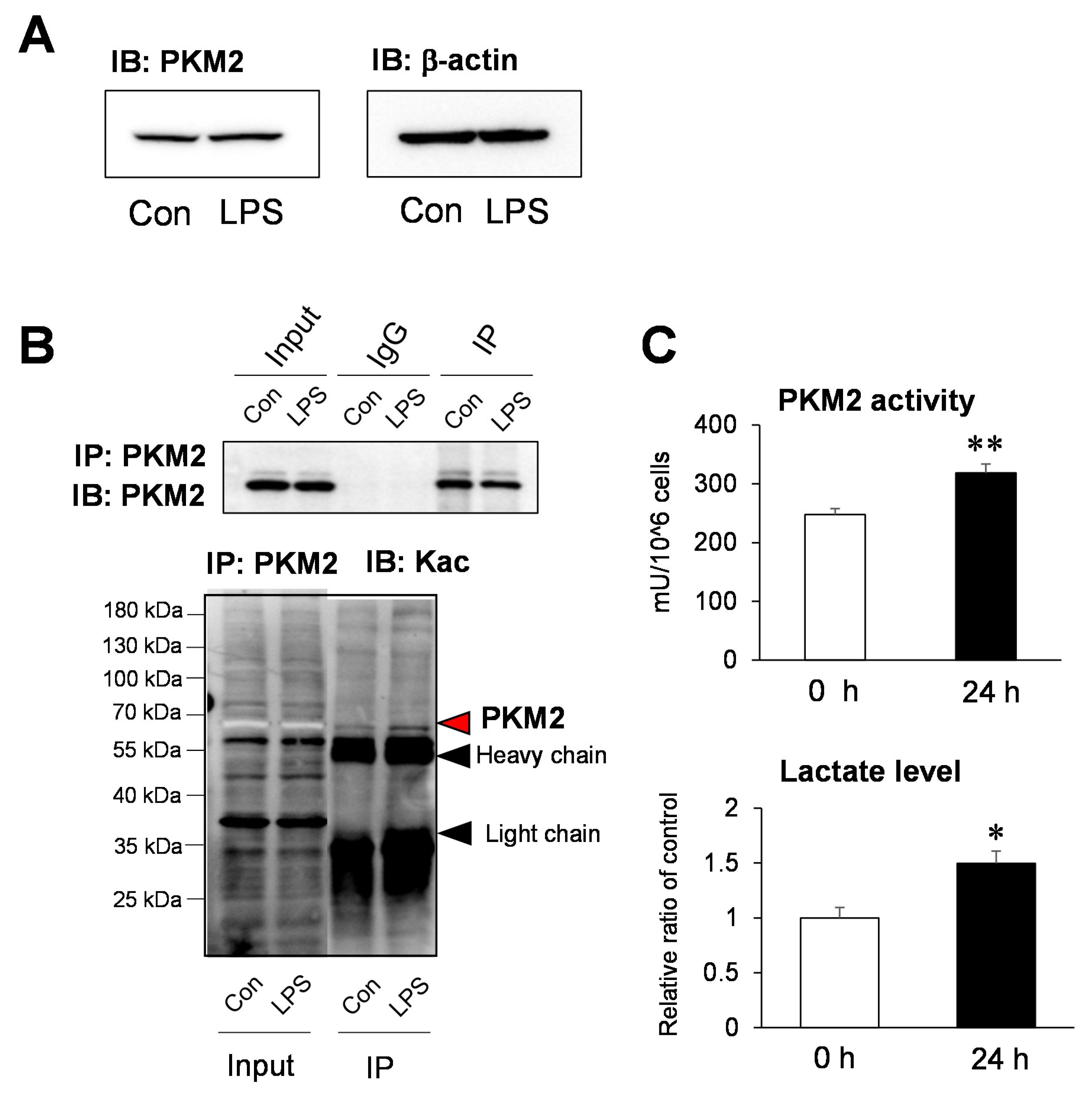
2.4. Functional Analysis of the Acetylation Site of PKM2 on Glycolysis
3. Discussion
4. Materials & Methods
4.1. Cell Culture and Chemicals
4.2. RNA Preparation and Quantitative Real-Time PCR
4.3. Western Blot
4.4. SILAC Label and Proteomics Sample Preparation for Global Proteomes
4.5. Global Immunoprecipitation
4.6. Nano LC–MS/MS Analysis
4.7. Protein Identification and Quantification Using SILAC Labeling by LC–MS
4.8. Pyruvate Kinase Activity Assay
4.9. Sample Preparation for Lactate Quantification
4.10. Determination of Lactate Level by LC–MS/MS
4.11. Bioinformatics
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.F.; Brown, C.M.; El Gazzar, M.; McPhail, L.; Millet, P.; Rao, A.; Vachharajani, V.T.; Yoza, B.K.; McCall, C.E. Fueling the flame: Bioenergy couples metabolism and inflammation. J. Leukoc Biol. 2012, 92, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Calandra, T.; Echtenacher, B.; Roy, D.L.; Pugin, J.; Metz, C.N.; Hültner, L.; Heumann, D.; Männel, D.; Bucala, R.; Glauser, M.P. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 2000, 6, 164–170. [Google Scholar] [CrossRef]
- Baranova, I.N.; Souza, A.C.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Kost, Y.; Remaley, A.T.; et al. Human SR-BI and SR-BII potentiate lipopolysaccharide-induced inflammation and acute liver and kidney injury in mice. J. Immunol. 2016, 196, 3135–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumanayagam, S.; Arunmani, M. Hepatoprotective and antibacterial activity of Lippia nodiflora Linn. against lipopolysaccharides on HepG2 cells. Pharmacogn. Mag. 2015, 11, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhainaut, J.F.; Marin, N.; Mignon, A.; Vinsonneau, C.; Sprung, C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit. Care Med. 2001, 29, S42–S47. [Google Scholar] [CrossRef]
- Kramer, L.; Jordan, B.; Druml, W.; Bauer, P.; Metnitz, P.G.H. Incidence and prognosis of early hepatic dysfunction in critically ill patients—A prospective multicenter study. Crit. Care Med. 2007, 35, 1099–1104. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin in sepsis and systemic inflammation: A harmful biomarker and a therapeutic target. Br. J. Pharmacol. 2010, 159, 253–264. [Google Scholar] [CrossRef]
- Audagnotto, M.; Peraro, M.D. Protein post-translational modifications: In silico prediction tools and molecular modeling. CSBJ 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Sabari, B.R.; Zhang, D.; Allis, C.D.; Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017, 18, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.G.; Xie, X.; Basisty, N.; Byrnes, J.; McSweeney, S.; Schilling, B.; Wolfe, A.J. Post-translational protein acetylation: An elegant mechanism for bacteria to dynamically regulate metabolic functions. Front. Microbiol. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ramy, R.; Magroun, N.; Messadecq, N.; Gauthier, L.R.; Boussin, F.D.; Kolthur-Seetharam, U.; Schreiber, V.; McBurney, M.W.; Sassone-Corsi, P.; Dantzer, F. Functional interplay between Parp-1 and SirT1 in genome integrity and chromatin-based processes. Cell. Mol. Life Sci. 2009, 66, 3219–3234. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013, 23, 746–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Buechler, N.L.; Woodruff, A.G.; Long, D.L.; Zabalawi, M.; Yoza, B.K.; McCall, C.E.; Vachharajani, V. Sirtuins and immuno-metabolism of sepsis. Int. J. Mol. Sci 2018, 19, 2738. [Google Scholar] [CrossRef] [Green Version]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Huang, C.; Huang, Y.; Hong, L.; Wang, H.; Zhou, Z.; Qiu, Y. SIRT4 suppresses inflammatory responses in human umbilical vein endothelial cells. Cardiovasc. Toxicol. 2015, 15, 217–223. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011, 334, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Anoopkumar-Dukie, S.; Mallik, S.B.; Davey, A.K. SIRT1 and SIRT2 modulators reduce LPS-induced inflammation in HAPI microglial cells and protect SH-SY5Y neuronal cells in vitro. J. Neural Transm. 2021, 128, 631–644. [Google Scholar] [CrossRef]
- Li, F.L.; Liu, J.P.; Bao, R.X.; Yan, G.; Feng, X.; Xu, Y.P.; Sun, Y.P.; Yan, W.; Ling, Z.Q.; Xiong, Y.; et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat. Commun. 2018, 9, 508. [Google Scholar] [CrossRef] [Green Version]
- Miyai, M.; Kanayama, T.; Hyodo, F.; Kinoshita, T.; Ishihara, T.; Okada, H.; Suzuki, H.; Takashima, S.; Wu, Z.; Hatano, Y.; et al. Glucose transporter Glut1 controls diffuse invasion phenotype with perineuronal satellitosis in diffuse glioma microenvironment. Neurooncol. Adv. 2021, 3, vdaa150. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Z.; Fu, W.; Cai, S.; Zeng, Z. Emerging evidence concerning the role of sirtuins in sepsis. Crit. Care Res. Pract. 2018, 2018, 5489571. [Google Scholar] [CrossRef] [PubMed]
- Vachharajani, V.T.; Liu, T.; Wang, X.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins link inflammation and metabolism. J. Immunol. Res. 2016, 2016, 8167273. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, A.; Iwasaka, H.; Hagiwara, S.; Asai, N.; Nishida, T.; Noguchi, T. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. J. Surg. Res. 2012, 174, 136–141. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Y.; Yao, J.; Li, Z.; Chen, Z.; Wang, G.; Jing, H.; Zhang, X.; Li, M.; Peng, J.; et al. Novel role of resveratrol: Suppression of high-mobility group protein box 1 nucleocytoplasmic translocation by the upregulation of sirtuin 1 in sepsis-induced liver injury. Shock 2014, 42, 440–447. [Google Scholar] [CrossRef]
- Hwang, J.S.; Choi, H.S.; Ham, S.A.; Yoo, T.; Lee, W.J.; Paek, K.S.; Seo, H.G. Deacetylation-mediated interaction of SIRT1-HMGB1 improves survival in a mouse model of endotoxemia. Sci. Rep. 2015, 5, 15971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.F.; Yoza, B.K.; El Gazzar, M.; Vachharajani, V.T.; McCall, C.E. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J. Biol. Chem. 2011, 286, 9856–9864. [Google Scholar] [CrossRef] [Green Version]
- Vachharajani, V.T.; Liu, T.; Brown, C.M.; Wang, X.; Buechler, N.L.; Wells, J.D.; Yoza, B.K.; McCall, C.E. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J. Leukoc. Biol. 2014, 96, 785–796. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Fievez, L.; Neyrinck, A.M.; Delzenne, N.M.; Bureau, F.; Vanbever, R. Sirtuin inhibition attenuates the production of inflammatory cytokines in lipopolysaccharide-stimulated macrophages. Biochem. Biophys. Res. Commun. 2012, 420, 857–861. [Google Scholar] [CrossRef]
- Mahlknecht, U.; Voelter-Mahlknecht, S. Fluorescence in situ hybridization and chromosomal organization of the sirtuin 4 gene (Sirt4) in the mouse. Biochem. Biophys. Res. Commun. 2009, 382, 685–690. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; He, Y.; Yuan, C.; Chen, Q.; Li, G.; Chen, X. Sirtuin 5: A review of structure, known inhibitors and clues for developing new inhibitors. Sci. China Life Sci. 2017, 60, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine 2021, 100, e24835. [Google Scholar] [CrossRef]
- Rivas, A.M.; Nugent, K. Hyperglycemia, insulin, and insulin resistance in sepsis. Am. J. Med. Sci. 2021, 361, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Gao, T.; Cheng, Y.; Cheng, M.; Su, T.; Xi, F.; Wu, C.; Yu, W. Hypothalamic AMPK-induced autophagy ameliorates hypercatabolism in septic rats by regulating POMC expression. Biochem. Biophys. Res. Commun. 2018, 497, 1089–1096. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.W.; Kim, J.H.; Kim, H.J.; Um, J.; Jung, D.W.; Williams, D.R. Lithium chloride protects against sepsis-induced skeletal muscle atrophy and cancer cachexia. Cells 2021, 10, 1017. [Google Scholar] [CrossRef]
- Rello, J.; Valenzuela-Sánchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A review of advances in management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [Green Version]
- Levy, B.; Desebbe, O.; Montemont, C.; Gibot, S. Increased aerobic glycolysis through β2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 2008, 30, 417–421. [Google Scholar] [CrossRef]
- Zahra, K.; Dey, T.; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The role of PKM2 in promoting tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xie, M.; Yang, M.; Yu, Y.; Zhu, S.; Hou, W.; Kang, R.; Lotze, M.T.; Billiar, T.R.; Wang, H.; et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 2014, 5, 4436. [Google Scholar] [CrossRef]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Sepsis-associated hyperlactatemia. Crit. Care 2014, 18, 503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.H.; Zhang, H.; Wang, Y.R.; Liu, X.L.; Huang, H.; Xu, X.H. SIRT 1 binding with PKM and NSE and modulate their acetylation and activities. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 794–801. [Google Scholar] [CrossRef]
- Xiong, Y.; Lei, Q.Y.; Zhao, S.; Guan, K.L. Regulation of glycolysis and gluconeogenesis by acetylation of PKM and PEPCK. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 285–289. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Xu, W.; Zhao, S.; Ye, D.; Wang, Y.; Xu, Y.; Zhou, L.; Chu, Y.; Zhang, C.; et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 2017, 19, 2331–2344. [Google Scholar] [CrossRef] [Green Version]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]
| No. | Protein Name | Proteins | Gene Name | Modified Sequence | Score | Positions | Charge | Ratio * |
|---|---|---|---|---|---|---|---|---|
| 1 | Calreticulin | P27797 | CALR | _GK(ac)NVLINK_ | 76.478 | K153 | 2 | 5.57 |
| 2 | Myosin-9 | P35579 | MYH9 | _K(ac)FDQLLAEEK_ | 63.473 | K1445 | 2 | 3.20 |
| 3 | Nucleoside diphosphate kinase | Q32Q12 | NME1-NME2 | _FEQK(ac)GFR_ | 100.19 | K56 | 2 | 3.05 |
| 4 | CCAAT/enhancer-binding protein gamma | P53567 | CEBPG | _AVAPSK(ac)QSK_ | 104.89 | K50 | 2 | 2.75 |
| 5 | Aldo-keto reductase family 1 member C3 | P42330 | AKR1C3 | _SK(ac)IADGSVK_ | 72.2 | K68 | 2 | 2.63 |
| 6 | Heterogeneous nuclear ribonucleoprotein U | A0A1 × 7SBS1 | HNRNPU | _K(ac)AEVEGKDLPEHAVLK_ | 68.751 | K539 | 3 | 2.57 |
| 7 | 60S ribosomal protein L35 | P42766 | RPL35 | _LNK(ac)HEENLK_ | 75.652 | K97 | 3 | 2.48 |
| 8 | 60S ribosomal protein L3 | P39023 | RPL3 | _FIDTTSK(ac)FGHGR_ | 41.242 | K373 | 3 | 2.30 |
| 9 | Protein disulfide-isomerase | P07237 | P4HB | _TAAESFK(ac)GK_ | 76.228 | K283 | 2 | 2.18 |
| 10 | 40S ribosomal protein SA | C9J9K3 | RPSA | _AVLK(ac)FAAATGATPIAGR_ | 118.66 | K89 | 2 | 2.08 |
| 11 | Peroxiredoxin-2 | P32119 | PRDX2 | _ATAVVDGAFK(ac)EVK_ | 119.88 | K26 | 2 | 2.06 |
| 12 | Alpha-enolase | P06733 | ENO1 | _IDK(ac)LM(ox)IEM(ox)DGTENK_ | 171.15 | K92 | 2 | 2.04 |
| 13 | Fructose-bisphosphate aldolase A | P04075 | ALDOA | _AAQEEYVK(ac)R_ | 113.62 | K330 | 2 | 2.04 |
| 14 | Heat shock protein HSP 90-alpha | P07900 | HSP90AA1 | _FYEQFSK(ac)NIK_ | 66.056 | K443 | 2 | 2.03 |
| 15 | Pyruvate kinase PKM | P14618 | PKM | _EM(ox)IK(ac)SGM(ox)NVAR_ | 43.68 | K66 | 2 | 2.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, A.-Y.; Paudel, S.; Choi, S.; Lee, J.H.; Kim, M.-S.; Bae, J.-S.; Lee, S. Global Lysine Acetylome Analysis of LPS-Stimulated HepG2 Cells Identified Hyperacetylation of PKM2 as a Metabolic Regulator in Sepsis. Int. J. Mol. Sci. 2021, 22, 8529. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168529
Na A-Y, Paudel S, Choi S, Lee JH, Kim M-S, Bae J-S, Lee S. Global Lysine Acetylome Analysis of LPS-Stimulated HepG2 Cells Identified Hyperacetylation of PKM2 as a Metabolic Regulator in Sepsis. International Journal of Molecular Sciences. 2021; 22(16):8529. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168529
Chicago/Turabian StyleNa, Ann-Yae, Sanjita Paudel, Soyoung Choi, Jun Hyung Lee, Min-Sik Kim, Jong-Sup Bae, and Sangkyu Lee. 2021. "Global Lysine Acetylome Analysis of LPS-Stimulated HepG2 Cells Identified Hyperacetylation of PKM2 as a Metabolic Regulator in Sepsis" International Journal of Molecular Sciences 22, no. 16: 8529. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168529






