Metabolomics as a Tool to Elucidate the Sensory, Nutritional and Safety Quality of Wheat Bread—A Review
Abstract
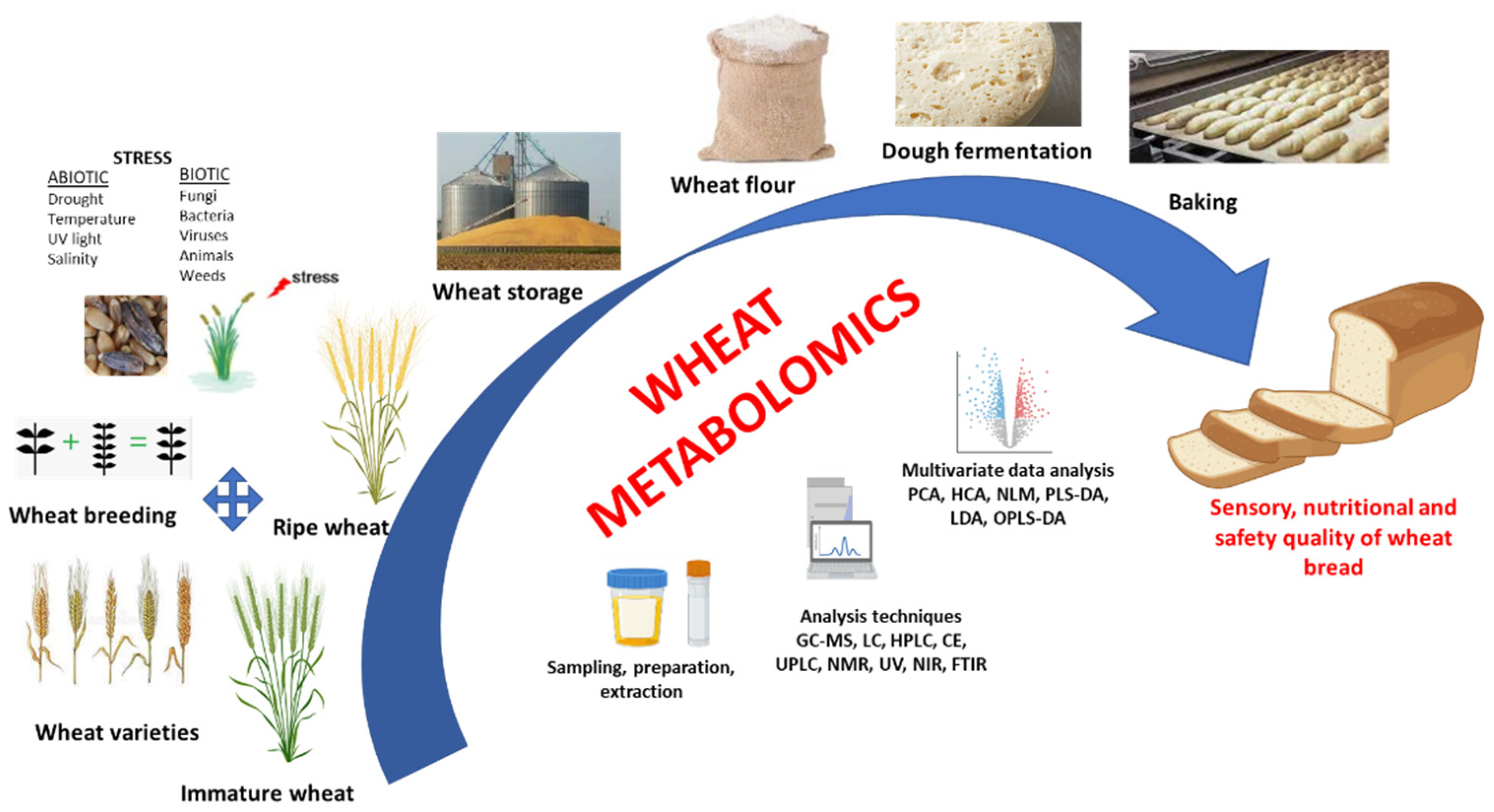
:1. Introduction
2. Metabolomics Approaches for Wheat Production and Processing
3. Metabolite Profiling during Wheat Dough Fermentation
4. Application of Metabolomics for Wheat and Bread Safety Control
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cauvain, S.P.; Young, L.S. Baked Products: Science, Technology and Practice; Blackwell Publishing: Oxford, UK, 2006; pp. 1–8. [Google Scholar]
- Martínez-Monzó, J.; García-Segovia, P.; Albors-Garrigos, J. Trends and innovations in bread, bakery, and pastry. J. Culin. Sci. Technol. 2013, 11, 56–65. [Google Scholar] [CrossRef]
- Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Popa, M.E. Chapter 7—Trends of innovation in bread and bakery production. In Trends in Wheat and Bread Making; Galanakis, C.M., Ed.; Elsevier: Edinburgh, UK, 2021; pp. 199–226. [Google Scholar]
- Cifuentes, A. Food analysis in the postgenomic era. Foodomics. Electrophor. 2012, 33, 2199–2200. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Valdes, A.; Leon, C.; Cifuentes, A. Chapter 1—Foodomics, fundamentals, state of the art and future trends. In Foodomics: Omic Strategies and Applications in Food Science; Barros-Velázquez, J., Ed.; The Royal Society of Biochemistry: Croydon, UK, 2021; pp. 1–53. [Google Scholar]
- Jacobs, D.M.; Van den Berg, M.A.; Hall, R.D. Towards superior plant-based foods using metabolomics. Curr. Opin. Biotechnol. 2021, 70, 23–28. [Google Scholar] [CrossRef]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics provides valuable insight for the study of durum wheat: A review. J. Agric. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef]
- Paul, A.; Harrington, P.B. Chemometric applications in metabolomic studies using chromatography-mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116165. [Google Scholar] [CrossRef]
- Villate, A.; San Nicolas, M.; Gallastegi, M.; Aulas, P.A.; Olivares, M.; Usobiaga, A.; Etxebarria, N.; Aizpurua-Olaizola, O. Review: Metabolomics as a prediction tool for plants performance under environmental stress. Plant Sci. 2020, 303, 110789. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Reisdorph, N.A.; Walmsley, S.; Reisdorph, R. A perspective and framework for developing sample type specific databases for lc/ms-based clinical metabolomics. Metabolites 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus Plantarum strains via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Saa, D.L.T.; Nissen, L.; Gianotti, A. Metabolomic approach to study the impact of flour type and fermentation process on volatile profile of bakery products. Food Res. Int. 2019, 119, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Longin, F.; Beck, H.; Gütler, H.; Heilig, W.; Kleinert, M.; Rapp, M.; Philipp, N.; Erban, A.; Brilhaus, D.; Mettler-Altmann, T.; et al. Aroma and quality of breads baked from old and modern wheat varieties and their prediction from genomic and flour-based metabolite profiles. Food Res. Int. 2020, 129, 108748. [Google Scholar] [CrossRef] [PubMed]
- Guerzoni, M.E.; Vernocchi, P.; Ndagijimana, M.; Gianotti, A.; Lanciotti, R. Generation of aroma compounds in sourdough: Effects of stress exposure and lactobacilli-yeasts interactions. Food Microbiol. 2007, 24, 139–148. [Google Scholar] [CrossRef]
- Quílez, J.; Ruiz, J.A.; Romero, M.P. Relationships between sensory flavor evaluation and volatile and nonvolatile compounds in commercial wheat bread type baguette. J. Food Sci. 2006, 71, S423–S427. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Paterson, A.; Piggott, J.R. Flavour in sourdough breads: A review. Trends Food Sci. Technol. 2006, 17, 557–566. [Google Scholar] [CrossRef]
- Vernocchi, P.; Ndagijimana, M.; Serrazanetti, D.; Gianotti, A.; Vallicelli, M.; Guerzoni, M.E. Influence of starch addition and dough microstructure on fermentation aroma production by yeasts and lactobacilli. Food Chem. 2008, 108, 1217–1225. [Google Scholar] [CrossRef]
- Brescia, M.A.; Sgaramella, A.; Ghelli, S.; Sacco, A. 1H HR-MAS NMR and isotopic investigation of bread and flour samples produced in southern Italy. J. Sci. Food Agric. 2003, 83, 1463–1468. [Google Scholar] [CrossRef]
- Balestra, F.; Laghi, L.; Saa, D.T.; Gianotti, A.; Rocculi, P.; Pinnavaia, G.G. Physico-chemical and metabolomic characterization of KAMUT® Khorasan and durum wheat fermented dough. Food Chem. 2015, 187, 451–459. [Google Scholar] [CrossRef]
- Makhoul, S.; Romano, A.; Capozzi, V.; Spano, G.; Aprea, E.; Cappellin, L.; Benozzi, E.; Scampicchio, M.; Märk, T.D.; Gasperi, F.; et al. Volatile compound production during the bread-making process: Effect of flour, yeast and their interaction. Food Bioprocess Technol. 2015, 8, 1925–1937. [Google Scholar] [CrossRef]
- Weckx, S.; Van Kerrebroeck, S.; Vuyst, L.D. Omics approaches to understand sourdough fermentation processes. Int. J. Food Microbiol. 2019, 302, 90–102. [Google Scholar] [CrossRef]
- Singh, A. Tools for metabolomics. Nat. Methods 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, S.; Dong, K.; Deng, X.; Zhou, J.; Xu, X.; Han, C.; Zhang, W.; Xu, Y.; Wang, Z.; Yan, Y. Dynamic metabolome profiling reveals significant metabolic changes during grain development of bread wheat (Triticum Aestivum, L.). J. Sci. Food Agric. 2016, 96, 3731–3740. [Google Scholar] [CrossRef]
- Matros, A.; Liu, G.; Hartmann, A.; Jiang, Y.; Zhao, Y.; Wang, H.; Ebmeyer, E.; Korzun, V.; Schachschneider, R.; Kazman, E.; et al. Genome-metabolite associations revealed low heritability, high genetic complexity, and causal relations for leaf metabolites in winter wheat (Triticum Aestivum). J. Exp. Bot. 2017, 68, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Beleggia, R.; Rau, D.; Laidò, G.; Platani, C.; Nigro, F.; Fragasso, M.; De Vita, P.; Scossa, F.; Fernie, A.R.; Nikoloski, Z.; et al. Evolutionary metabolomics reveals domestication-associated changes in tetraploid wheat kernels. Mol. Biol. Evol. 2016, 33, 1740–1753. [Google Scholar] [CrossRef] [Green Version]
- Francki, M.G.; Hayton, S.; Gummer, J.P.A.; Rawlinson, C.; Trengove, R.D. Metabolomic profiling and genomic analysis of wheat aneuploid lines to identify genes controlling biochemical pathways in mature grain. Plant Biotechnol. J. 2016, 14, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, A.; Fragasso, M.; Beleggia, R.; Nigro, F.; Papa, R. Evolution of the crop rhizosphere: Impact of domestication on root exudates in tetraploid wheat (Triticum Turgidum, L.). Front. Plant Sci. 2017, 8, 2124. [Google Scholar] [CrossRef] [Green Version]
- Biyiklioglu, S.; Alptekin, B.; Akpinar, B.A.; Varella, A.C.; Hofland, M.L.; Weaver, D.K.; Bothner, B.; Budak, H. A large-scale multiomics analysis of wheat stem solidness and the wheat stem sawfly feeding response, and syntenic associations in barley, brachypodium, and rice. Funct. Integr. Genom. 2018, 18, 241–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef]
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4947. [Google Scholar] [CrossRef] [PubMed]
- Righetti, L.; Rubert, J.; Galaverna, G.; Folloni, S.; Ranieri, R.; Stranska-Zachariasova, M.; Hajslov, J.; Dall’Asta, C. Characterization and discrimination of ancient grains: A metabolomics approach. Int. J. Mol. Sci. 2016, 17, 1217. [Google Scholar] [CrossRef] [Green Version]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Feil, R.; Lunn, J.E.; Watanabe, M.; Arrivault, A.; Stitt, M.; Hoefgen, R.; Morcuende, R. Metabolic and transcriptional analysis of durum wheat responses to elevated CO2 at low and high nitrate supply. Plant Cell Physiol. 2016, 57, 2133–2146. [Google Scholar] [CrossRef] [Green Version]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Kage, U.; Yogendra, K.N.; Kushalappa, A.C. TaWRKY70 Transcription factor in wheat QTL-2DL regulates downstream metabolite biosynthetic genes to resist Fusarium Graminearum infection spread within spike. Sci. Rep. 2017, 7, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righetti, L.; Rubert, J.; Galaverna, G.; Hurkova, K.; Dall’Asta, C.; Hajslova, J.; Stranska-Zachariasova, M. A novel approach based on untargeted lipidomics reveals differences in the lipid pattern among durum and common wheat. Food Chem. 2018, 240, 775–783. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.M.; Wang, X.C.; Liu, J.H.; Huang, B.I.; Guo, X.Y.; Xiong, S.P.; La, G.X. UPLC-QTOF Analysis reveals metabolomic changes in the flag leaf of wheat (Triticum Aestivum, L.) under low-nitrogen stress. Plant Physiol. Biochem. 2017, 111, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Thomason, K.; Babar, M.A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; MacDonald, G. Comparative physiological and metabolomics analysis of wheat (Triticum Aestivum, L.) following post-anthesis heat stress. PLoS ONE 2018, 13, e0197919. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.X.; Jiao, Y.; Li, M.X.; Zhong, X.L.; Gu, F.X.; Liu, X.; Xia, X.; Li, H.R. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vergara-Diaz, O.; Vatter, T.; Vicente, R.; Obata, T.; Nieto-Taladriz, M.T.; Aparicio, N.; Kefauver, S.C.; Fernie, A.; Araus, J.L. Metabolome profiling supports the key role of the spike in wheat yield performance. Cells 2020, 9, 1025. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics response for drought stress tolerance in chinese wheat genotypes (Triticum Aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Corol, D.I.; Jones, J.D.; Beale, M.H.; Ward, J.L. Defining genetic and chemical diversity in wheat grain by 1H-NMR spectroscopy of polar metabolites. Mol. Nutr. Food Res. 2017, 61, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.V.; Ruth, W. Wheat leaf lipids during heat stress: II. Lipids experiencing coordinated metabolism are detected by analysis of lipid co-occurrence. Plant Cell Env. 2016, 39, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal, C.; Barion, C.G.; Visioli, G.; Mattarozzi, M.; Mosca, G.; Vamerali, T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ. 2017, 247, 396–408. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steingass, C.B.; Longin, C.F.H.; Würschum, T.; Carle, R.; Schweiggert, R.M. Alkylresorcinol composition allows the differentiation of Triticum spp. having different degrees of ploidy. J. Cereal Sci. 2015, 65, 244–251. [Google Scholar] [CrossRef]
- Varzakas, T. Quality and safety aspects of cereals (wheat) and their products. Crit. Rev. Food Sci. Nutr. 2016, 56, 2495–2510. [Google Scholar] [CrossRef] [PubMed]
- Cauvain, S. Breadmaking Improving Quality, 2nd ed.; Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2012; 832p. [Google Scholar]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Front. Nutr. 2019, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough technology as a tool for the development of healthier grain-based products: An update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Peñas, E.; Diana, M.; Frias, J.; Quílez, J.; Martínez-Villaluenga, C. A multistrategic approach in the development of sourdough bread targeted towards blood pressure reduction. Plant Foods Hum. Nutr. 2015, 70, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Li, K.J.; Brouwer-Brolsma, E.M.; Burton-Pimentel, K.J.; Vergères, G.; Feskens, E.J.M. A Systematic review to identify biomarkers of intake for fermented food products. Genes Nutr. 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Chiş, M.; Păucean, A.; Man, S.; Vodnar, D.; Teleky, B.-E.; Pop, C.; Stan, L.; Borsai, O.; Kadar, C.; Urcan, A.; et al. Quinoa sourdough fermented with Lactobacillus Plantarum ATCC 8014 designed for gluten-free muffins—A powerful tool to enhance bioactive compounds. Appl. Sci. 2020, 10, 7140. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Pizarro, F.; Franco, F. Volatile organic compounds at early stages of sourdough preparation via static headspace and GC/MS analysis. Curr. Res. Nutr. Food Sci. 2017, 5, 89–99. [Google Scholar] [CrossRef]
- Nakamura, T.; Tomita, S.; Saito, K. Metabolite profiling in dough during fermentation. Food Sci. Technol. Res. 2018, 24, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The effect of sourdough fermentation on Triticum Dicoccum from Garfagnana: 1H NMR characterization and analysis of the antioxidant activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef]
- Koca, N.; Karaman, Ș. The effects of plant growth regulators and L-phenylalanine on phenolic compounds of sweet basil. Food Chem. 2015, 166, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Moroni, A.; Zannini, E. Medical nutrition therapy: Use of sourdough lactic acid bacteria as a cell factory for delivering functional biomolecules and food ingredients in gluten free bread. Microb. Cell Factories 2011, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chiș, M.S.; Păucean, A.; Stan, L.; Suharoschi, R.; Socaci, S.A.; Man, S.M.; Pop, C.R.; Muste, S. Impact of protein metabolic conversion and volatile derivatives on gluten-free muffins made with quinoa sourdough. CYTA J. Food 2019, 17, 744–753. [Google Scholar] [CrossRef]
- Lamberts, L.; Joye, I.J.; Beliën, T.; Delcour, J.A. Dynamics of γ-aminobutyric acid in wheat flour bread making. Food Chem. 2012, 130, 896–901. [Google Scholar] [CrossRef]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT Food Sci. Technol. 2018, 91, 557–567. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Khosrowshahi, A.Y.; Shamloo, H.B.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, I.; Fuciños, P.; Charalampopoulos, D.; Pandiella, S.S. Volatile compounds produced by the probiotic strain lactobacillus plantarum NCIMB 8826 in cereal-based substrates. Food Chem. 2009, 117, 265–271. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic profiling of sourdough fermented wheat and rye bread. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Galli, V.; Venturi, M.; Guerrini, S.; Blandino, M.; Luti, S.; Pazzagli, L.; Granchi Lisa. Antioxidant properties of sourdoughs made with whole grain flours of hull-less barley or conventional and pigmented wheat and by selected lactobacilli strains. Foods 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavoring production in Kamut®, quinoa and wheat doughs fermented by lactobacillus paracasei, lactobacillus plantarum, and lactobacillus brevis: A SPME-GC/MS study. Front. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Struyf, N.; Van der Maelen, E.; Hemdane, S.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Bread dough and baker’s yeast: An uplifting synergy. Compr. Rev. Food Sci. Food Saf. 2017, 16, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Sadiq, F.A.; Cai, Y.; Fan, D.; Zhang, H.; Zhao, J.; Chen, W. Identification of key aroma compounds in type I sourdough-based Chinese steamed bread: Application of untargeted metabolomics analysisp. Int. J. Mol. Sci. 2019, 20, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Sun, Y.; Sadiq, F.A.; Sakandar, H.A.; He, G. Evaluation of the effect of saccharomyces cerevisiae on fermentation characteristics and volatile compounds of sourdough. J. Food Sci. Technol. 2018, 55, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Erban, A.; Fehrle, I.; Martinez-Seidel, F.; Brigante, F.; Más, A.L.; Baroni, V.; Wunderlin, D.; Kopka, J. Discovery of food identity markers by metabolomics and machine learning technology. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocchetti, G.; Rizzi, C.; Cervini, M.; Rainero, G.; Bianchi, F.; Giuberti, G.; Lucini, L.; Simonato, B. Impact of grape pomace powder on the phenolic bioaccessibility and on in vitro starch digestibility of wheat based bread. Foods 2021, 10, 507. [Google Scholar] [CrossRef]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Bianchi, A.; Sgherri, C.; Quartacci, M.F.; De Leo, M.; Pistelli, L.; Palla, F.; et al. Bread fortified with cooked purple potato flour and citrus albedo: An evaluation of its compositional and sensorial properties. Foods 2021, 10, 942. [Google Scholar] [CrossRef]
- Nissen, L.; Bordoni, A.; Gianotti, A. Shift of volatile organic compounds (VOCs) in gluten-free hemp-enriched sourdough bread: A metabolomic approach. Nutrients 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinu, F.R. Metabolomics—The new frontier in food safety and quality research. Food Res. Int. 2015, 72, 80–81. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Green, E.; Adebiyi, J.A.; Ogundele, O.M.; Gbashi, S.; Adefisoye, M.A.; Oyeyinka, S.A.; Adebo, O.A. Metabolomic approaches for the determination of metabolites from pathogenic microorganisms: A review. Food Res. Int. 2021, 140, 110042. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wu, J.E.; He, Y.; Yang, H. Elucidating antimicrobial mechanism of nisin and grape seed extract against Listeria monocytogenes in broth and on shrimp through NMR-based metabolomics approach. Int. J. Food Microbiol. 2020, 319, 108494. [Google Scholar] [CrossRef]
- Dropler, M.; Kluger, B.; Bueschl, C.; Steiner, B.; Buerstmayr, H.; Lemmens, M.; Krska, R.; Adam, G.; Schuhmacher, R. Stable isotope-assisted plant metabolomics: Investigation of phenylalanine-related metabolic response in wheat upon treatment with the Fusarium virulence factor deoxynivalenol. Front. Plant Sci. 2019, 10, 1137. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnaiah, R.; Kushalappa, A.C. Metabolomics deciphers the host resistance mechanisms in wheat cultivar sumai-3, against trichothecene producing and non-producing isolates of Fusarium Graminearum. Plant Physiol. Biochem. 2014, 83, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Piacentini, K.C.; Tibola, C.S.; Santos, K.; Maria, G.S.; Scussel, V.M. Deoxynivalenol in the wheat milling process and wheat-based products and daily intake estimates for the southern Brazilian population. Food Control 2016, 62, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EC), No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; O. L. EU. L346; Commission of the European Communities: Brussels, Belgium, 2006; p. 5e24.
- European Commission. European Commission Regulation No1126/2007 amending Regulation No1881/2006 setting maximum levels for certain contaminants sin foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union L 2007, 255, 14. [Google Scholar]
- De Dominicis, E.; Commissati, I.; Suman, M. Targeted screening of pesticides, veterinary drugs and mycotoxins in bakery ingredients and food commodities by liquid chromatography-high-resolution single-stage orbitrap Mass Spectrometry. J. Mass Spectrom. 2012, 47, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Huang, J.; Ma, L.; Chen, Z.; Wang, F. Aflatoxin B1 and sterigmatocystin in wheat and wheat products from supermarkets in China. Food Addit. Contam. Part B Surveill. 2018, 11, 9–14. [Google Scholar] [CrossRef]
- Syed, A.A.; Nazir, S.; Adnan, M.; Azad, Z.R.A.A. UPLC-MS: An emerging novel technology and its application in food safety; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Mastovska, K.; Dorweiler, K.J.; Lehotay, S.J.; Wegscheid, J.S.; Szpylka, K.A. Pesticide multiresidue analysis in cereal grains using modified QuEChERS method combined with automated direct sample introduction GC-TOFMS and UPLC-MS/MS techniques. J. Agric. Food Chem. 2010, 58, 5959–5972. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Mastovska, K.; Dorweiler, K.J.; Leepipatpiboon, N. Extension of the QuEChERS method for pesticide residues in cereals to flaxseeds, peanuts, and doughs. J. Agric. Food Chem. 2010, 58, 5950–5958. [Google Scholar] [CrossRef]
- Lacina, O.; Zachariasova, M.; Urbanova, J.; Vaclavikova, M.; Cajka, T.; Hajslova, J. Critical assessment of extraction methods for the simultaneous determination of pesticide residues and mycotoxins in fruits, cereals, spices and oil seeds employing ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, L.V.; Fraser, P.; Stewart, D. Metabolomics: A second-generation platform for crop and food analysis. Bioanalysis 2011, 3, 1143–1159. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.M.; Hawkins, N.D.; Ward, J.L.; Lovegrove, A.; Napier, J.A.; Shewry, P.R.; Beale, M.H. A Metabolomic study of substantial equivalence of field-grown genetically modified wheat. Plant Biotechnol. J. 2006, 4, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Shewry, P.R. Future prospects for the analysis of bioactive components in cereal grain. Cereal Foods World 2010, 55, 71–75. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păucean, A.; Mureșan, V.; Maria-Man, S.; Chiș, M.S.; Mureșan, A.E.; Șerban, L.R.; Pop, A.; Muste, S. Metabolomics as a Tool to Elucidate the Sensory, Nutritional and Safety Quality of Wheat Bread—A Review. Int. J. Mol. Sci. 2021, 22, 8945. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168945
Păucean A, Mureșan V, Maria-Man S, Chiș MS, Mureșan AE, Șerban LR, Pop A, Muste S. Metabolomics as a Tool to Elucidate the Sensory, Nutritional and Safety Quality of Wheat Bread—A Review. International Journal of Molecular Sciences. 2021; 22(16):8945. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168945
Chicago/Turabian StylePăucean, Adriana, Vlad Mureșan, Simona Maria-Man, Maria Simona Chiș, Andruța Elena Mureșan, Larisa Rebeca Șerban, Anamaria Pop, and Sevastița Muste. 2021. "Metabolomics as a Tool to Elucidate the Sensory, Nutritional and Safety Quality of Wheat Bread—A Review" International Journal of Molecular Sciences 22, no. 16: 8945. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168945








