Production of a Potentially Probiotic Product for Animal Feed and Evaluation of Some of Its Probiotic Properties
Abstract
:1. Introduction
2. Results and Discussion
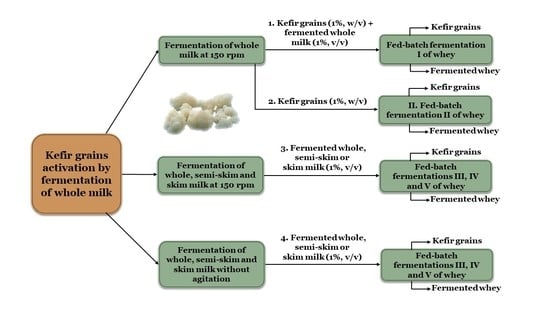
2.1. Kinetics of Whey Cultures Inoculated with Different Inocula
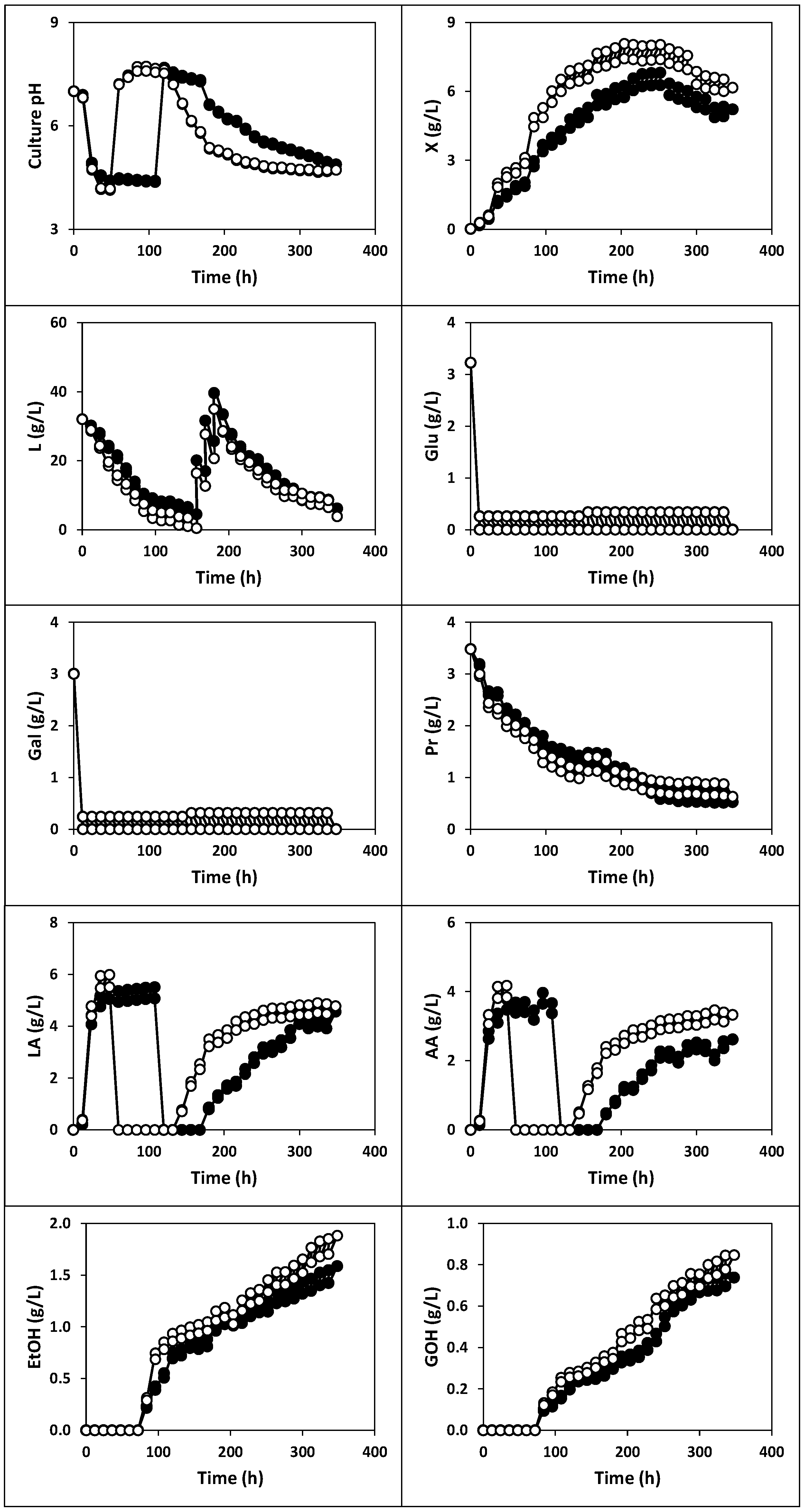
2.1.1. Fermentation of Whey with Kefir Grains and Fermented Whole Milk (Fermentation I)
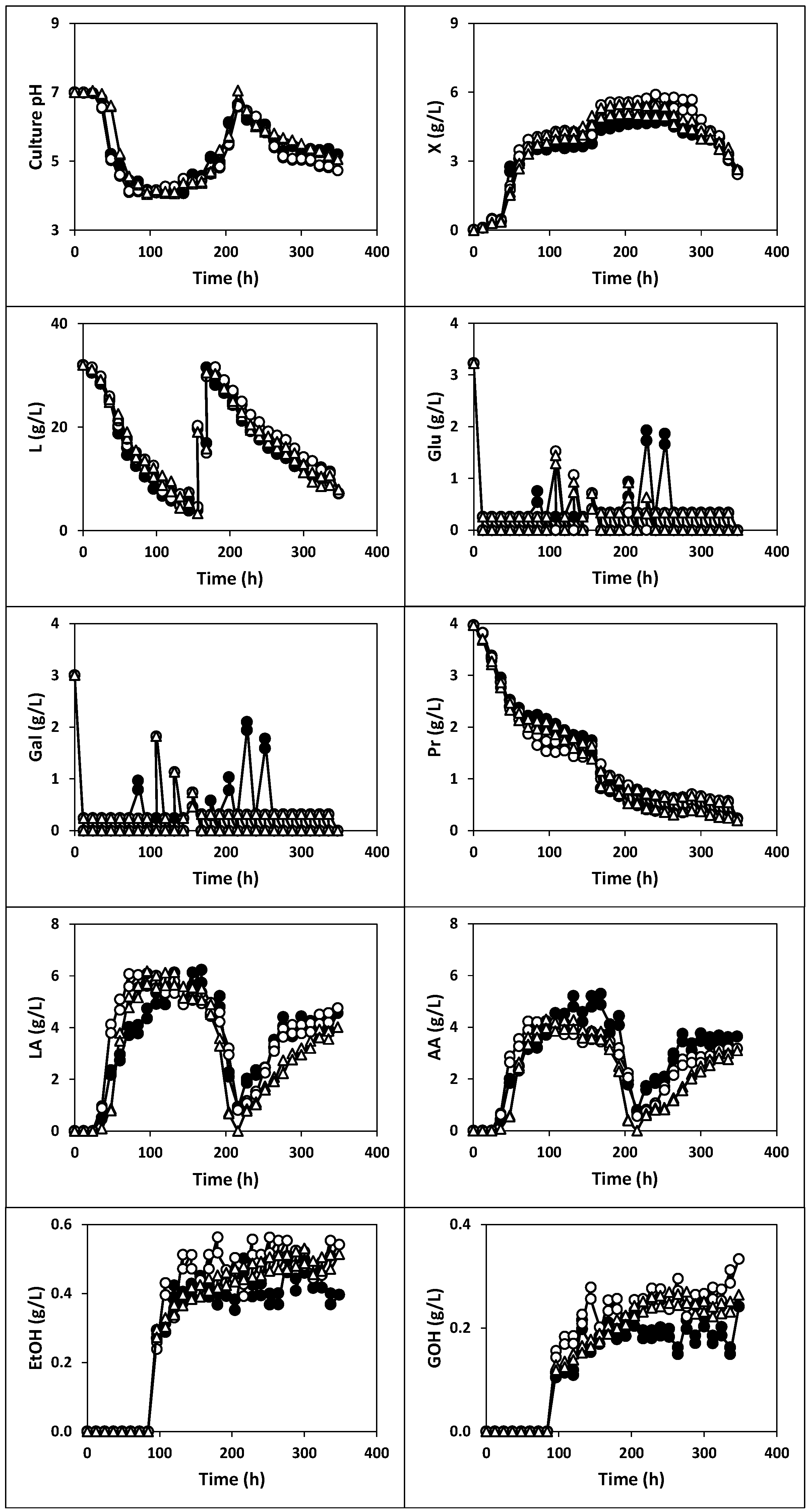
2.1.2. Fermentation of Whey with Kefir Grains (Fermentation II)
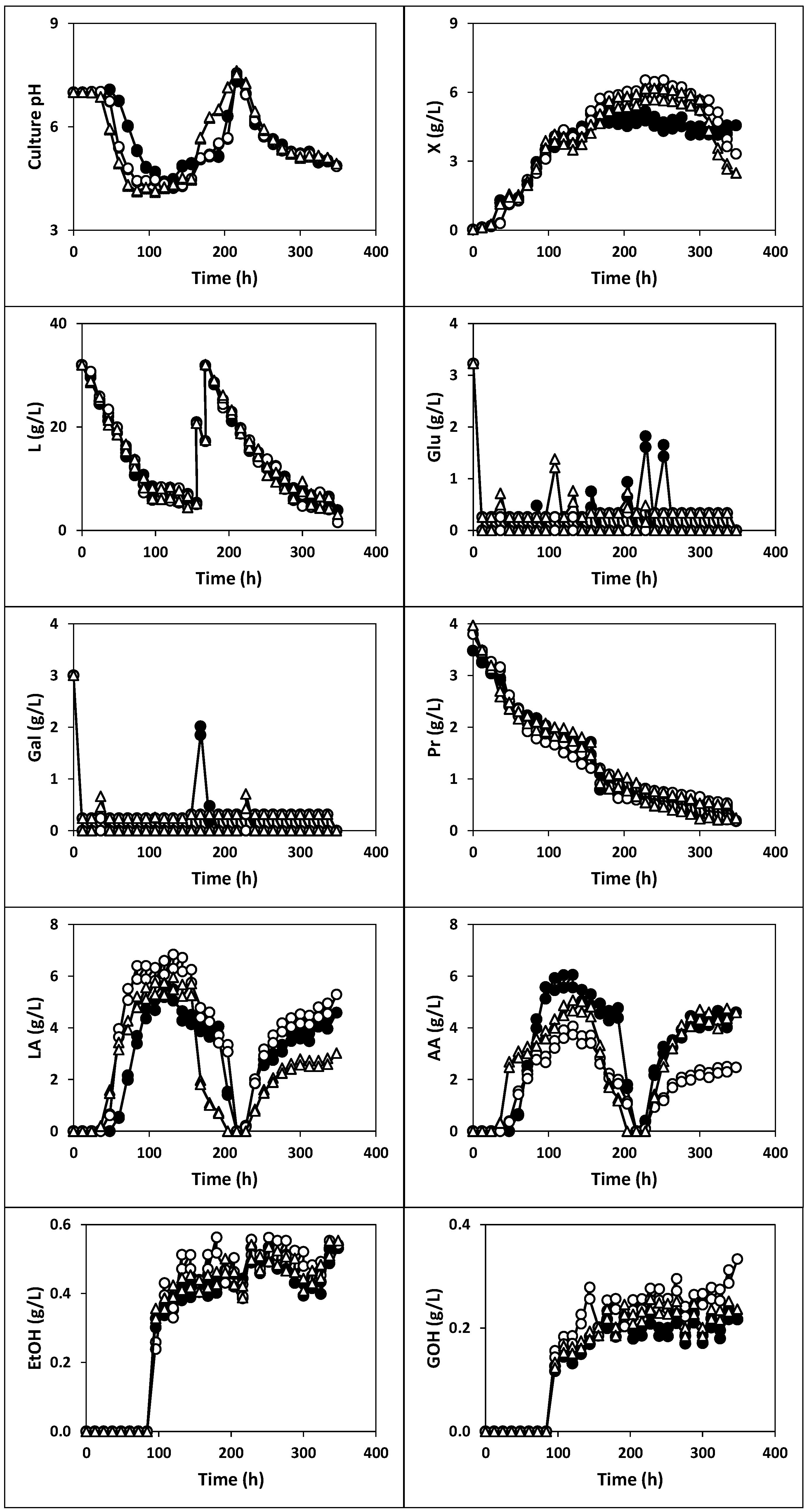
2.1.3. Fermentation of Whey with Fermented Milk (Fermentations III, IV and V)
2.2. Evaluation of Some Probiotic Properties of the Kefir Cultures
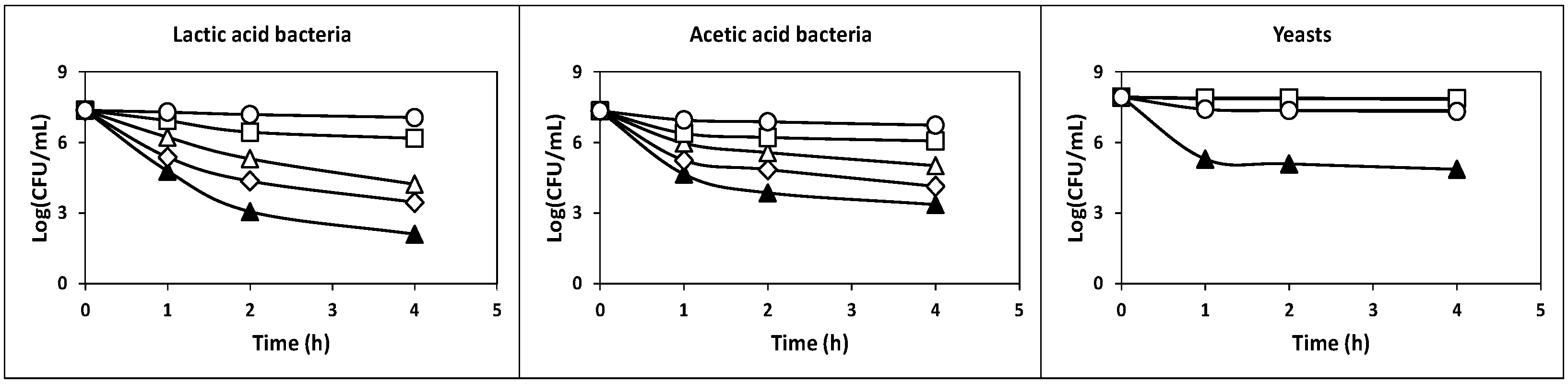
2.2.1. Survival at Acidic pH Values
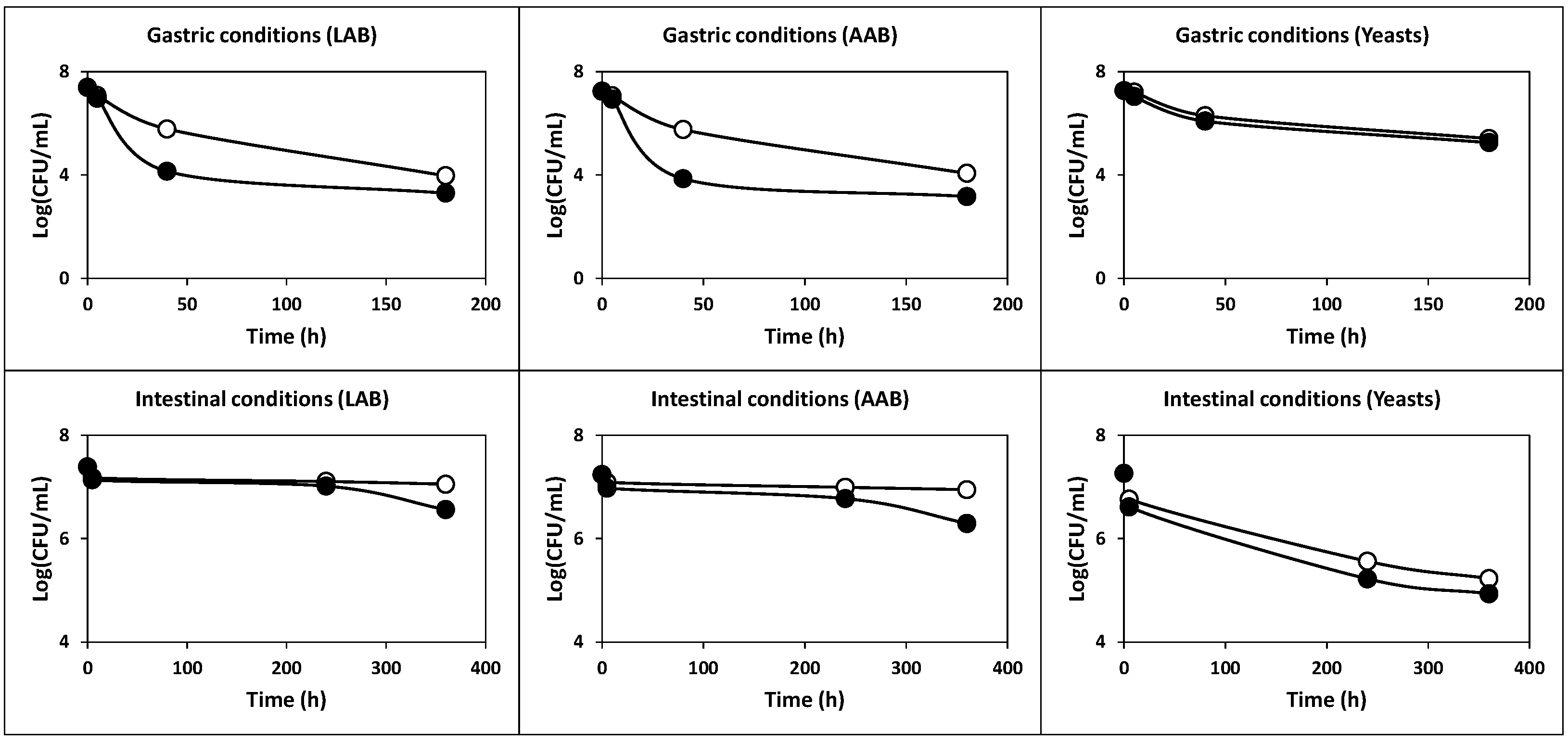
2.2.2. Survival under Simulated Gastric and Intestinal Transit
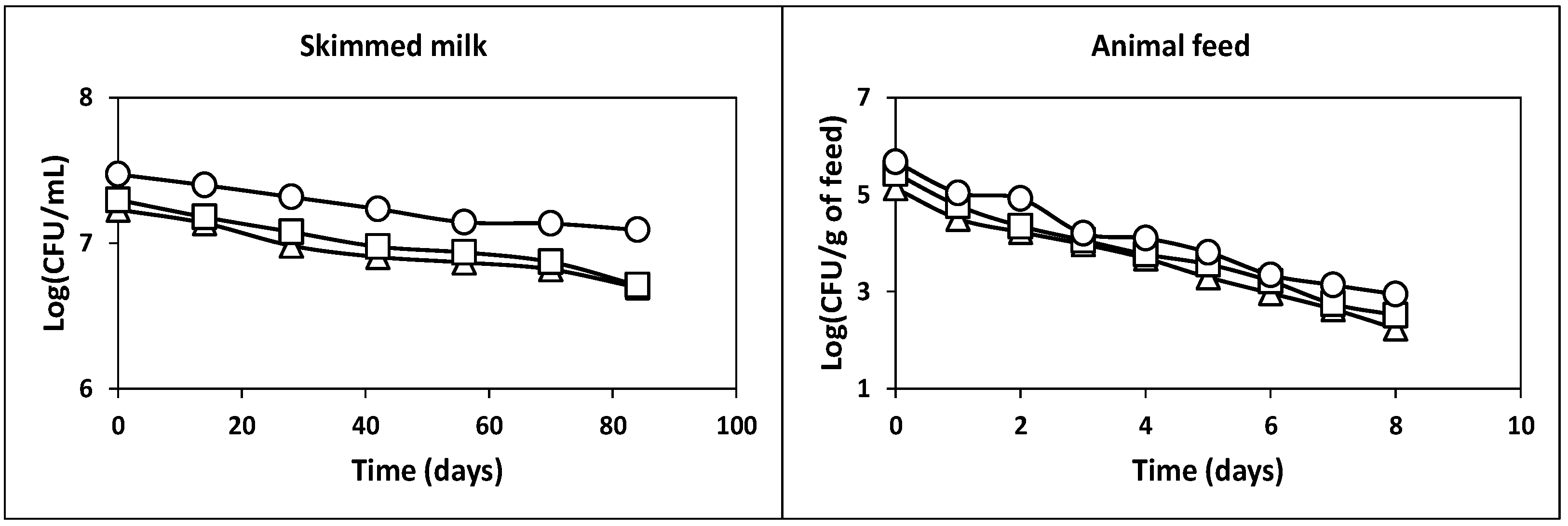
2.2.3. Survival after Freezing in Skim Milk at −20 °C
2.2.4. Survival in Commercial Piglet Feed at Room Temperature
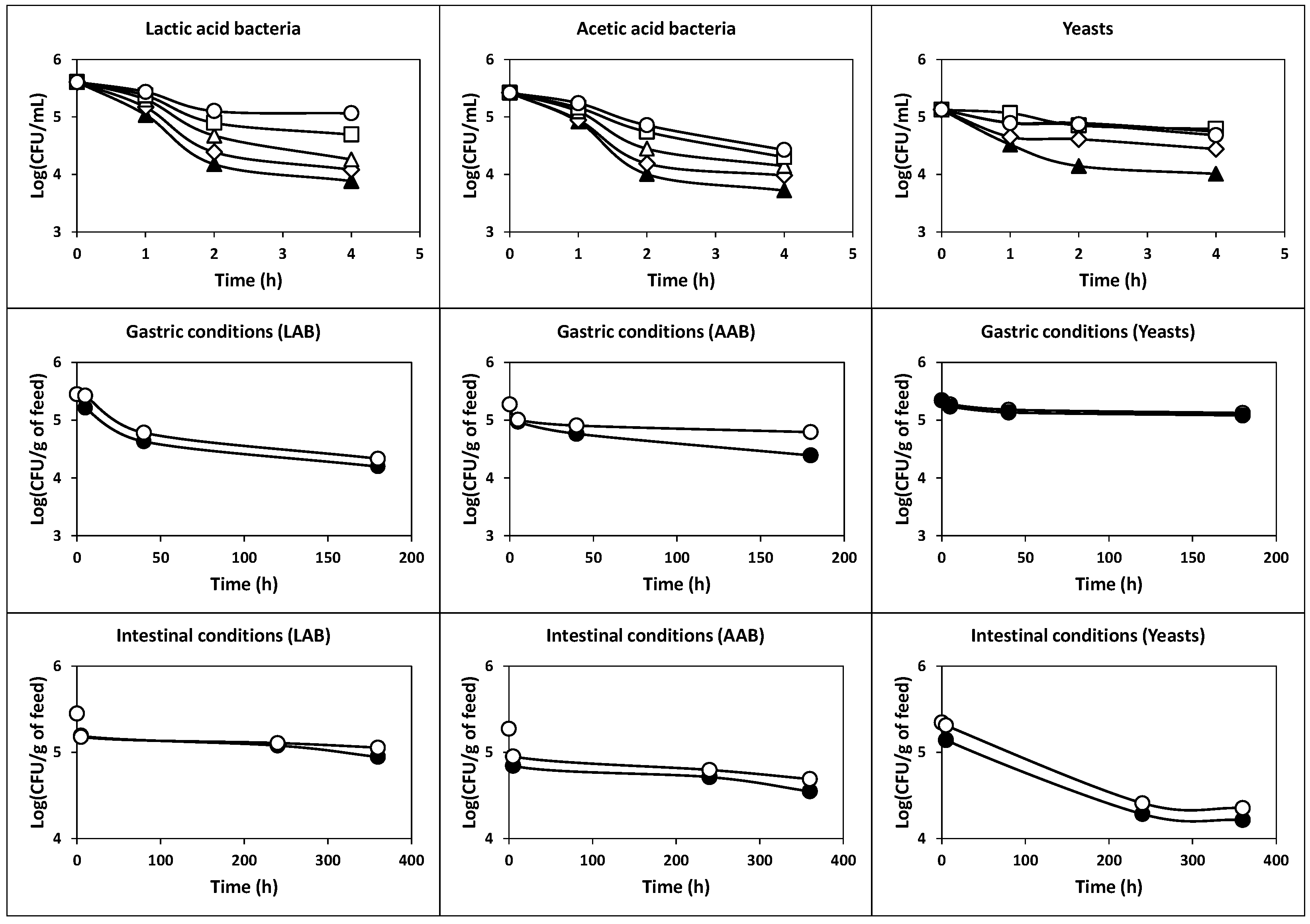
2.2.5. Survival of Cells Protected with Skim Milk and Piglet Feed at Low pH Values and Simulated Gastrointestinal Conditions
3. Materials and Methods
3.1. Inoculum Preparation, Culture Media and Fermentation Conditions
3.2. Analytical Determinations
3.3. Antibacterial Activity Quantification
3.4. Determination of Total Viable Counts
3.5. Tolerance to Acidic pH
3.6. Preparation of Simulated Gastric and Pancreatic Juices
3.7. Tolerance to Simulated Gastrointestinal Juices
3.8. Survival of the Microorganisms from Kefir Grains during Freezing Storage
3.9. Survival of Microorganisms from Kefir Grains in the Piglet Feed stored at Room Temperature
3.10. Effects of Skim Milk and Feed on the Tolerance of Cells to Acidic pH and Gastrointestinal Conditions
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Cao, M.; Zhou, J.; Yan, S.; Peng, J.; Yu, Z.; Zhang, A.; Wu, J.; Yan, X.; Zhao, J. Lactobacillus casei T1 from kurut against Helicobacter pylori-induced inflammation and the gut microbial disorder. J. Funct. Foods 2021, 85, 104611. [Google Scholar] [CrossRef]
- Fajardo, P.; Rodríguez, I.; Pastrana, L.; Guerra, N.P. Production of a potentially probiotic culture of Lactobacillus casei subsp. casei CECT 4043 in whey. Int. Dairy J. 2008, 18, 1057–1065. [Google Scholar]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- Guerra, N.P.; Bernárdez, P.F.; Méndez, J.; Cachaldora, P.; Castro, L.P. Production of four potentially probiotic lactic acid bacteria and their evaluation as feed additives for weaned piglets. Anim. Feed. Sci. Technol. 2007, 134, 89–107. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0282-tab-03-ref-19-joint-faowho-vol219.pdf (accessed on 10 July 2021).
- Pedroso, A.A.; Hurley-Bacon, A.L.; Zedek, A.S.; Kwan, T.W.; Jordan, A.P.O.; Avellaneda, G.; Hofacre, C.L.; Oakley, B.B.; Collett, S.R.; Maurer, J.J.; et al. Can Probiotics Improve the Environmental Microbiome and Resistome of Commercial Poultry Production? Int. J. Environ. Res. Public Health 2013, 10, 4534–4559. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xie, Q.M.; Ji, J.; Yang, W.H.; Wu, Y.B.; Li, C.; Ma, J.Y.; Bi, Y.Z. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult. Sci. 2012, 91, 2755–2760. [Google Scholar] [CrossRef] [PubMed]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002, 28, 1–6. [Google Scholar] [CrossRef]
- Arslan, S. A review: Chemical, microbiological and nutritional characteristics of kefir. CyTA—J. Food 2015, 13, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Farnworth, E.R. Kefir a complex probiotic. In Food Science and Technology Bulletin: Functional Foods, 1st ed.; Gibson, G.R., Ed.; International Food Information Service Publishing: Reading, UK, 2006; Volume 2, pp. 1–17. [Google Scholar]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Mahmoud, N.S.; Rushton, D.G.; Arab, F. Potential Environmental and Health Impacts of High Land Application of Cheese Whey. Am. J. Agric. Biol. Sci. 2007, 2, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadis, I.; Boskou, D.; Kanellaki, M.; Kiosseoglou, V.; Koutinas, A.A. Whey Liquid Waste of the Dairy Industry as Raw Material for Potable Alcohol Production by Kefir Granules. J. Agric. Food Chem. 2002, 50, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, I.; Paraskevopoulou, A.; Blekas, G.; Kiosseoglou, V. Development of a novel whey beverage by fermentation with kefir granules. Effect of various treatments. Biotechnol. Prog. 2004, 20, 1091–1095. [Google Scholar] [CrossRef]
- Magalhães, K.T.; Dragone, G.; de Melo Pereira, G.V.; Oliveira, J.M.; Domingues, L.; Teixeira, J.A.; e Silva, J.B.A.; Schwan, R.F. Comparative study of the biochemical changes and volatile compound formations during the production of novel whey-based kefir beverages and traditional milk kefir. Food Chem. 2011, 126, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.L.; Abraham, A.G.; de Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef]
- Costas, M.; Alonso, E.; Outeiriño, D.; Guerra, N.P. Production of a highly concentrated probiotic culture of Lactococcus lactis CECT 539 containing high amounts of nisin. 3 Biotech 2018, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Costas, M.; Alonso, E.; Bazán, D.L.; Bendaña, R.J.; Guerra, N.P. Batch and fed-batch production of probiotic biomass and nisin in nutrient-supplemented whey media. Braz. J. Microbiol. 2019, 50, 915–925. [Google Scholar]
- Felipe, M.D.G.A.; Vieira, D.C.; Vitolo, M.; da Silva, S.S.; Roberto, I.C.; Manchilha, I.M. Effect of acetic acid on xylose fermentation to xylitol by Candida guilliermondii. J. Basic Microbiol. 1995, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kirtadze, E.; Nutsubidze, N. Metabolic potential of alcoholic fermentation yeasts. Bull. Georg. Natl. Acad. Sci. 2009, 3, 110–116. [Google Scholar]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Enhanced kefiran production of Lactobacillus kefiranofaciens by mixed culture with Sac-charomyces cerevisiae. J. Biotechnol. 2003, 100, 43–53. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shoji, H.; Shimizu, H.; Shioya, S. Interactions between Lactobacillus kefiranofaciens and Saccharomyces cerevisiae in mixed culture for kefiran production. J. Biosci. Bioeng. 2003, 96, 279–284. [Google Scholar] [CrossRef]
- Tada, S.; Katakura, Y.; Ninomiya, K.; Shioya, S. Fed-batch coculture of Lactobacillus kefiranofaciens with Saccharomyces cerevisiae for effective production of kefiran. J. Biosci. Bioeng. 2007, 103, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Radchabut, S. Use of whey lactose from dairy industry for economical kefiran production by Lactobacillus kefiranofaciens in mixed cultures with yeasts. New Biotechnol. 2011, 28, 574–580. [Google Scholar] [CrossRef]
- Upadhyay, V.K.; McSweeney, P.L.H.; Magboul, A.A.A.; Fox, P.F. Proteolysis in Cheese during Ripening. In Cheese Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic Press: London, UK, 2004; Volume 1, pp. 391–434. [Google Scholar]
- Poolman, B.; Konings, W.N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J. Bacteriol. 1988, 170, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Noé, F.; Orlid, S.; Querol, A.; Barrio, E. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 2009, 131, 120–127. [Google Scholar]
- Yalcin, S.K.; Ozbas, Z.Y. Effects of ph and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from turkey. Braz. J. Microbiol. 2008, 39, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Costas, M.; Alonso, E.; Guerra, N.P. Nisin production in realkalized fed-batch cultures in whey with feeding with lactose- or glucose-containing substrates. Appl. Microbiol. Biotechnol. 2016, 100, 7899–7908. [Google Scholar] [CrossRef]
- Guerra, N.P.; Fajardo, P.; Pastrana, L. Modelling the stress inducing biphasic growth and pediocin production by Pediococcus acidilactici NRRL B-5627 in re-alkalized fed-batch cultures. Biochem. Eng. J. 2008, 40, 465–472. [Google Scholar] [CrossRef]
- Costas, M.; Alonso, E.; Outeiriño, D.; Fajardo, P.; Guerra, N.P. Combination of food wastes for an efficient production of nisin in realkalized fed-batch cultures. Biochem. Eng. J. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Costas, M.; Alonso, E.; Bendaña, R.J.; Guerra, N.P. The joint effect of pH gradient and glucose feeding on the growth kinetics of Lactococcus lactis CECT 539 in glucose-limited fed-batch cultures. Polish J. Microbiol. 2019, 68, 269–280. [Google Scholar]
- Chen, H.-C.; Wang, S.-Y.; Chen, M.-J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoglu, U.; Cakar, A.; Dogan, H.; Abaci, N.; Ustek, D.; Sayood, K.; Can, H. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014, 41, 42–51. [Google Scholar] [CrossRef]
- Walsh, A.M.; Crispie, F.; Kilcawley, K.; O’Sullivan, O.; O’Sullivan, M.G.; Claesson, M.J.; Cotter, P.D. Microbial Succession and Flavor Production in the Fermented Dairy Beverage Kefir. mSystems 2016, 1, e00052-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, N.P.; Torrado, A.; Lopez, C.; Fajardo, P.; Pastrana, L. Dynamic mathematical models to describe the growth and nisin production by Lactococcus lactis subsp. lactis CECT 539 in both batch and re-alkalized fed-batch cultures. J. Food Eng. 2007, 82, 103–113. [Google Scholar] [CrossRef]
- Guerra, N.P. A Review on Some Chemical Engineering and Microbiological Aspects Considered in the Production of Highly Concentrated Probiotic Cultures and Bacteriocins by Lactococci and Lactobacilli. ISRN Chem. Eng. 2012, 2012, 836381. [Google Scholar] [CrossRef]
- Guerra, N.P.; Rua, M.L.; Pastrana, L. Nutritional factors affecting the production of two bacteriocins from lactic acid bacteria on whey. Int. J. Food Microbiol. 2001, 70, 267–281. [Google Scholar] [CrossRef]
- Guerra, N.P.; Pastrana, L. Modelling the influence of pH on the kinetics of both nisin and pediocin production and charac-terization of their functional properties. Process Biochem. 2002, 37, 1005–1015. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Psarianos, C.; Koutinas, A.A.; Kanellaki, M.; Banat, I.M.; Marchant, R. Continuous Whey Fermentation Using Kefir Yeast Immobilized on Delignified Cellulosic Material. J. Agric. Food Chem. 2002, 50, 2543–2547. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, C.Y.; Teng, W.L.; Ouwehand, A.C.; Tuomola, E.M.; Salminen, S. Quantitative Approach in the Study of Adhesion of Lactic Acid Bacteria to Intestinal Cells and Their Competition with Enterobacteria. Appl. Environ. Microbiol. 2000, 66, 3692–3697. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Cabo, M.L.; González, M.P.; Murado, M.A. The role of amino acids in nisin and pediocin production by two lactic acid bacteria: A factorial study. Enzym. Microb. Technol. 2004, 34, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Matijasić, B.B.; Stojković, S.; Salobir, J.; Malovrh, S.; Rogelj, I. Evaluation of the Lactobacillus gasseri K7 and LF221 strains in weaned piglets for their possible probiotic use and their detection in the faeces. Anim. Res. 2004, 53, 35–44. [Google Scholar] [CrossRef]
- Pascual, M.; Hugas, M.; Badiola, J.I.; Monfort, J.M.; Garriga, M. Lactobacillus salivarius CTC2197 Prevents Salmonella enteritidis Colonization in Chickens. Appl. Environ. Microbiol. 1999, 65, 4981–4986. [Google Scholar] [CrossRef] [Green Version]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Jagatić Korenika, A.M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile Profile Characterization of Croatian Commercial Sparkling Wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef] [PubMed]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef] [PubMed]


| Inoculum | LAB (CFU/mL) | AAB (CFU/mL) | Yeasts (CFU/mL) |
|---|---|---|---|
| Kefir grains AGK1 | 9.9 × 107 ± 1.3 × 107 * | 6.8 × 106 ± 1.0 × 106 * | 7.8 × 107 ± 1.6 × 107 * |
| Fermented whole milk at 150 rpm | 3.3 × 108 ± 2.5 × 1.07 | 3.8 × 108 ± 2.2 × 1.07 | 1.7 × 106 ± 5.7 × 1.04 |
| Fermented semi-skim milk at 150 rpm | 1.1 × 108 ± 1.4 × 1.07 | 3.1 × 108 ± 7.1 × 1.07 | 2.4 × 106 ± 2.5 × 1.05 |
| Fermented skim milk at 150 rpm | 2.6 × 108 ± 4.4 × 1.07 | 3.2 × 108 ± 2.8 × 1.07 | 2.3 × 106 ± 2.0 × 1.05 |
| Fermented whole milk without agitation | 1.0 × 108 ± 7.8 × 1.06 | 1.2 × 108 ± 2.7 × 1.07 | 2.4 × 105 ± 5.0 × 1.04 |
| Fermented semi-skim milk without agitation | 2.6 × 107 ± 1.1 × 1.07 | 1.1 × 108 ± 5.3 × 1.06 | 2.2 × 105 ± 7.8 × 1.04 |
| Fermented skim milk without agitation | 2.8 × 108 ± 9.9 × 1.06 | 4.8 × 107 ± 1.4 × 1.06 | 1.6 × 105 ± 5.4 × 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agregán-Pérez, R.; Alonso-González, E.; Mejuto, J.C.; Pérez-Guerra, N. Production of a Potentially Probiotic Product for Animal Feed and Evaluation of Some of Its Probiotic Properties. Int. J. Mol. Sci. 2021, 22, 10004. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810004
Agregán-Pérez R, Alonso-González E, Mejuto JC, Pérez-Guerra N. Production of a Potentially Probiotic Product for Animal Feed and Evaluation of Some of Its Probiotic Properties. International Journal of Molecular Sciences. 2021; 22(18):10004. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810004
Chicago/Turabian StyleAgregán-Pérez, Rubén, Elisa Alonso-González, Juan Carlos Mejuto, and Nelson Pérez-Guerra. 2021. "Production of a Potentially Probiotic Product for Animal Feed and Evaluation of Some of Its Probiotic Properties" International Journal of Molecular Sciences 22, no. 18: 10004. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810004