FAK Shutdown: Consequences on Epithelial Morphogenesis and Biomarker Expression Involving an Innovative Biomaterial for Tissue Regeneration
Abstract
:1. Introduction
2. Results
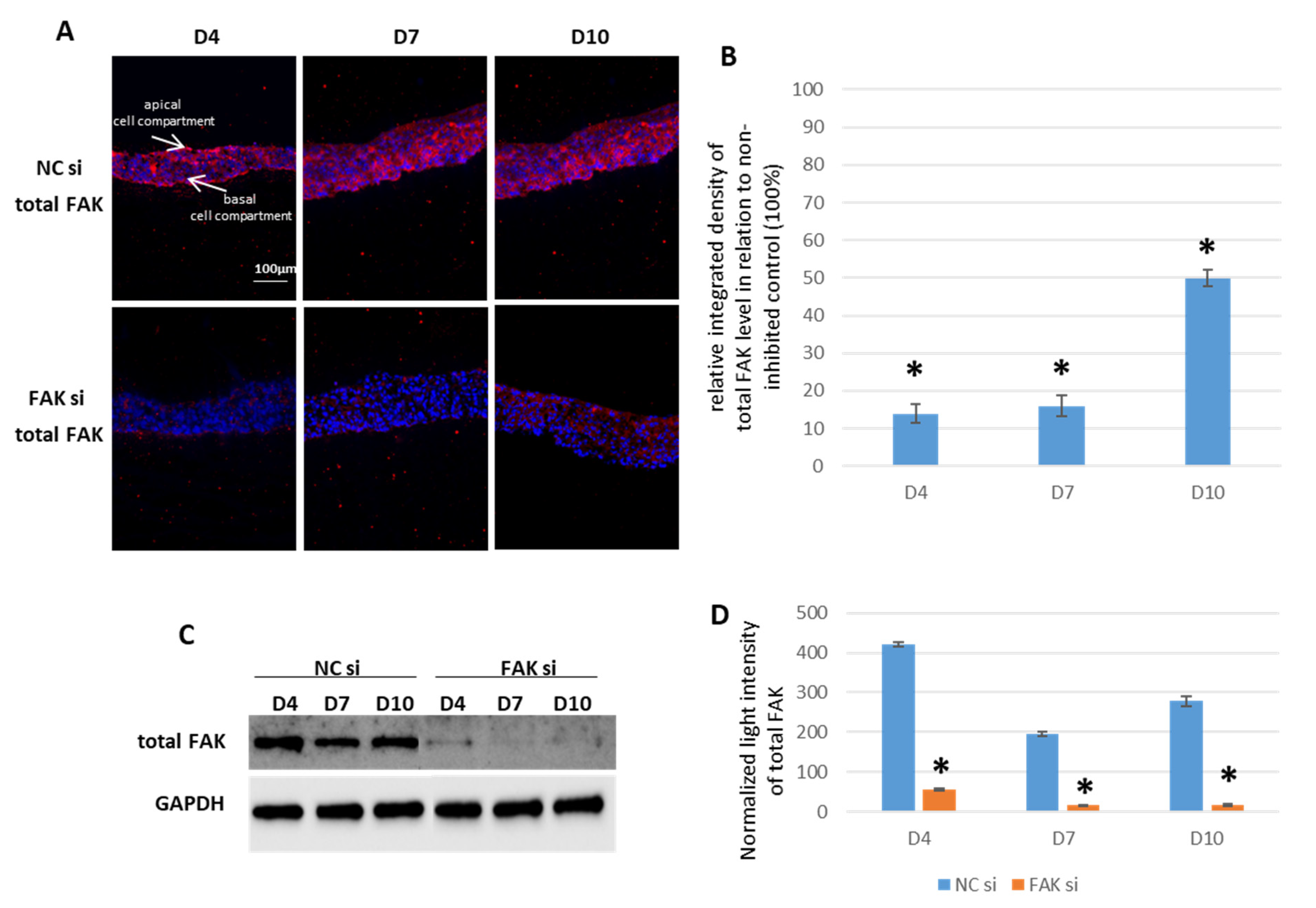
2.1. siRNA-Mediated FAK Shutdown
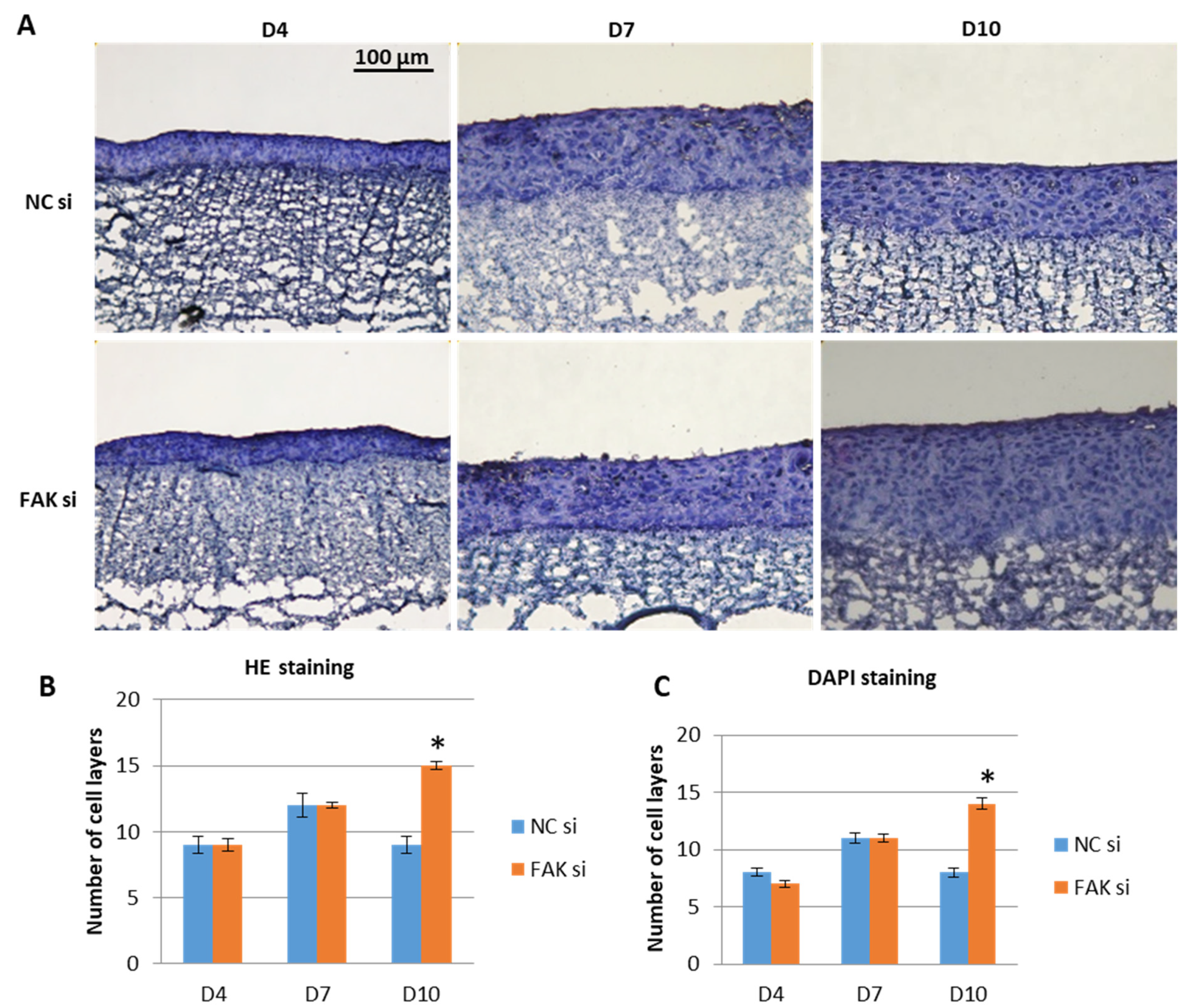
2.2. Effect of FAK-siRNA on Oral Epithelial Morphogenesis
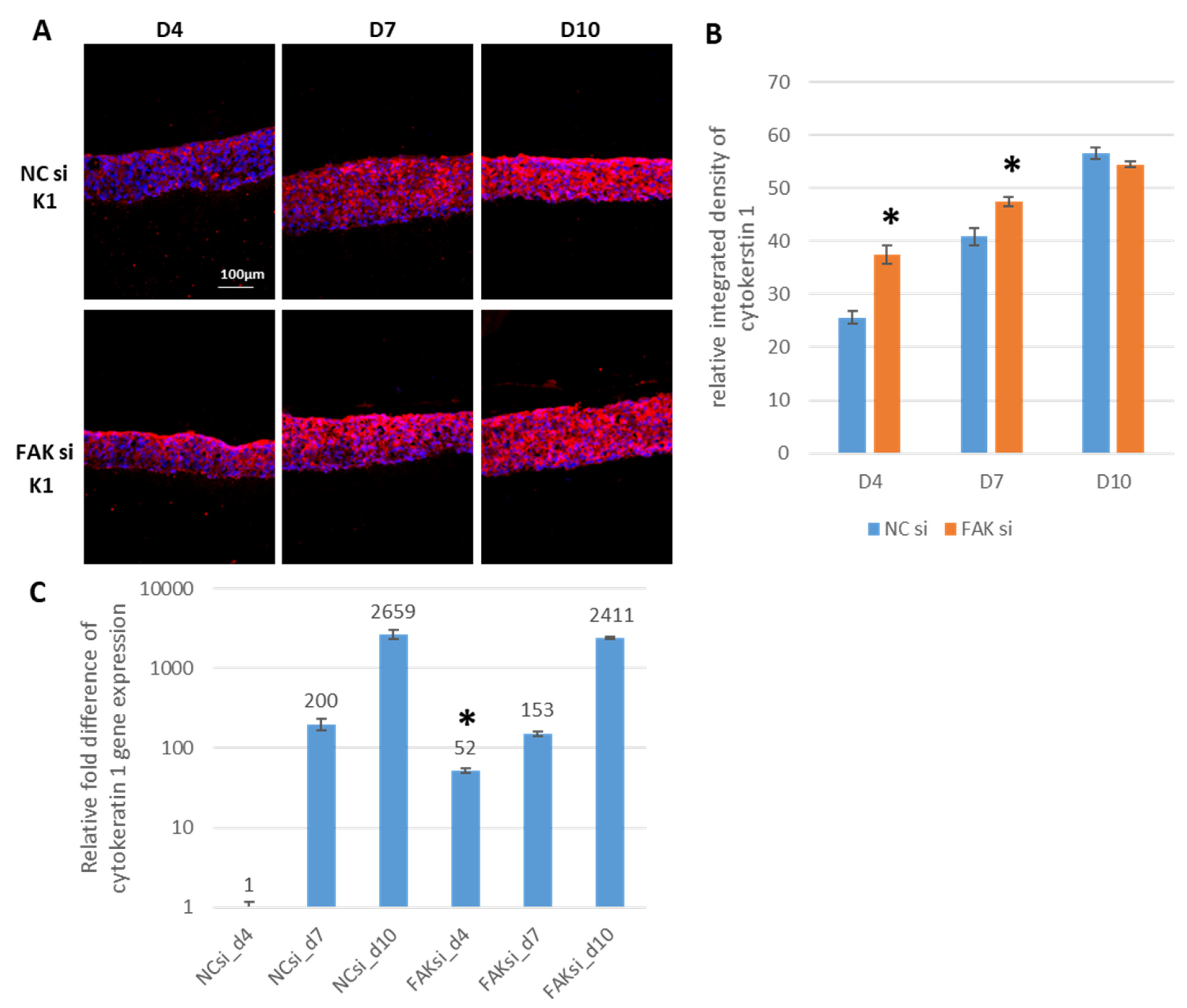
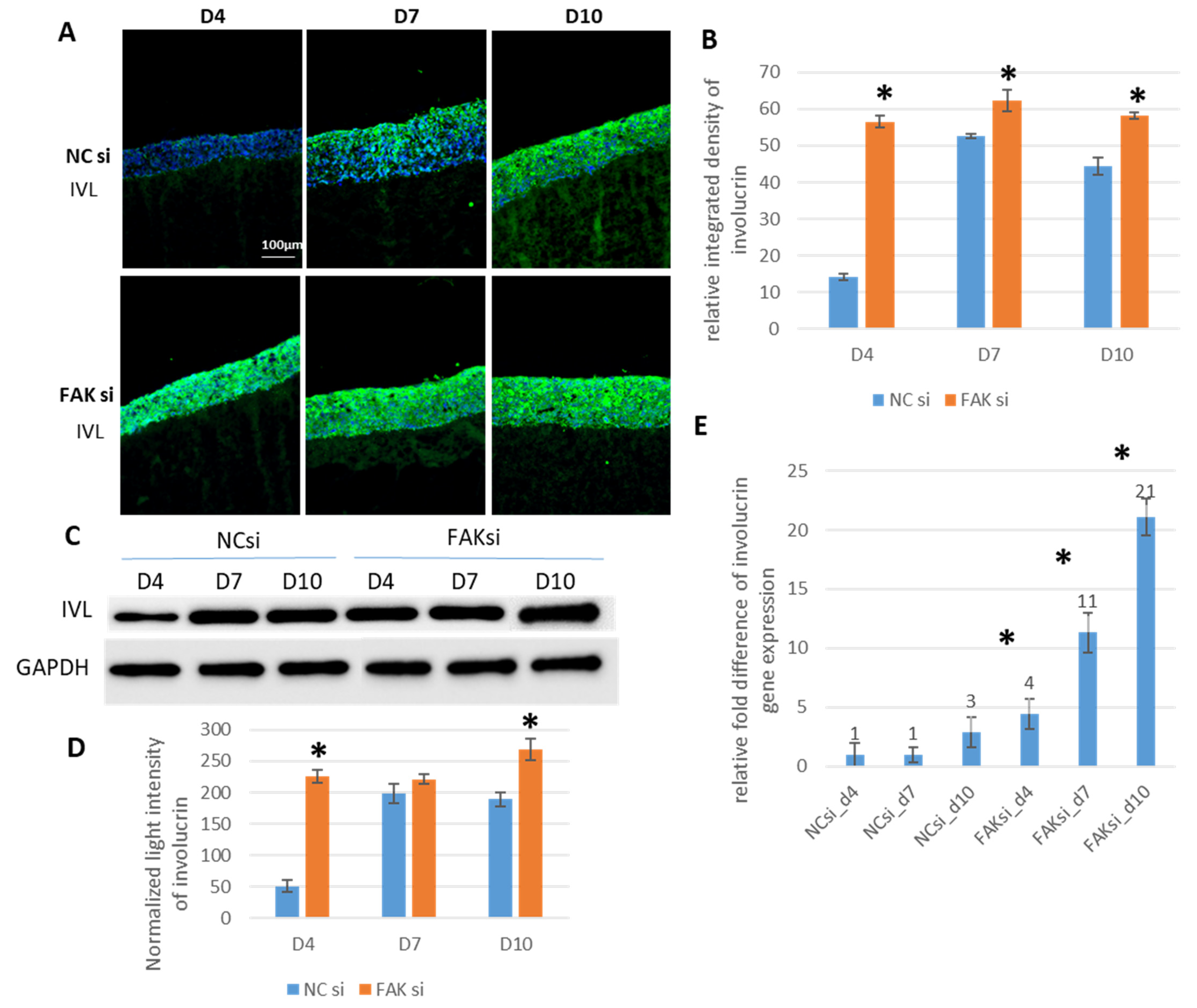
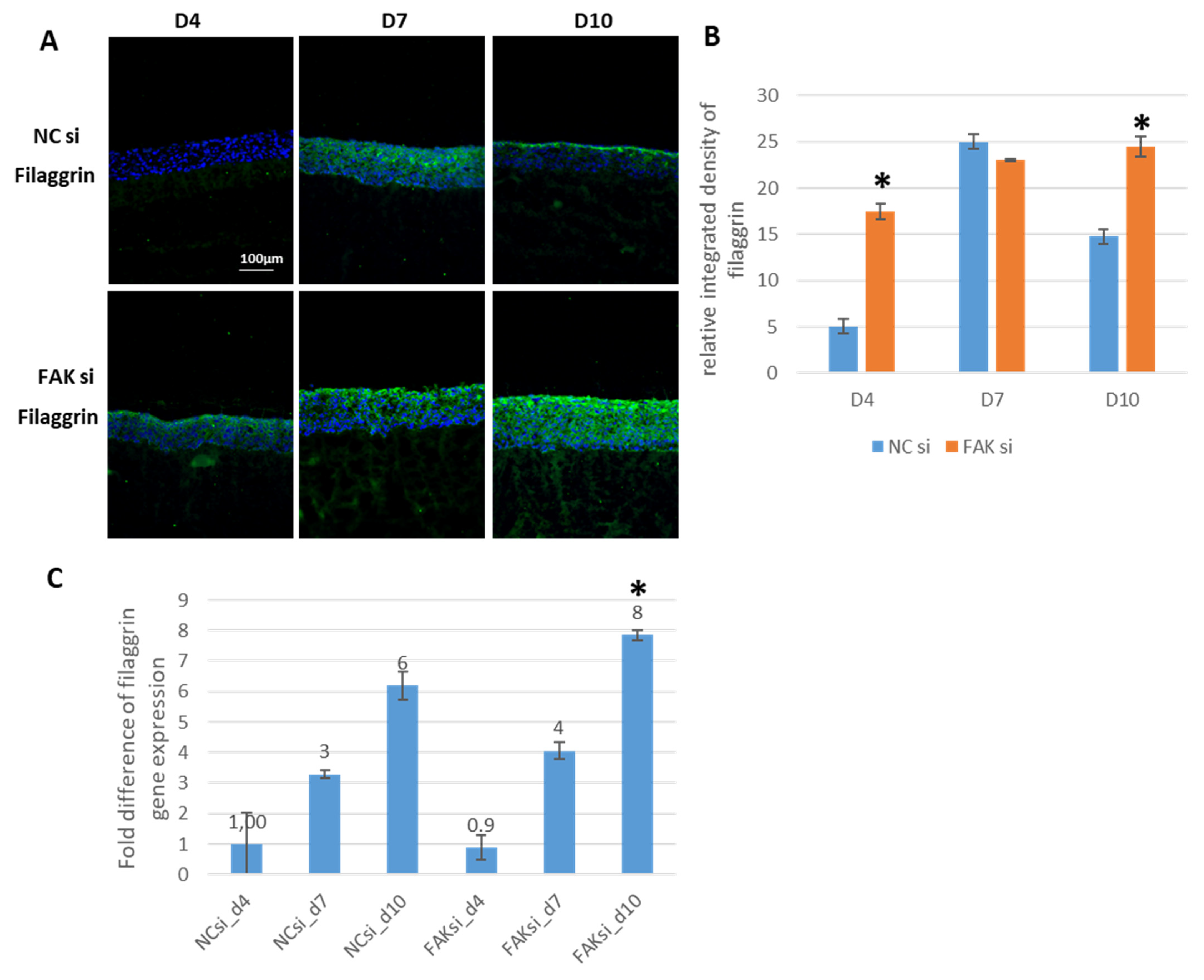
2.3. Consequences of FAK Knockdown on Differentiation Biomarkers
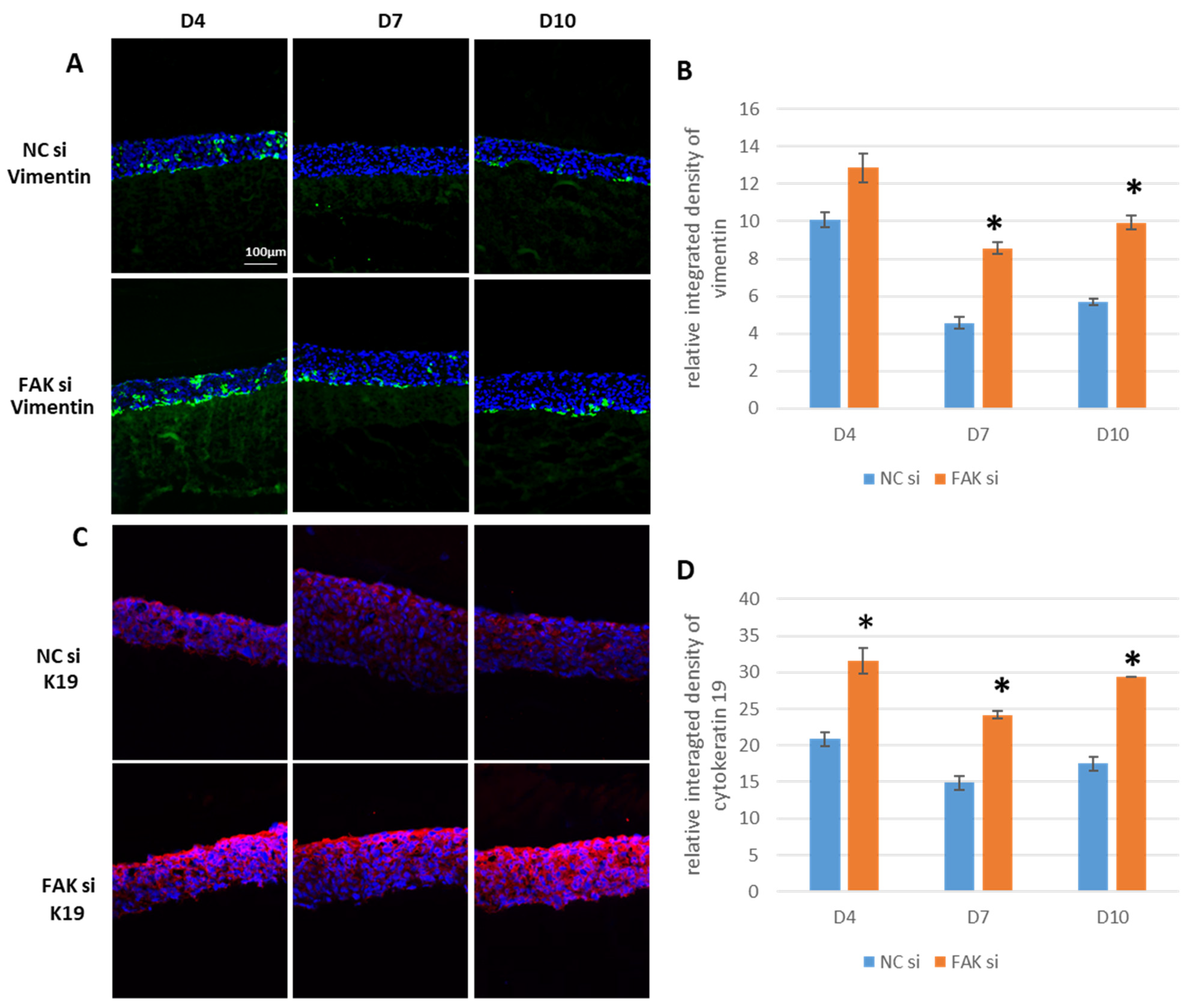
2.4. Impact of FAK-siRNA on Vimentin and K19
2.5. Effect of FAK RNAi on Vinculin and Phosphorylated ROCK-IISer1366
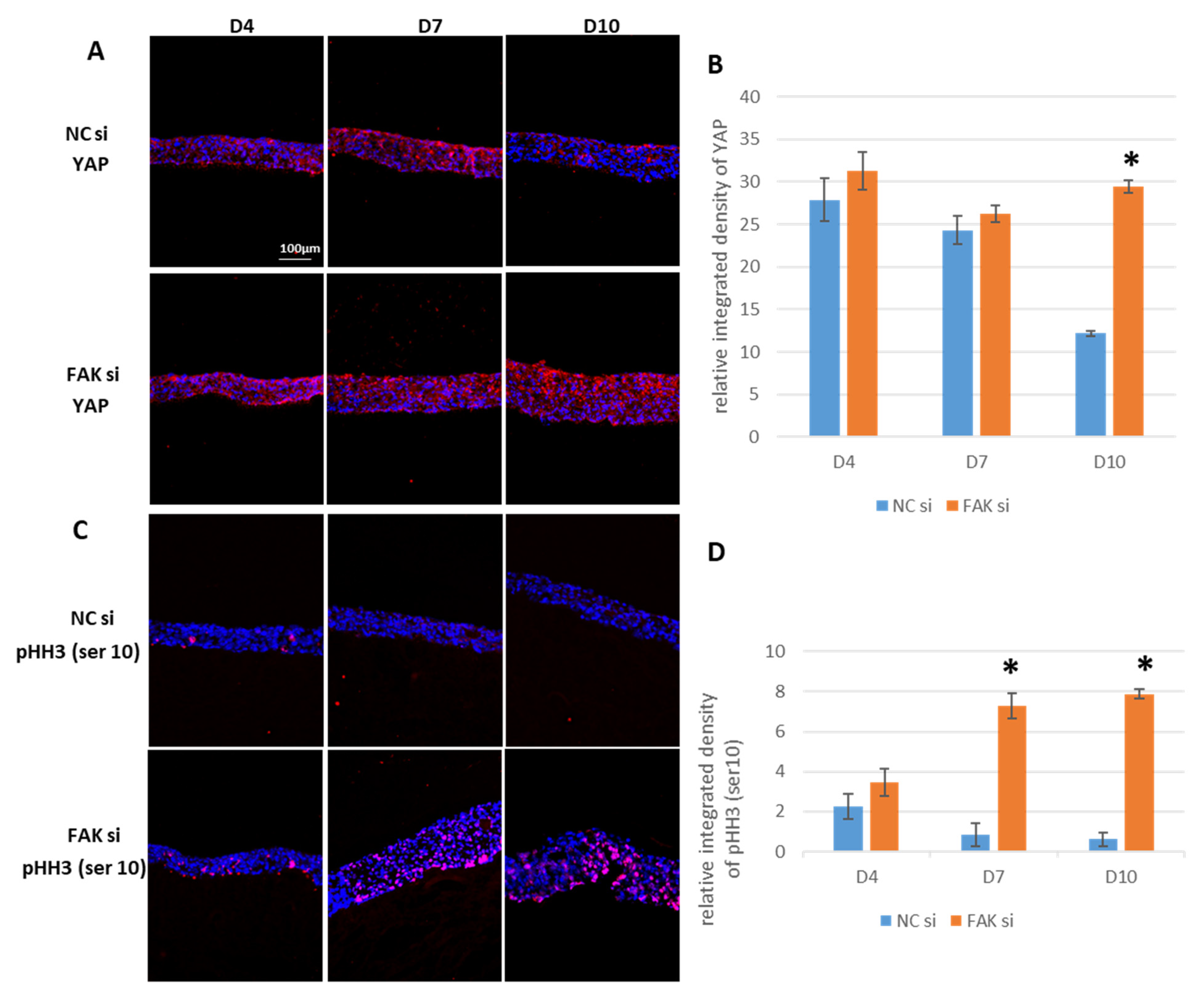
2.6. Maintenance of YAP and pHH3Ser10 in FAK-siRNA-Treated Epithelial Equivalents
3. Discussion
- (i)
- In vitro gingival epithelial morphogenesis with proper spatiotemporal expression of important GK biomarkers in the respective epithelial layers is feasible and reproducible with the herein presented biomaterial-based approach.
- (ii)
- The presented experimental platform can therefore be used for various animal-free applications in the study of signaling networks within the 3D environment of epithelia or even to screen for organ-specific drugs. As mentioned above, as the membrane is biocompatible, the concept can also be used in future translational approaches, where patient-derived cells can be seeded on the scaffold, cultivated/expanded in the laboratory, and then be transplanted into the patient for defect coverage in critical-size injuries of the gingiva.
- (iii)
- FAK-siRNA treatment in this 3D organotypic coculture leads to efficient FAK shutdown, whereas Y15 inhibitor treatments lead to paradoxical findings most likely due to signaling from the underlying fibroblasts.
- (iv)
- Inhibition of FAK protein synthesis results in hyperplastic epithelia, which are more proliferative when compared to the control samples as indicated by proteins such as YAP, ROCK-IISer1366, and pHH3Ser10. Additionally, differentiation biomarkers such as involucrin and filaggrin are more abundant, and their expression is more pronounced in basal cell layers.
- (v)
- FAK knockdown in the gingival epithelial equivalents leads to aberrant expression of vimentin and K19, which should be studied further in the context of EMT and carcinogenesis.
- (vi)
- As a consequence of (iii) and (iv), FAK signaling should not be inhibited via siRNA in the context of gingival tissue engineering and oral regenerative medicine, since FAK is necessary for proper epithelial morphogenesis and biomarker expression. Contrary to many cancer cell models, siRNA-based FAK inhibition is probably not a useful strategy to prevent epithelial overgrowth and the dysbalanced expression of GKs differentiation markers, as can be concluded from our data. Based on our findings, the role of FAK inhibition for the treatment of premalignant oral lesions needs to be studied in more detail.
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guan, J.-L.; Trevithick, J.E.; Hynes, R. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kda protein. Cell Regul. 1991, 2, 951–964. [Google Scholar] [CrossRef] [Green Version]
- Hanks, S.K.; Calalb, M.B.; Harper, M.C.; Patel, S.K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA 1992, 89, 8487–8491. [Google Scholar] [CrossRef] [Green Version]
- Schaller, M.D.; Borgman, C.A.; Cobb, B.S.; Vines, R.R.; Reynolds, A.B.; Parsons, J.T. Pp125fak a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA 1992, 89, 5192–5196. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.D. Apico-basal polarity in polycystic kidney disease epithelia. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2011, 1812, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.-J.; Liu, X.-J.; Liu, Y.; Liu, W.-B.; Li, Y.-R.; Yu, G.-X.; Tian, X.-Y.; Zhang, Y.-B.; Song, J.; Jin, C.-Y. Drug discovery targeting focal adhesion kinase (fak) as a promising cancer therapy. Molecules 2021, 26, 4250. [Google Scholar] [CrossRef] [PubMed]
- Canel, M.; Serrels, A.; Miller, D.; Timpson, P.; Serrels, B.; Frame, M.C.; Brunton, V.G. Quantitative in vivo imaging of the effects of inhibiting integrin signaling via src and fak on cancer cell movement: Effects on e-cadherin dynamics. Cancer Res. 2010, 70, 9413–9422. [Google Scholar] [CrossRef] [Green Version]
- Serrels, A.; Canel, M.; Brunton, V.G.; Frame, M.C. Src/fak-mediated regulation of e-cadherin as a mechanism for controlling collective cell movement: Insights from in vivo imaging. Cell Adhes. Migr. 2011, 5, 360–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Fischer, T.; Puder, S.; Kunschmann, T.; Soetje, B.; Ziegler, W.H. Focal adhesion kinase activity is required for actomyosin contractility-based invasion of cells into dense 3d matrices. Sci. Rep. 2017, 7, 42780. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.T.; Pyun, J.-C.; Lee, S.-G.; Kang, M.-J. Identification of new binding proteins of focal adhesion kinase using immunoprecipitation and mass spectrometry. Sci. Rep. 2019, 9, 12908. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Kato, K.; Miyazawa, H.; Kobayashi, H.; Noguchi, N.; Kawashiri, S. Focal adhesion kinase (fak) overexpression and phosphorylation in oral squamous cell carcinoma and their clinicopathological significance. Pathol. Oncol. Res. 2020, 26, 1659–1667. [Google Scholar] [CrossRef]
- Leavitt, T.; Hu, M.S.; Marshall, C.D.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Scarless wound healing: Finding the right cells and signals. Cell Tissue Res. 2016, 365, 483–493. [Google Scholar] [CrossRef]
- Su, L.; Li, X.; Wu, X.; Hui, B.; Han, S.; Gao, J.; Li, Y.; Shi, J.; Zhu, H.; Zhao, B. Simultaneous deactivation of fak and src improves the pathology of hypertrophic scar. Sci. Rep. 2016, 6, 26023. [Google Scholar] [CrossRef] [Green Version]
- Urciuoli, E.; Peruzzi, B. Involvement of the fak network in pathologies related to altered mechanotransduction. Int. J. Mol. Sci. 2020, 21, 9426. [Google Scholar] [CrossRef]
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2012, 18, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Duperret, E.; Ridky, T.W. Focal adhesion complex proteins in epidermis and squamous cell carcinoma. Cell Cycle 2013, 12, 3272–3285. [Google Scholar] [CrossRef]
- Essayem, S.; Kovacic-Milivojevic, B.; Baumbusch, C.; McDonagh, S.; Dolganov, G.; Howerton, K.; Larocque, N.; Mauro, T.; Ramirez, A.; Ramos, D. Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of fak. Oncogene 2006, 25, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Guan, J.-L. Focal adhesion kinase: From in vitro studies to functional analyses in vivo. Curr. Protein Pept. Sci. 2011, 12, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, R.A.; Serrels, B.; Mason, S.; Kinnaird, A.; Muir, M.; Patel, H.; Muller, W.J.; Sansom, O.J.; Brunton, V.G. Focal adhesion kinase is required for β-catenin-induced mobilization of epidermal stem cells. Carcinogenesis 2012, 33, 2369–2376. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schröder, E.; Zhou, J.; Brunton, V.G. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 2011, 19, 776–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januszyk, M.; Kwon, S.H.; Wong, V.W.; Padmanabhan, J.; Maan, Z.N.; Whittam, A.J.; Major, M.R.; Gurtner, G.C. The role of focal adhesion kinase in keratinocyte fibrogenic gene expression. Int. J. Mol. Sci. 2017, 18, 1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-Y.; Zhou, X.; Rowe, R.G.; Hu, Y.; Schlaepfer, D.D.; Ilić, D.; Dressler, G.; Park, A.; Guan, J.-L.; Weiss, S.J. Snail1 controls epithelial–mesenchymal lineage commitment in focal adhesion kinase–null embryonic cells. J. Cell Biol. 2011, 195, 729–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, J.; Auernheimer, V.; Strissel, P.L.; Goldmann, W.H. Influence of focal adhesion kinase on the mechanical behavior of cell populations. Biochem. Biophys. Res. Commun. 2013, 436, 246–251. [Google Scholar] [CrossRef]
- Choma, D.P.; Milano, V.; Pumiglia, K.M.; DiPersio, C.M. Integrin α3β1-dependent activation of fak/src regulates rac1-mediated keratinocyte polarization on laminin-5. J. Investig. Dermatol. 2007, 127, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gates, R.E.; King Jr, L.E.; Hanks, S.K.; Nanney, L.B. Potential role for focal adhesion kinase in migrating and proliferating keratinocytes near epidermal wounds and in culture. Cell Growth Differ. Publ. Am. Assoc. Cancer Res. 1994, 5, 891–900. [Google Scholar]
- Buskermolen, J.K.; Reijnders, C.M.; Spiekstra, S.W.; Steinberg, T.; Kleverlaan, C.J.; Feilzer, A.J.; Bakker, A.D.; Gibbs, S. Development of a full-thickness human gingiva equivalent constructed from immortalized keratinocytes and fibroblasts. Tissue Eng. Part C Methods 2016, 22, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Garzon, I.; Sánchez-Quevedo, M.; Moreu, G.; González-Jaranay, M.; González-Andrades, M.; Montalvo, A.; Campos, A.; Alaminos, M. In vitro and in vivo cytokeratin patterns of expression in bioengineered human periodontal mucosa. J. Periodontal Res. 2009, 44, 588–597. [Google Scholar] [CrossRef]
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cells Mater. 2019, 38, 63–78. [Google Scholar] [CrossRef]
- Vriens, A.P.; Waaijman, T.; Van Den Hoogenband, H.M.; De Boer, E.M.; Scheper, R.J.; Gibbs, S. Comparison of autologous full-thickness gingiva and skin substitutes for wound healing. Cell Transplant. 2008, 17, 1199–1209. [Google Scholar] [CrossRef]
- Gibbs, S.; Ponec, M. Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch. Oral Biol. 2000, 45, 149–158. [Google Scholar] [CrossRef]
- Weinmann, J.; Meyer, J. Types of keratinization in the human gingiva. J. Investig. Dermatol. 1959, 32, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedrusik, N.; Meyen, C.; Finkenzeller, G.; Stark, G.B.; Meskath, S.; Schulz, S.D.; Steinberg, T.; Eberwein, P.; Strassburg, S.; Tomakidi, P. Nanofibered gelatin-based nonwoven elasticity promotes epithelial histogenesis. Adv. Healthc. Mater. 2018, 7, 1700895. [Google Scholar] [CrossRef] [PubMed]
- Boink, M.A.; Roffel, S.; Breetveld, M.; Thon, M.; Haasjes, M.S.; Waaijman, T.; Scheper, R.J.; Blok, C.S.; Gibbs, S. Comparison of advanced therapy medicinal product gingiva and skin substitutes and their in vitro wound healing potentials. J. Tissue Eng. Regen. Med. 2018, 12, e1088–e1097. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.P.; Romer, L.H. Inhibition of focal adhesion kinase (fak) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell 1996, 7, 1209–1224. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Nikoloudaki, G.E.; Michelsons, S.; Creber, K.; Hamilton, D.W. Fibronectin synthesis, but not α-smooth muscle expression, is regulated by periostin in gingival healing through fak/jnk signaling. Sci. Rep. 2019, 9, 2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Jiang, M.; Li, H.; Li, C.; Su, R.; Huang, K. Knockdown of fak inhibits the invasion and metastasis of tca-8113 cells in vitro. Mol. Med. Rep. 2013, 8, 703–707. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X. Role of focal adhesion kinase in head and neck squamous cell carcinoma and its therapeutic prospect. OncoTargets Ther. 2020, 13, 10207. [Google Scholar] [CrossRef]
- Jedrusik, N.; Steinberg, T.; Husari, A.; Volk, L.; Wang, X.; Finkenzeller, G.; Strassburg, S.; Tomakidi, P. Gelatin nonwovens-based epithelial morphogenesis involves a signaling axis comprising egf-receptor, map kinases erk 1/2, and β1 integrin. J. Biomed. Mater. Res. Part A 2019, 107, 663–677. [Google Scholar] [CrossRef]
- Tomakidi, P.; Fusenig, N.; Kohl, A.; Komposch, G. Histomorphological and biochemical differentiation capacity in organotypic co-cultures of primary gingival cells. J. Periodontal Res. 1997, 32, 388–400. [Google Scholar] [CrossRef]
- Tomakidi, P.; Breitkreutz, D.; Fusenig, N.E.; Zöller, J.; Kohl, A.; Komposch, G. Establishment of oral mucosa phenotype in vitro in correlation to epithelial anchorage. Cell Tissue Res. 1998, 292, 355–366. [Google Scholar] [CrossRef]
- Roesch-Ely, M.; Steinberg, T.; Bosch, F.X.; Müssig, E.; Whitaker, N.; Wiest, T.; Kohl, A.; Komposch, G.; Tomakidi, P. Organotypic co-cultures allow for immortalized human gingival keratinocytes to reconstitute a gingival epithelial phenotype in vitro. Differentiation 2006, 74, 622–637. [Google Scholar] [CrossRef]
- Eckert, R.L.; Crish, J.F.; Efimova, T.; Dashti, S.R.; Deucher, A.; Bone, F.; Adhikary, G.; Huang, G.; Gopalakrishnan, R.; Balasubramanian, S. Regulation of involucrin gene expression. J. Investig. Dermatol. 2004, 123, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.; Aho, S.; Bosko, C. Filaggrin—Revisited. Int. J. Cosmet. Sci. 2013, 35, 412–423. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Syed, D.N.; Adhami, V.M.; Liovic, M.; Mukhtar, H. Keratin gene mutations in disorders of human skin and its appendages. Arch. Biochem. Biophys. 2011, 508, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Feghali-Assaly, M.; Sawaf, M.; Serres, G.; Forest, N.; Ouhayoun, J. Cytokeratin profile of the junctional epithelium in partially erupted teeth. J. Periodontal Res. 1994, 29, 185–195. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Peng, C.; Qin, Y.; Gao, T.; Jing, J.; Zhao, H. Brafv600e-induced krt19 expression in thyroid cancer promotes lymph node metastasis via emt. Oncol. Lett. 2019, 18, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Müssig, E.; Steinberg, T.; Kohl, A.; Chamulitrat, W.; Komposch, G.; Tomakidi, P. Discrimination of epithelium-like and fibroblast-like phenotypes derived from ethanol-treated immortalised human gingival keratinocytes in epithelial equivalents. Cell Tissue Res. 2008, 332, 57–71. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Xiao, Y.; Wu, L.; Peng, Y.; Tang, W.; Liu, G.; Sun, Y.; Wang, J.; Zhu, H. Coexpression of foxk1 and vimentin promotes emt, migration, and invasion in gastric cancer cells. J. Mol. Med. 2019, 97, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havel, L.S.; Kline, E.R.; Salgueiro, A.M.; Marcus, A.I. Vimentin regulates lung cancer cell adhesion through a vav2–rac1 pathway to control focal adhesion kinase activity. Oncogene 2015, 34, 1979–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef] [Green Version]
- Eberwein, P.; Laird, D.; Schulz, S.; Reinhard, T.; Steinberg, T.; Tomakidi, P. Modulation of focal adhesion constituents and their down-stream events by egf: On the cross-talk of integrins and growth factor receptors. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2015, 1853, 2183–2198. [Google Scholar] [CrossRef] [Green Version]
- Hülter-Hassler, D.; Wein, M.; Schulz, S.D.; Proksch, S.; Steinberg, T.; Jung, B.A.; Tomakidi, P. Biomechanical strain-induced modulation of proliferation coincides with an erk1/2-independent nuclear yap localization. Exp. Cell Res. 2017, 361, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of yap and taz in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Liu, H.; Du, S.; Lei, T.; Wang, H.; He, X.; Tong, R.; Wang, Y. Multifaceted regulation and functions of yap/taz in tumors. Oncol. Rep. 2018, 40, 16–28. [Google Scholar] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. Yap/taz upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Hu, J.K.-H.; Du, W.; Shelton, S.J.; Oldham, M.C.; DiPersio, C.M.; Klein, O.D. An fak-yap-mtor signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell 2017, 21, 91–106.e106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husari, A.; Steinberg, T.; Dieterle, M.P.; Prucker, O.; Rühe, J.; Jung, B.; Tomakidi, P. On the relationship of yap and fak in hmscs and osteosarcoma cells: Discrimination of fak modulation by nuclear yap depletion or yap silencing. Cell Signal. 2019, 63, 109382. [Google Scholar] [CrossRef]
- Wong, W.Y.; Gilman, K.; Limesand, K.H. Yap activation in irradiated parotid salivary glands is regulated by rock activity. PLoS ONE 2020, 15, e0232921. [Google Scholar] [CrossRef]
- Kümper, S.; Mardakheh, F.K.; McCarthy, A.; Yeo, M.; Stamp, G.W.; Paul, A.; Worboys, J.; Sadok, A.; Jørgensen, C.; Guichard, S. Rho-associated kinase (rock) function is essential for cell cycle progression, senescence and tumorigenesis. Elife 2016, 5, e12203. [Google Scholar] [CrossRef]
- Torsoni, A.S.; Marin, T.M.; Velloso, L.A.; Franchini, K.G. Rhoa/rock signaling is critical to fak activation by cyclic stretch in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1488–H1496. [Google Scholar] [CrossRef] [Green Version]
- McMullan, R.; Lax, S.; Robertson, V.H.; Radford, D.J.; Broad, S.; Watt, F.M.; Rowles, A.; Croft, D.R.; Olson, M.F.; Hotchin, N.A. Keratinocyte differentiation is regulated by the rho and rock signaling pathway. Curr. Biol. 2003, 13, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Soares, D.G.; Pansani, T.N.; Turrioni, A.P.S.; Scheffel, D.L.; Hebling, J.; Costa, C.A.D.S. Response of a co-culture model of epithelial cells and gingival fibroblasts to zoledronic acid. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delcourt-Huard, A.; Corlu, A.; Joffre, A.; Magloire, H.; Bonnaure-Mallet, M. Reconstituted human gingival epithelium: Nonsubmerged in vitro model. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 30–36. [Google Scholar] [CrossRef]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.; van den Bogaard, E.H. 3d skin models for 3r research: The potential of 3d reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, Q.; Zheng, Y.; Nan, L.; Liao, N.; Mo, S. Potential role of integrin α5β1/focal adhesion kinase (fak) and actin cytoskeleton in the mechanotransduction and response of human gingival fibroblasts cultured on a 3-dimension lactide-co-glycolide (3d plga) scaffold. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921626-1. [Google Scholar]
- Guo, B.; Tang, C.; Wang, M.; Zhao, Z.; Shokoohi-Tabrizi, H.A.; Shi, B.; Andrukhov, O.; Rausch-Fan, X. In vitro biocompatibility of biohybrid polymers membrane evaluated in human gingival fibroblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2590–2598. [Google Scholar] [CrossRef] [Green Version]
- Strassburg, S.; Caduc, M.; Stark, G.B.; Jedrusik, N.; Tomakidi, P.; Steinberg, T.; Simunovic, F.; Finkenzeller, G. In vivo evaluation of an electrospun gelatin nonwoven mat for regeneration of epithelial tissues. J. Biomed. Mater. Res. Part A 2019, 107, 1605–1614. [Google Scholar] [CrossRef]
- Heim, J.B.; McDonald, C.A.; Wyles, S.P.; Sominidi-Damodaran, S.; Squirewell, E.J.; Li, M.; Motsonelidze, C.; Böttcher, R.T.; van Deursen, J.; Meves, A. Fak auto-phosphorylation site tyrosine 397 is required for development but dispensable for normal skin homeostasis. PLoS ONE 2018, 13, e0200558. [Google Scholar] [CrossRef]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. Fak integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriram, G.; Bigliardi, P.L.; Bigliardi-Qi, M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 2015, 94, 483–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auernheimer, V.; Lautscham, L.A.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Fabry, B.; Goldmann, W.H. Vinculin phosphorylation at residues y100 and y1065 is required for cellular force transmission. J. Cell Sci. 2015, 128, 3435–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchini, C.; Manara, M.C.; Cristalli, C.; Carrabotta, M.; Greco, S.; Pinca, R.S.; Ferrari, C.; Landuzzi, L.; Pasello, M.; Lollini, P.-L. Rock2 deprivation leads to the inhibition of tumor growth and metastatic potential in osteosarcoma cells through the modulation of yap activity. J. Exp. Clin. Cancer Res. 2019, 38, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, C.; Boerboom, D.; Paquet, M. Expression of the hippo signalling effectors yap and taz in canine mammary gland hyperplasia and malignant transformation of mammary tumours. Vet. Comp. Oncol. 2018, 16, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Alt-Holland, A.; Sowalsky, A.G.; Szwec-Levin, Y.; Shamis, Y.; Hatch, H.; Feig, L.A.; Garlick, J.A. Suppression of e-cadherin function drives the early stages of ras-induced squamous cell carcinoma through upregulation of fak and src. J. Investig. Dermatol. 2011, 131, 2306–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, E.M.C.; Hobbs, C.; Dyer, C.; Ghuman, M.; Hughes, F.J. Differential regulation of epithelial growth by gingival and periodontal fibroblasts in vitro. J. Periodontal Res. 2020, 55, 859–867. [Google Scholar] [CrossRef]
- Müssig, E.; Tomakidi, P.; Steinberg, T. Gingival fibroblasts established on microstructured model surfaces: Their influence on epithelial morphogenesis and other tissue-specific cell functions in a co-cultured epithelium. J. Orofac. Orthop. Fortschr. der Kieferorthopädie 2009, 70, 351. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Dahal, A.; Ridky, T.W. Focal-adhesion-independent integrin-αv regulation of fak and c-myc is necessary for 3d skin formation and tumor invasion. J. Cell Sci. 2015, 128, 3997–4013. [Google Scholar]
- Dieterle, M.P.; Husari, A.; Steinberg, T.; Wang, X.; Ramminger, I.; Tomakidi, P. From the matrix to the nucleus and back: Mechanobiology in the light of health, pathologies, and regeneration of oral periodontal tissues. Biomolecules 2021, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.H.; Lee, S.K.; Hwang, Y.S.; Choi, M.G.; Lee, S.K.; Kim, E.C. Differential expression of periodontal ligament-specific markers and osteogenic differentiation in human papilloma virus 16-immortalized human gingival fibroblasts and periodontal ligament cells. J. Periodontal Res. 2007, 42, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Eberwein, P.; Steinberg, T.; Schulz, S.; Zimmermann, D.; Accardi, R.; Beck, D.; Reinhard, T.; Tomakidi, P. Expression of keratinocyte biomarkers is governed by environmental biomechanics. Eur. J. Cell Biol. 2011, 90, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Steinberg, T.; Beck, D.; Tomakidi, P.; Accardi, R.; Tommasino, M.; Reinhard, T.; Eberwein, P. Generation and evaluation of a human corneal model cell system for ophthalmologic issues using the hpv16 e6/e7 oncogenes as uniform immortalization platform. Differentiation 2013, 85, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamulitrat, W.; Schmidt, R.; Chunglok, W.; Kohl, A.; Tomakidi, P. Epithelium and fibroblast-like phenotypes derived from hpv16 e6/e7-immortalized human gingival keratinocytes following chronic ethanol treatment. Eur. J. Cell Biol. 2003, 82, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Velez-delValle, C.; Marsch-Moreno, M.; Castro-Muñozledo, F.; Galván-Mendoza, I.J.; Kuri-Harcuch, W. Epithelial cell migration requires the interaction between the vimentin and keratin intermediate filaments. Sci. Rep. 2016, 6, 24389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlowe, T.A.; Lenzo, F.L.; Figel, S.A.; Grapes, A.T.; Cance, W.G. Oncogenic receptor tyrosine kinases directly phosphorylate focal adhesion kinase (fak) as a resistance mechanism to fak-kinase inhibitors. Mol. Cancer Ther. 2016, 15, 3028–3039. [Google Scholar] [CrossRef] [Green Version]
- Rudelius, M.; Rosenfeldt, M.T.; Leich, E.; Rauert-Wunderlich, H.; Solimando, A.G.; Beilhack, A.; Ott, G.; Rosenwald, A. Inhibition of focal adhesion kinase overcomes resistance of mantle cell lymphoma to ibrutinib in the bone marrow microenvironment. Haematologica 2018, 103, 116. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Choi, B.Y.; Cho, Y.-Y.; Mizuno, H.; Kang, B.S.; Bode, A.M.; Dong, Z. Phosphorylation of histone h3 at serine 10 is indispensable for neoplastic cell transformation. Cancer Res. 2005, 65, 5818–5827. [Google Scholar] [CrossRef] [Green Version]
- Cortés, A.; Muñoz-Antoli, C.; Martín-Grau, C.; Esteban, J.G.; Grencis, R.K.; Toledo, R. Differential alterations in the small intestine epithelial cell turnover during acute and chronic infection with echinostoma caproni (trematoda). Parasites Vectors 2015, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Pirone, D.M.; Liu, W.F.; Ruiz, S.A.; Gao, L.; Raghavan, S.; Lemmon, C.A.; Romer, L.H.; Chen, C.S. An inhibitory role for fak in regulating proliferation: A link between limited adhesion and rhoa-rock signaling. J. Cell Biol. 2006, 174, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Manso, A.M.; Kang, S.-M.; Plotnikov, S.V.; Thievessen, I.; Oh, J.; Beggs, H.E.; Ross, R.S. Cardiac fibroblasts require focal adhesion kinase for normal proliferation and migration. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H627–H638. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.-H.; Yang, C.-H.; Tsay, Y.-G.; Hsu, C.-Y.; Tseng, L.-M.; Chang, Z.-F.; Lee, H.-H. Rockii ser1366 phosphorylation reflects the activation status. Biochem. J. 2012, 443, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognoni, E.; Walko, G. The roles of yap/taz and the hippo pathway in healthy and diseased skin. Cells 2019, 8, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Park, J.; Feng, A.; Awasthi, P.; Wang, Z.; Chen, Q.; Iglesias-Bartolome, R. Yap1/taz-tead transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting klf4 activity. Nat. Commun. 2020, 11, 1472. [Google Scholar] [CrossRef] [Green Version]
- Totaro, A.; Castellan, M.; Battilana, G.; Zanconato, F.; Azzolin, L.; Giulitti, S.; Cordenonsi, M.; Piccolo, S. Yap/taz link cell mechanics to notch signalling to control epidermal stem cell fate. Nat. Commun. 2017, 8, 15206. [Google Scholar] [CrossRef]
- Noethel, B.; Ramms, L.; Dreissen, G.; Hoffmann, M.; Springer, R.; Rübsam, M.; Ziegler, W.H.; Niessen, C.M.; Merkel, R.; Hoffmann, B. Transition of responsive mechanosensitive elements from focal adhesions to adherens junctions on epithelial differentiation. Mol. Biol. Cell 2018, 29, 2317–2325. [Google Scholar] [CrossRef]
- Young, P.; Boussadia, O.; Halfter, H.; Grose, R.; Berger, P.; Leone, D.P.; Robenek, H.; Charnay, P.; Kemler, R.; Suter, U. E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J. 2003, 22, 5723–5733. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Cuff, L.E.; Lawton, C.D.; DeMali, K.A. Vinculin regulates cell-surface e-cadherin expression by binding to β-catenin. J. Cell Sci. 2010, 123, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Konishi, S.; Yano, T.; Tanaka, H.; Mizuno, T.; Kanoh, H.; Tsukita, K.; Namba, T.; Tamura, A.; Yonemura, S.; Gotoh, S. Vinculin is critical for the robustness of the epithelial cell sheet paracellular barrier for ions. Life Sci. Alliance 2019, 2, 4. [Google Scholar] [CrossRef]
- Golji, J.; Wendorff, T.; Mofrad, M.R. Phosphorylation primes vinculin for activation. Biophys. J. 2012, 102, 2022–2030. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.M.; Rodriguez, Y.A.; Jeong, K.; Ahn, E.-Y.E.; Lim, S.-T.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Bambang, I.F.; Lu, D.; Li, H.; Chiu, L.-L.; Lau, Q.C.; Koay, E.; Zhang, D. Cytokeratin 19 regulates endoplasmic reticulum stress and inhibits erp29 expression via p38 mapk/xbp-1 signaling in breast cancer cells. Exp. Cell Res. 2009, 315, 1964–1974. [Google Scholar] [CrossRef]
- Sharma, P.; Alsharif, S.; Bursch, K.; Parvathaneni, S.; Anastasakis, D.G.; Chahine, J.; Fallatah, A.; Nicolas, K.; Sharma, S.; Hafner, M. Keratin 19 regulates cell cycle pathway and sensitivity of breast cancer cells to cdk inhibitors. Sci. Rep. 2019, 9, 14650. [Google Scholar] [CrossRef] [PubMed]
- Moers, K.; Steinberg, T.; Schlunck, G.; Reinhard, T.; Tomakidi, P.; Eberwein, P. Substrate elasticity as biomechanical modulator of tissue homeostatic parameters in corneal keratinocytes. Exp. Cell Res. 2013, 319, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Husari, A.; Dieterle, M.P.; Fontaine, S.; Prucker, O.; Tomakidi, P.; Rühe, J. Pnba/pdmaa-based iron-loaded micropillars allow for discrete cell adhesion and analysis of actuation-related molecular responses. Adv. Mater. Interfaces 2020, 7, 1901806. [Google Scholar] [CrossRef]
- Belgardt, E.; Steinberg, T.; Husari, A.; Dieterle, M.P.; Hülter-Hassler, D.; Jung, B.; Tomakidi, P. Force-responsive zyxin modulation in periodontal ligament cells is regulated by yap rather than taz. Cell Signal. 2020, 72, 109662. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Steinberg, T.; Dieterle, M.P.; Ramminger, I.; Husari, A.; Tomakidi, P. FAK Shutdown: Consequences on Epithelial Morphogenesis and Biomarker Expression Involving an Innovative Biomaterial for Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 9774. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189774
Wang X, Steinberg T, Dieterle MP, Ramminger I, Husari A, Tomakidi P. FAK Shutdown: Consequences on Epithelial Morphogenesis and Biomarker Expression Involving an Innovative Biomaterial for Tissue Regeneration. International Journal of Molecular Sciences. 2021; 22(18):9774. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189774
Chicago/Turabian StyleWang, Xiaoling, Thorsten Steinberg, Martin P. Dieterle, Imke Ramminger, Ayman Husari, and Pascal Tomakidi. 2021. "FAK Shutdown: Consequences on Epithelial Morphogenesis and Biomarker Expression Involving an Innovative Biomaterial for Tissue Regeneration" International Journal of Molecular Sciences 22, no. 18: 9774. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189774






