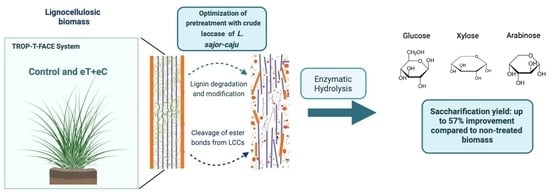
Enzymatic Pretreatment with Laccases from Lentinus sajor-caju Induces Structural Modification in Lignin and Enhances the Digestibility of Tropical Forage Grass (Panicum maximum) Grown under Future Climate Conditions
Abstract
:1. Introduction
2. Results
2.1. Evaluation Enzymes Produced by L. sajor caju in Orange Waste Solid-State Cultures
2.2. Laccase Activity Optimal Parameters
2.3. Optimization of Crude Laccase Pretreatment by Central Composite Design (CCD) and Response Surface Analysis
2.4. Chemical Composition
2.5. Validation of Crude Laccase Pretreatment at Determined Optimal Conditions
2.6. Hydrolysis Studies of Crude Laccase Pretreated P. maximum Biomass
2.6.1. At Lower Protein Load
2.6.2. At Different Solid Load
2.7. Simon’s Staning
2.8. GC-MS Analysis of Lignin Degradation Products
2.9. Physical Characterization of Optimized Laccase Pretreated Biomass
2.9.1. CLSM and SEM Analysis
2.9.2. FTIR Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents and Raw Materials
4.2. Growth of Panicum maximum under Simulate Future Climate Conditions
4.3. Fungal Strain, Culture Conditions, and Enzyme Extraction
4.4. Enzyme Assays
4.5. Protein Content Determination, Electrophoresis, and Zymogram Analysis
4.6. Purification and Identification of Laccase by Mass Spectrometer Analyses
4.7. Effect of the Temperature and pH on Laccase Activity and Stability
4.8. Optimization of Laccase Pretreatment
4.9. Chemical Characterization
4.10. Enzymatic Hydrolysis Studies of Crude Laccase Pretreated Biomass
4.11. Determination of Monosaccharides
4.12. Simon’s Stain
4.13. GC-MS Analysis of Lignin Degradatory Products
4.14. Physical Characterization of Optimized Laccase Pretreated Biomass
4.14.1. SEM and FTIR Analysis
4.14.2. Confocal Microscopy
4.15. Statistics and Numerical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.K.; Kalia, V.C.; Lee, J.K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products, Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Contato, A.; de Oliveira, T.; Aranha, G.; de Freitas, E.; Vici, A.; Nogueira, K.; de Lucas, R.; Scarcella, A.; Buckeridge, M.; Silva, R.; et al. Prospection of fungal lignocellulolytic enzymes produced from jatoba (Hymenaea courbaril) and tamarind (Tamarindus indica) seeds: Scaling for bioreactor and saccharification profile of sugarcane bagasse. Microorganisms 2021, 9, 533. [Google Scholar] [CrossRef]
- Chundawat, S.P.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Patel, S.K.S.; Gupta, R.K.; Otari, S.; Gao, H.; Lee, J.; Zhang, L. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol. J. 2019, 14, e1800468. [Google Scholar] [CrossRef] [PubMed]
- Santo, M.C.E.; Fockink, D.H.; Pellegrini, V.O.; Guimaraes, F.E.; Deazevedo, E.R.; Ramos, L.P.; Polikarpov, I. Physical techniques shed light on the differences in sugarcane bagasse structure subjected to steam explosion pretreatments at equivalent combined severity factors. Ind. Crop. Prod. 2020, 158, 113003. [Google Scholar] [CrossRef]
- Fillat, U.; Ibarra, D.; Eugenio, M.E.; Moreno, A.D.; Tomás-Pejó, E.; Martín-Sampedro, R. Laccases as a potential tool for the efficient conversion of lignocellulosic biomass: A review. Fermentation 2017, 3, 17. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Banerjee, R.; Chintagunta, A.D.; Ray, S. Laccase mediated delignification of pineapple leaf waste: An ecofriendly sustainable attempt towards valorization. BMC Chem. 2019, 13, 58. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase production from lignocellulosic agricultural wastes by solid-state fermentation: A review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef] [Green Version]
- Munk, L.; Sitarz, A.K.; Kalyani, D.; Mikkelsen, J.D.; Meyer, A.S. Can laccases catalyze bond cleavage in lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef]
- Rencoret, J.; Pereira, A.; del Río, J.C.; Martínez, T.; Gutiérrez, A. Delignification and saccharification enhancement of sugarcane byproducts by a laccase-based pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 7145–7154. [Google Scholar] [CrossRef]
- Rajeswari, G.; Jacob, S. Deciphering the aloe vera leaf rind as potent feedstock for bioethanol through enzymatic delignification and its enhanced saccharification. Ind. Crop. Prod. 2019, 143, 111876. [Google Scholar] [CrossRef]
- Zhang, R.; Lv, C.; Lu, J. Studies on laccase mediated conversion of lignin from ginseng residues for the production of sugars. Bioresour. Technol. 2020, 317, 123945. [Google Scholar] [CrossRef]
- Giacobbe, S.; Pezzella, C.; Lettera, V.; Sannia, G.; Piscitelli, A. Laccase pretreatment for agrofood wastes valorization. Bioresour. Technol. 2018, 265, 59–65. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Sosa, L.L.; Jozami, E.; Oakley, L.J.; Montero, G.; Ferreras, L.A.; Venturi, G.; Feldman, S.R. Using C4 perennial rangeland grasses for bioenergy. Biomass Bioenergy 2019, 128, 105299. [Google Scholar] [CrossRef]
- Lima, M.A.; Gomez, L.D.; Steele-King, C.G.; Simister, R.; Bernardinelli, O.D.; Carvalho, M.A.; Rezende, C.A.; Labate, C.A.; De Azevêdo, E.R.; McQueen-Mason, S.J.; et al. Evaluating the composition and processing potential of novel sources of Brazilian biomass for sustainable biorenewables production. Biotechnol. Biofuels 2014, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Reilly, E.C.; Jungers, J.M.; Chen, J.; Smith, T.M. Climate benefits of increasing plant diversity in perennial bioenergy crops. One Earth 2019, 1, 434–445. [Google Scholar] [CrossRef]
- Sage, R.F.; Way, D.A.; Kubien, D.S. Rubisco, Rubisco activase, and global climate change. J. Exp. Bot. 2008, 59, 1581–1595. [Google Scholar] [CrossRef] [Green Version]
- Daniel, R.M.; Danson, M.J.; Eisenthal, R.; Lee, C.K.; Peterson, M.E. The effect of temperature on enzyme activity: New insights and their implications. Extremophiles 2007, 12, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Shi, W.; Sun, W.; Li, X.; Wang, F.; Zhao, J.; Qu, Y. Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol. Biofuels 2014, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Chandra, R.; Saddler, J. Alkali–oxygen treatment prior to the mechanical pulping of hardwood enhances enzymatic hydrolysis and carbohydrate recovery through selective lignin modification. Sustain. Energy Fuels 2018, 3, 227–236. [Google Scholar] [CrossRef]
- Du, J.; Cao, Y.; Liu, G.; Zhao, J.; Li, X.; Qu, Y. Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour. Technol. 2017, 229, 88–95. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R.P.; Lee, J.-S.; Lu, C.; Saddler, J.N. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Avanthi, A.; Banerjee, R. A strategic laccase mediated lignin degradation of lignocellulosic feedstocks for ethanol production. Ind. Crop. Prod. 2016, 92, 174–185. [Google Scholar] [CrossRef]
- Sherpa, K.; Ghangrekar, M.M.; Banerjee, R. A green and sustainable approach on statistical optimization of laccase mediated delignification of sugarcane tops for enhanced saccharification. J. Environ. Manag. 2018, 217, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.M.O.; Vici, A.C.; Messias, J.M.; Heinen, P.R.; Pinheiro, V.E.; Rechia, C.G.V.; Buckeridge, M.S.; Polizeli, M.L.T.M. Increased Malbranchea pulchella β-glucosidase production and its application in agroindustrial residue hydrolysis: A research based on experimental designs. Biotechnol. Rep. 2021, 30, e00618. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, A.A.; Tyhoda, L.; Brienzo, M. Sugarcane biomass conversion influenced by lignin. Biofuels Bioprod. Biorefining 2019, 14, 469–480. [Google Scholar] [CrossRef]
- Takada, M.; Chandra, R.P.; Saddler, J.N. The influence of lignin migration and relocation during steam pretreatment on the enzymatic hydrolysis of softwood and corn stover biomass substrates. Biotechnol. Bioeng. 2019, 116, 2864–2873. [Google Scholar] [CrossRef]
- Brar, K.K.; Santo, M.E.; Pellegrini, V.O.; Deazevedo, E.R.; Guimaraes, F.E.; Polikarpov, I.; Chadha, B.S. Enhanced hydrolysis of hydrothermally and autohydrolytically treated sugarcane bagasse and understanding the structural changes leading to improved saccharification. Biomass Bioenergy 2020, 139, 105639. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Abualreesh, M.; Ahmed, K.; Razak, A.A. Enzymatic delignification of biomass for enhanced fermentable sugars production. Energy Technol. 2015, 3, 121–127. [Google Scholar] [CrossRef]
- Rencoret, J.; Pereira, A.; Marques, G.; del Río, J.C.; Martínez, T.; Gutiérrez, A. A commercial laccase-mediator system to delignify and improve saccharification of the fast-growing Paulownia fortunei (Seem.) Hemsl. Holzforschung 2018, 73, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, A.; Rencoret, J.; Cadena, E.M.; Rico, A.; Barth, D.; del Río, J.C.; Martínez, A.T. Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour. Technol. 2012, 119, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanirun, Z.; Bahrin, E.K.; Lai-Yee, P.; Hassan, M.A.; Abd-Aziz, S. Enhancement of fermentable sugars production from oil palm empty fruit bunch by ligninolytic enzymes mediator system. Int. Biodeterior. Biodegrad. 2015, 105, 13–20. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass—A review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef]
- Rencoret, J.; Pereira, A.; del Río, J.C.; Martínez, A.T.; Gutiérrez, A. Laccase-Mediator Pretreatment of wheat straw degrades lignin and improves saccharification. BioEnergy Res. 2016, 9, 917–930. [Google Scholar] [CrossRef] [Green Version]
- van der Zwan, T.; Chandra, R.P.; Saddler, J.N. Laccase-mediated hydrophilization of lignin decreases unproductive enzyme binding but limits subsequent enzymatic hydrolysis at high substrate concentrations. Bioresour. Technol. 2019, 292, 121999. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.N.; Khatri, V.; Contin, D.; Oliveira, T.; Contato, A.; dos Santos, W.R.P.; Martínez, C.; Saddler, J.; Polizeli, M.L.T. Climate change affects cell wall structure and hydrolytic performance of a tropical forage grass as an energy crop. Prepr. Author. 2020. [Google Scholar] [CrossRef]
- Castoldi, R.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Corrêa, R.C.G.; Peralta, R.; de Fátima Peralta MunizMoreira, R.; Polizeli, M.L.T.M.; de Souza, C.G.M.; Peralta, R.M. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar] [CrossRef]
- Santo, M.E.; Rezende, C.A.; Bernardinelli, O.D.; Pereira, N.; Curvelo, A.A.; Deazevedo, E.R.; Guimarães, F.E.; Polikarpov, I. Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crop. Prod. 2018, 113, 64–74. [Google Scholar] [CrossRef]
- Auxenfans, T.; Terryn, C.; Paës, G. Seeing biomass recalcitrance through fluorescence. Sci. Rep. 2017, 7, 8838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajak, R.C.; Banerjee, R. Enzyme mediated biomass pretreatment and hydrolysis: A biotechnological venture towards bioethanol production. RSC Adv. 2016, 6, 61301–61311. [Google Scholar] [CrossRef]
- Habermann, E.; Martin, J.A.B.S.; Contin, D.R.; Bossan, V.P.; Barboza, A.; Braga, M.R.; Groppo, M.; Martinez, C.A. Increasing atmospheric CO2 and canopy temperature induces anatomical and physiological changes in leaves of the C4 forage species Panicum maximum. PLoS ONE 2019, 14, e0212506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Assis Prado, C.H.B.; De Camargo-Bortolin, L.H.G.; Castro, E.; Martínez, C.A. Leaf dynamics of Panicum maximum under future climatic changes. PLoS ONE 2016, 11, e0149620. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014 Synthesis Report Summary Chapter for Policymakers; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Freitas, E.N.; Bubna, G.A.; Brugnari, T.; Kato, C.G.; Nolli, M.; Rauen, T.G.; de FátimaPeralta Muniz Moreira, R.; Peralta, R.; Bracht, A.; de Souza, C.G.; et al. Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem. Eng. J. 2017, 330, 1361–1369. [Google Scholar] [CrossRef]
- Vogel, H. A convenient growth medium for Neurospora (Medium N). Microb. Genet. Bull. 1956, 14, 42–43. [Google Scholar]
- Starcher, B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal. Biochem. 2001, 292, 125–129. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis. Biotechnol. Bioeng. 2010, 105, 871–879. [Google Scholar] [CrossRef]
- Sluiter, D.; Hames, A.; Ruiz, B.; Scarlata, R.; Sluiter, C.; Templeton, J. Determination of Ash in Biomass; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Chandra, R.P.; Saddler, J.N. Use of the Simons’ staining technique to assess cellulose accessibility in pretreated substrates. Ind. Biotechnol. 2012, 8, 230–237. [Google Scholar] [CrossRef]
- Tahir, A.A.; Barnoh, N.F.M.; Yusof, N.; Said, N.N.M.; Utsumi, M.; Yen, A.M.; Hashim, H.; Noor, M.J.M.M.; Akhir, F.N.M.; Mohamad, S.E.; et al. Microbial diversity in decaying oil palm empty fruit bunches (opefb) and isolation of lignin-degrading bacteria from a tropical environment. Microbes Environ. 2019, 34, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Component (%) | Non-Treated | LacPT c | ||
|---|---|---|---|---|
| C a | eT+eC b | C | eT+eC | |
| Anhydrousglucose | 26.2 ± 0.5 a | 29.7 ± 0.1 b | 30.4 ± 0.2 b | 33.4 ± 0.3 c |
| Anhydrousxylose | 17 ± 0.3 a | 17.3 ± 0.2 a | 16.3 ± 0.2 a | 15.7 ± 0.4 b |
| Anhydrousarabinose | 2.4 ± 0.12 a | 2.3 ± 0.15 a | 2.4 ± 0.12 a | 2.2 ± 0.0 a |
| Anhydrousgalactose | 1.15 ± 0.05 a | 1.12 ± 0.03 a | 1.08 ± 0.05 a | 1.04 ± 0.07 a |
| Lignin | 26.3 ± 0.8 a | 29.8 ± 0.9 b | 21.2 ± 1.2 c | 20.5 ± 1.4 c |
| Ash | 10.8 ± 0.2 a | 11.5 ± 0.4 a | 9.7 ± 0.4a | 9.2 ± 0.6 a |
| Solid yield | N/A | N/A | 73.4 | 78.2 |
| Glucan recovery | N/A | N/A | 92.5 | 90.8 |
| Lignin removal | N/A | N/A | 40.8 | 46.2 |
| Biomass | Adsorption of Direct Orange (mg g−1) | |
|---|---|---|
| C a | eT+eC b | |
| Non-treated | 63.7 ± 1.4 a | 76.4 ± 1.7 a |
| LacPT c | 79.9 ± 3.0 b | 84.7 ± 2.9 b |
| No | Intermediate Compounds | Control (C) | eT+eC | Retention Time (min) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 6 h | 1 h | 4 h | 6 h | |||
| 1 | 2,4-imethyl-benzaldehyde | + | + | – | – | + | + | 8.60 |
| 2 | 2-ethylhexyl ester,3-phenylpropionic acid | – | – | – | + | + | – | 8.65 |
| 3 | 4,6-dimethyldodecane | – | – | – | – | – | + | 8.81 |
| 4 | 2-isopropyl-5-methylhexyl acetate | – | – | – | – | – | + | 9.59 |
| 5 | 2,3-dimethyldodecane | – | + | + | – | – | – | 12.33 |
| 6 | 2-isopropyl-5-methyl-1-heptanol | – | + | + | – | – | – | 13.39 |
| 7 | 5,5,8a-trimethyl-3,5,6,7,8,8a-hexahydro-2H-chromene | – | + | + | – | + | + | 16.65 |
| 8 | 2,5-di-tert-butyl-p-quinone | – | – | + | – | + | + | 17.84 |
| 9 | 5-methyl-indole | + | + | + | + | + | + | 22.57 |
| 10 | 2-hydroxy-1,3-propanediyl ester-cctadecanoic acid | – | + | – | – | + | – | 22.76 |
| Laccase Source | Condition for Maximal Lignin Degradation | Delignification (%) | Reference |
|---|---|---|---|
| Commercial from Trametes versicolor | Use of HBT (hydroxybenzotriazole) as a mediator | 9 | [31] |
| Commercial from Myceliophthora thermophila | Laccase-mediated pretreatment with methyl syringate (MeS) combined with alkaline extraction with NaOH | 24 | [32] |
| Commercial Trametes villosa laccase | Laccase-mediated pretreatment (HBT) combined with alkaline extraction | 32 | [33] |
| Crude laccase from Pycnoporus sanguineus | Laccase-mediated pretreatment (HBT and ABTS) combined with alkaline extraction | up to 8 | [34] |
| Crude white-rot fungi locally isolated | Laccase load (922 U g−1) | 76 | [12] |
| Crude laccase from Lentinus sajor-caju | Laccase-mediated pretreatment (Vanillin) | up to 46.2 | This work |
| Independent Variables | Sugar Yield for C Group (g L−1) d | Sugar Yields for eT+eC Group (g L−1) e | |||||
|---|---|---|---|---|---|---|---|
| Run Order | Laccase (X1) a | Mediator (X2) b | Temperature (X3) c | Experimental | Predicted | Experimental | Predicted |
| 1 | 150(−1) | 0.34(−1) | 45(−1) | 8.46 | 9.03 | 8.34 | 8.95 |
| 2 | 300(+1) | 0.34(−1) | 45(−1) | 9.94 | 10.33 | 11.2 | 11.18 |
| 3 | 150(−1) | 1.34(+1) | 45(−1) | 8.3 | 8.11 | 8.21 | 8.56 |
| 4 | 300(+1) | 1.34(+1) | 45(−1) | 9.13 | 9.41 | 9.88 | 9.85 |
| 5 | 150(−1) | 0.34(−1) | 55(+1) | 9.91 | 9.76 | 9.54 | 9.8 |
| 6 | 300(+1) | 0.34(−1) | 55(+1) | 10.77 | 11.06 | 11.56 | 12.03 |
| 7 | 150(−1) | 1.34(+1) | 55(+1) | 8.33 | 8.84 | 9.42 | 9.41 |
| 8 | 300(+1) | 1.34(+1) | 55(+1) | 9.48 | 10.14 | 10.77 | 10.71 |
| 9 | 100(−1.68) | 0.84(0) | 50 (0) | 8.69 | 8.53 | 9.75 | 9.22 |
| 10 | 350(+1.68) | 0.84(0) | 50(0) | 11.39 | 10.71 | 12.20 | 12.17 |
| 11 | 225(0) | 0(−1.68) | 50(0) | 10.74 | 10.38 | 11.51 | 10.91 |
| 12 | 225(0) | 1.68(+1.68) | 50(0) | 9.32 | 8.84 | 9.43 | 9.46 |
| 13 | 225(0) | 0.84(0) | 41.6(−1.68) | 9.51 | 9.17 | 9.23 | 8.87 |
| 14 | 225(0) | 0.84(0) | 58.4(+1.68) | 10.89 | 10.39 | 10.51 | 10.31 |
| 15 | 225(0) | 0.84(0) | 50(0) | 11.03 | 10.98 | 11.84 | 11.67 |
| 16 | 225(0) | 0.84(0) | 50(0) | 10.98 | 10.98 | 11.59 | 11.67 |
| 17 | 225(0) | 0.84(0) | 50(0) | 10.52 | 10.98 | 11.40 | 11.67 |
| 18 | 225(0) | 0.84(0) | 50(0) | 11.25 | 10.98 | 11.75 | 11.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, E.N.d.; Alnoch, R.C.; Contato, A.G.; Nogueira, K.M.V.; Crevelin, E.J.; Moraes, L.A.B.d.; Silva, R.N.; Martínez, C.A.; Polizeli, M.d.L.T.M. Enzymatic Pretreatment with Laccases from Lentinus sajor-caju Induces Structural Modification in Lignin and Enhances the Digestibility of Tropical Forage Grass (Panicum maximum) Grown under Future Climate Conditions. Int. J. Mol. Sci. 2021, 22, 9445. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22179445
Freitas ENd, Alnoch RC, Contato AG, Nogueira KMV, Crevelin EJ, Moraes LABd, Silva RN, Martínez CA, Polizeli MdLTM. Enzymatic Pretreatment with Laccases from Lentinus sajor-caju Induces Structural Modification in Lignin and Enhances the Digestibility of Tropical Forage Grass (Panicum maximum) Grown under Future Climate Conditions. International Journal of Molecular Sciences. 2021; 22(17):9445. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22179445
Chicago/Turabian StyleFreitas, Emanuelle Neiverth de, Robson Carlos Alnoch, Alex Graça Contato, Karoline Maria V. Nogueira, Eduardo José Crevelin, Luiz Alberto Beraldo de Moraes, Roberto Nascimento Silva, Carlos Alberto Martínez, and Maria de Lourdes T. M. Polizeli. 2021. "Enzymatic Pretreatment with Laccases from Lentinus sajor-caju Induces Structural Modification in Lignin and Enhances the Digestibility of Tropical Forage Grass (Panicum maximum) Grown under Future Climate Conditions" International Journal of Molecular Sciences 22, no. 17: 9445. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22179445